Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease
Abstract
:1. Introduction
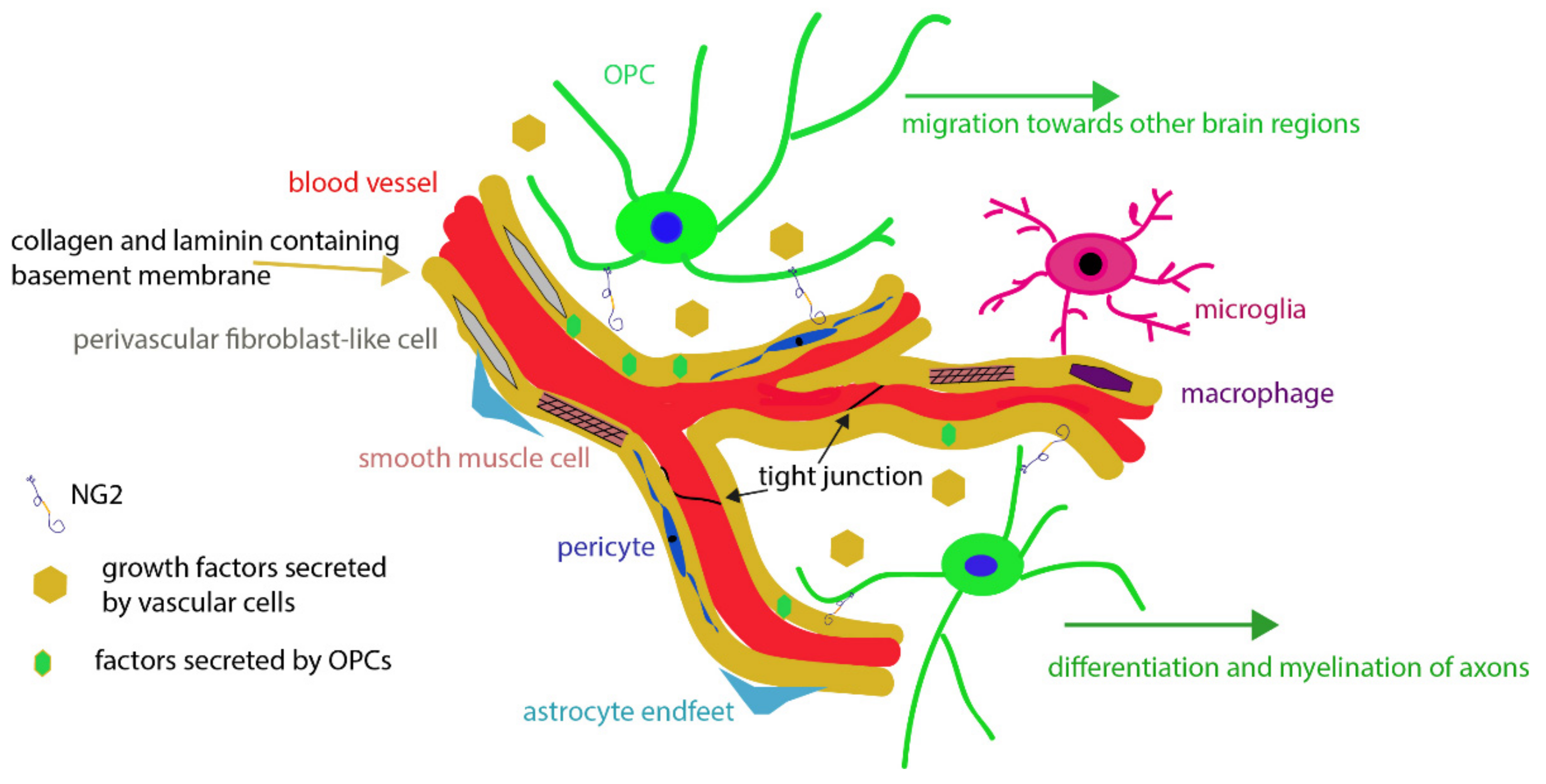
2. The Neurovascular Unit
2.1. Endothelial Cells
2.2. Mural Cells
2.3. Perivascular Fibroblast-Like Cells
2.4. Astrocytes
2.5. Basal Lamina
2.6. Perivascular Macrophages and Juxtavascular Microglia
3. Oligodendrocyte Precursor Cells (OPCs)
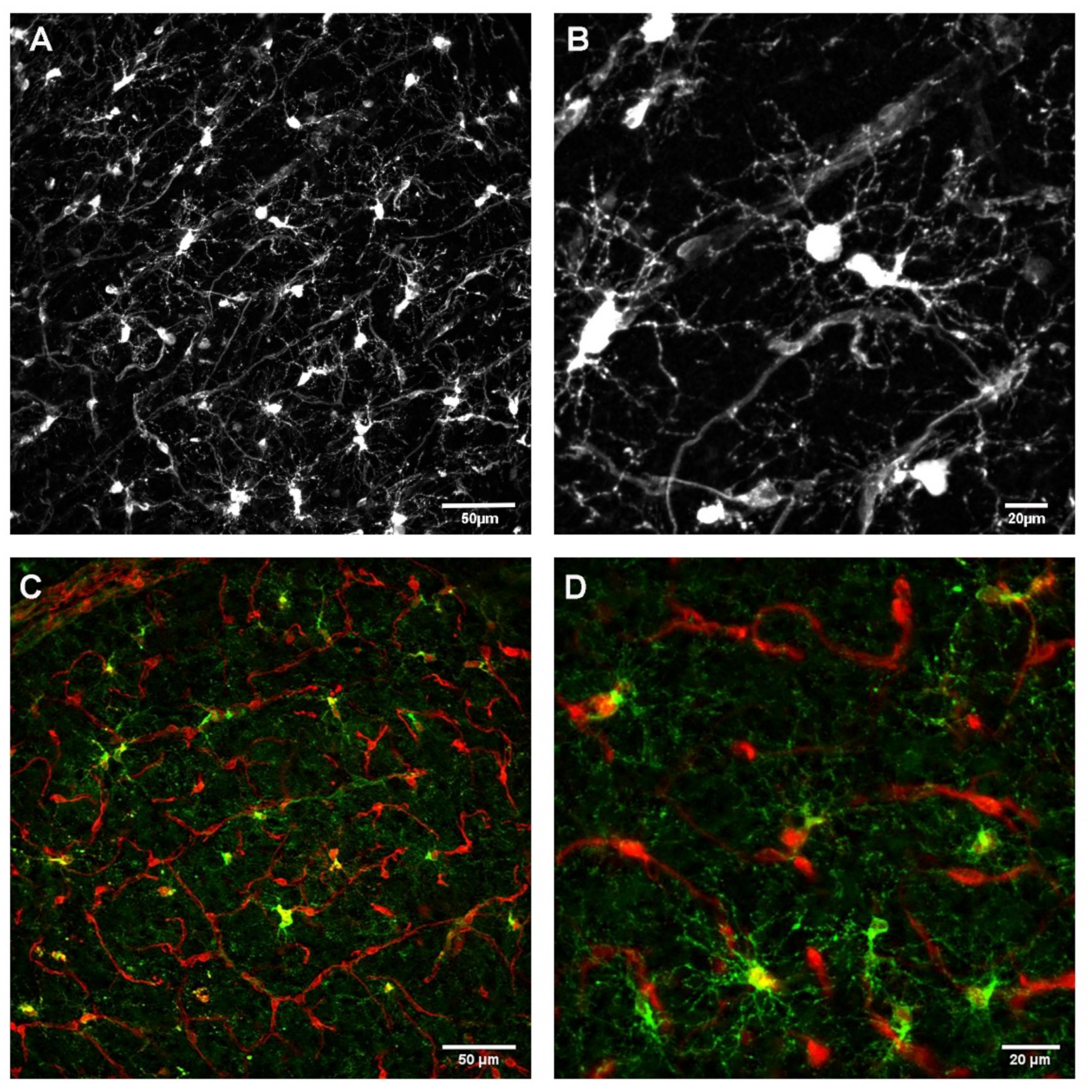
3.1. Relationship between OPC Processes and Vascular Elements
3.1.1. Development
3.1.2. Homeostasis
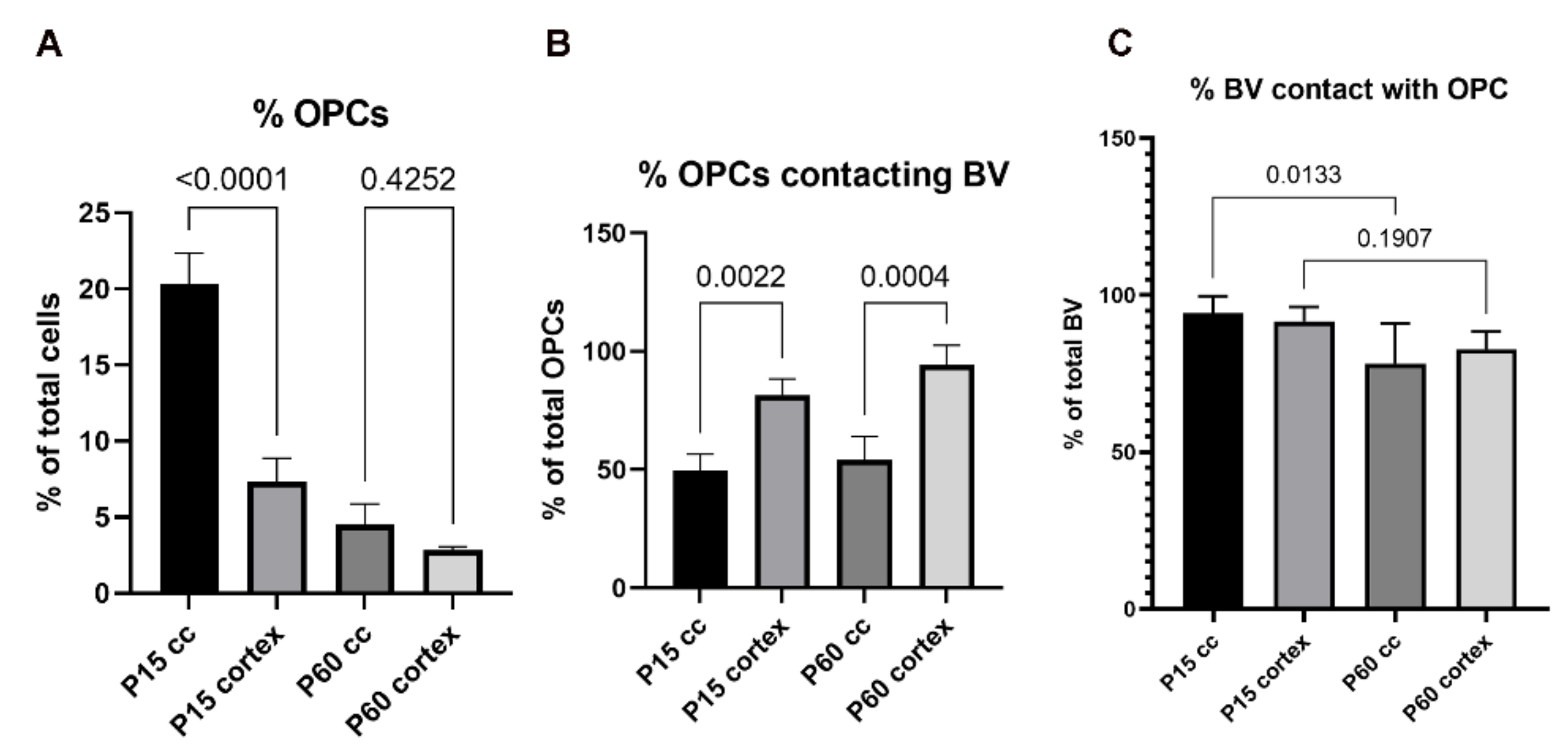
3.1.3. Influence of Brain Region
3.2. Vascular-Oligodendroglial Interactions in Disease
3.2.1. White Matter Injury
3.2.2. Inflammation
3.2.3. Multiple Sclerosis
4. The NG2 Chondroitin Sulfate Proteoglycan
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta. Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci 2020, 21, 416–432. [Google Scholar] [CrossRef]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharm. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030.e1016. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.A.; Tong, L.; Yuan, P.; Murikinati, S.; Gupta, S.; Grutzendler, J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron 2015, 87, 95–110. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Vanlandewijck, M.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Mendanha Falcao, A.; Xiao, L.; Li, H.; Haring, M.; Hochgerner, H.; Romanov, R.A.; et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef] [Green Version]
- Dang, T.C.; Ishii, Y.; Nguyen, V.; Yamamoto, S.; Hamashima, T.; Okuno, N.; Nguyen, Q.L.; Sang, Y.; Ohkawa, N.; Saitoh, Y.; et al. Powerful Homeostatic Control of Oligodendroglial Lineage by PDGFRalpha in Adult Brain. Cell Rep. 2019, 27, 1073–1089.e1075. [Google Scholar] [CrossRef] [Green Version]
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging links between cerebrovascular and neurodegenerative diseases-a special role for pericytes. EMBO Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Dorrier, C.E.; Aran, D.; Haenelt, E.A.; Sheehy, R.N.; Hoi, K.K.; Pintaric, L.; Chen, Y.; Lizama, C.O.; Cautivo, K.M.; Weiner, G.A.; et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 2021, 24, 234–244. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Hayakawa, K.; Pham, L.D.; Som, A.T.; Lee, B.J.; Guo, S.; Lo, E.H.; Arai, K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J. Neurosci. 2011, 31, 10666–10670. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Neuhaus, J.; Risau, W.; Wolburg, H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann. NY. Acad. Sci. 1991, 633, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Wolburg-Buchholz, K.; Mack, A.F.; Steiner, E.; Pfeiffer, F.; Engelhardt, B.; Wolburg, H. Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis. Acta. Neuropathol. 2009, 118, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, T.; Bechmann, I.; Engelhardt, B. Perivascular spaces and the two steps to neuroinflammation. J. Neuropathol. Exp. Neurol. 2008, 67, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.; Bix, G.; Yao, Y. Basal lamina changes in neurodegenerative disorders. Mol. Neurodegener 2021, 16, 81. [Google Scholar] [CrossRef]
- Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001, 153, 933–946. [Google Scholar] [CrossRef]
- Relucio, J.; Menezes, M.J.; Miyagoe-Suzuki, Y.; Takeda, S.; Colognato, H. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia 2012, 60, 1451–1467. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.E.; Lange, S.; Hinrichsen, B.; Philp, A.R.; Reyes, C.R.; Halabi, D.; Mansilla, J.B.; Rotheneichner, P.; Guzman de la Fuente, A.; Couillard-Despres, S.; et al. Pericytes Favor Oligodendrocyte Fate Choice in Adult Neural Stem Cells. Front. Cell Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Hyodo, M.; Hayashi, C.; Mabuchi, Y.; Sekimoto, K.; Onchi, C.; Sekiguchi, K.; Akazawa, C. Laminin alpha2, alpha4, and alpha5 Chains Positively Regulate Migration and Survival of Oligodendrocyte Precursor Cells. Sci. Rep. 2019, 9, 19882. [Google Scholar] [CrossRef]
- Burg, M.A.; Tillet, E.; Timpl, R.; Stallcup, W.B. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J. Biol. Chem. 1996, 271, 26110–26116. [Google Scholar] [CrossRef] [Green Version]
- Bonney, S.K.; Coelho-Santos, V.; Huang, S.F.; Takeno, M.; Kornfeld, J.; Keller, A.; Shih, A.Y. Public Volume Electron Microscopy Data: An Essential Resource to Study the Brain Microvasculature. Front. Cell Dev. Biol. 2022, 10, 849469. [Google Scholar] [CrossRef]
- Mildenberger, W.; Stifter, S.A.; Greter, M. Diversity and function of brain-associated macrophages. Curr. Opin. Immunol. 2022, 76, 102181. [Google Scholar] [CrossRef]
- Dudvarski Stankovic, N.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H.H. Microglia-blood vessel interactions: A double-edged sword in brain pathologies. Acta. Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef]
- Zhao, X.; Eyo, U.B.; Murugan, M.; Wu, L.J. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol. 2018, 78, 604–617. [Google Scholar] [CrossRef]
- Mondo, E.; Becker, S.C.; Kautzman, A.G.; Schifferer, M.; Baer, C.E.; Chen, J.; Huang, E.J.; Simons, M.; Schafer, D.P. A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature. J. Neurosci. 2020, 40, 6503–6521. [Google Scholar] [CrossRef]
- Grossmann, R.; Stence, N.; Carr, J.; Fuller, L.; Waite, M.; Dailey, M.E. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 2002, 37, 229–240. [Google Scholar] [CrossRef]
- Nishiyama, A.; Watanabe, M.; Yang, Z.; Bu, J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J. Neurocytol. 2002, 31, 437–455. [Google Scholar] [CrossRef]
- Zhu, X.; Hill, R.A.; Dietrich, D.; Komitova, M.; Suzuki, R.; Nishiyama, A. Age-dependent fate and lineage restriction of single NG2 cells. Development 2011, 138, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Boshans, L.; Goncalves, C.M.; Wegrzyn, J.; Patel, K.D. Lineage, fate, and fate potential of NG2-glia. Brain Res. 2016, 1638, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Lin, X.H.; Giese, N.; Heldin, C.H.; Stallcup, W.B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci Res. 1996, 43, 315–330. [Google Scholar] [CrossRef]
- Pringle, N.P.; Mudhar, H.S.; Collarini, E.J.; Richardson, W.D. PDGF receptors in the rat CNS: During late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 1992, 115, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Sherafat, A.; Pfeiffer, F.; Nishiyama, A. Shaping of Regional Differences in Oligodendrocyte Dynamics by Regional Heterogeneity of the Pericellular Microenvironment. Front. Cell Neurosci. 2021, 15, 721376. [Google Scholar] [CrossRef] [PubMed]
- Boshans, L.L.; Sherafat, A.; Nishiyama, A. The effects of developmental and current niches on oligodendrocyte precursor dynamics and fate. Neurosci. Lett 2020, 715, 134593. [Google Scholar] [CrossRef]
- Hill, R.A.; Patel, K.D.; Medved, J.; Reiss, A.M.; Nishiyama, A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 2013, 33, 14558–14566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, S.; van Bruggen, D.; Vanichkina, D.P.; Floriddia, E.M.; Munguba, H.; Varemo, L.; Giacomello, S.; Falcao, A.M.; Meijer, M.; Bjorklund, A.K.; et al. Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development. Dev. Cell 2018, 46, 504–517 e507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, R.G.; Lyons, D.A. On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function. J. Neurosci. 2017, 37, 10023–10034. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.W.; Monje, M. Wrapped to Adapt: Experience-Dependent Myelination. Neuron 2017, 95, 743–756. [Google Scholar] [CrossRef]
- Bonetto, G.; Kamen, Y.; Evans, K.A.; Karadottir, R.T. Unraveling Myelin Plasticity. Front. Cell Neurosci. 2020, 14, 156. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef]
- Nishiyama, A.; Suzuki, R.; Zhu, X. NG2 cells (polydendrocytes) in brain physiology and repair. Front. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef]
- Bonetto, G.; Belin, D.; Karadottir, R.T. Myelin: A gatekeeper of activity-dependent circuit plasticity? Science 2021, 374, eaba6905. [Google Scholar] [CrossRef]
- Bedner, P.; Jabs, R.; Steinhauser, C. Properties of human astrocytes and NG2 glia. Glia 2020, 68, 756–767. [Google Scholar] [CrossRef]
- Carmeliet, P.; Storkebaum, E. Vascular and neuronal effects of VEGF in the nervous system: Implications for neurological disorders. Semin. Cell Dev. Biol. 2002, 13, 39–53. [Google Scholar] [CrossRef]
- Le Bras, B.; Barallobre, M.J.; Homman-Ludiye, J.; Ny, A.; Wyns, S.; Tammela, T.; Haiko, P.; Karkkainen, M.J.; Yuan, L.; Muriel, M.P.; et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat. Neurosci. 2006, 9, 340–348. [Google Scholar] [CrossRef]
- Hiratsuka, D.; Kurganov, E.; Furube, E.; Morita, M.; Miyata, S. VEGF- and PDGF-dependent proliferation of oligodendrocyte progenitor cells in the medulla oblongata after LPC-induced focal demyelination. J. Neuroimmunol. 2019, 332, 176–186. [Google Scholar] [CrossRef]
- Kimura, I.; Dohgu, S.; Takata, F.; Matsumoto, J.; Watanabe, T.; Iwao, T.; Yamauchi, A.; Kataoka, Y. Oligodendrocytes upregulate blood-brain barrier function through mechanisms other than the PDGF-BB/PDGFRalpha pathway in the barrier-tightening effect of oligodendrocyte progenitor cells. Neurosci. Lett 2020, 715, 134594. [Google Scholar] [CrossRef]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [Green Version]
- Arai, K.; Lo, E.H. An oligovascular niche: Cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef]
- Miyamoto, N.; Pham, L.D.; Seo, J.H.; Kim, K.W.; Lo, E.H.; Arai, K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol. Life Sci. 2014, 71, 1055–1066. [Google Scholar] [CrossRef]
- Zerlin, M.; Goldman, J.E. Interactions between glial progenitors and blood vessels during early postnatal corticogenesis: Blood vessel contact represents an early stage of astrocyte differentiation. J. Comp. Neurol. 1997, 387, 537–546. [Google Scholar] [CrossRef]
- Paredes, I.; Vieira, J.R.; Shah, B.; Ramunno, C.F.; Dyckow, J.; Adler, H.; Richter, M.; Schermann, G.; Giannakouri, E.; Schirmer, L.; et al. Oligodendrocyte precursor cell specification is regulated by bidirectional neural progenitor-endothelial cell crosstalk. Nat. Neurosci. 2021, 24, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavali, M.; Ulloa-Navas, M.J.; Perez-Borreda, P.; Garcia-Verdugo, J.M.; McQuillen, P.S.; Huang, E.J.; Rowitch, D.H. Wnt-Dependent Oligodendroglial-Endothelial Interactions Regulate White Matter Vascularization and Attenuate Injury. Neuron 2020, 108, 1130–1145 e1135. [Google Scholar] [CrossRef] [PubMed]
- Minocha, S.; Valloton, D.; Brunet, I.; Eichmann, A.; Hornung, J.P.; Lebrand, C. NG2 glia are required for vessel network formation during embryonic development. Elife 2015, 4, e09102. [Google Scholar] [CrossRef]
- Delgado, A.C.; Maldonado-Soto, A.R.; Silva-Vargas, V.; Mizrak, D.; von Kanel, T.; Tan, K.R.; Paul, A.; Madar, A.; Cuervo, H.; Kitajewski, J.; et al. Release of stem cells from quiescence reveals gliogenic domains in the adult mouse brain. Science 2021, 372, 1205–1209. [Google Scholar] [CrossRef]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Sherafat, A.; Nishiyama, A. The Impact of Fixation on the Detection of Oligodendrocyte Precursor Cell Morphology and Vascular Associations. Cells 2021, 10, 1302. [Google Scholar] [CrossRef]
- Swire, M.; Kotelevtsev, Y.; Webb, D.J.; Lyons, D.A.; Ffrench-Constant, C. Endothelin signalling mediates experience-dependent myelination in the CNS. Elife 2019, 8, e49493. [Google Scholar] [CrossRef]
- Gross, P.M.; Sposito, N.M.; Pettersen, S.E.; Fenstermacher, J.D. Differences in function and structure of the capillary endothelium in gray matter, white matter and a circumventricular organ of rat brain. Blood Vessel. 1986, 23, 261–270. [Google Scholar] [CrossRef]
- Cavaglia, M.; Dombrowski, S.M.; Drazba, J.; Vasanji, A.; Bokesch, P.M.; Janigro, D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001, 910, 81–93. [Google Scholar] [CrossRef]
- Borowsky, I.W.; Collins, R.C. Histochemical changes in enzymes of energy metabolism in the dentate gyrus accompany deafferentation and synaptic reorganization. Neuroscience 1989, 33, 253–262. [Google Scholar] [CrossRef]
- Maki, T.; Maeda, M.; Uemura, M.; Lo, E.K.; Terasaki, Y.; Liang, A.C.; Shindo, A.; Choi, Y.K.; Taguchi, A.; Matsuyama, T.; et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci. Lett 2015, 597, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Sherafat, A.; Pfeiffer, F.; Reiss, A.M.; Wood, W.M.; Nishiyama, A. Microglial neuropilin-1 promotes oligodendrocyte expansion during development and remyelination by trans-activating platelet-derived growth factor receptor. Nat. Commun. 2021, 12, 2265. [Google Scholar] [CrossRef]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef]
- Savchenko, V.L.; McKanna, J.A.; Nikonenko, I.R.; Skibo, G.G. Microglia and astrocytes in the adult rat brain: Comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience 2000, 96, 195–203. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp. Transl. Stroke Med. 2009, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1646; discussion 1647. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M.V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction mapping. J. Cereb Blood Flow Metab 2018, 38, 919–931. [Google Scholar] [CrossRef]
- Rajani, R.M.; Quick, S.; Ruigrok, S.R.; Graham, D.; Harris, S.E.; Verhaaren, B.F.J.; Fornage, M.; Seshadri, S.; Atanur, S.S.; Dominiczak, A.F.; et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 2018, 10, eaam9507. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puentes, S.; Kurachi, M.; Shibasaki, K.; Naruse, M.; Yoshimoto, Y.; Mikuni, M.; Imai, H.; Ishizaki, Y. Brain microvascular endothelial cell transplantation ameliorates ischemic white matter damage. Brain Res. 2012, 1469, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Kurachi, M.; Shibasaki, K.; Naruse, M.; Puentes, S.; Imai, H.; Yoshimoto, Y.; Mikuni, M.; Ishizaki, Y. Transplanted microvascular endothelial cells promote oligodendrocyte precursor cell survival in ischemic demyelinating lesions. J. Neurochem. 2015, 135, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.D.; Maki, T.; Ayata, C.; Kim, K.W.; Lo, E.H.; Arai, K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Kishida, N.; Maki, T.; Takagi, Y.; Yasuda, K.; Kinoshita, H.; Ayaki, T.; Noro, T.; Kinoshita, Y.; Ono, Y.; Kataoka, H.; et al. Role of Perivascular Oligodendrocyte Precursor Cells in Angiogenesis After Brain Ischemia. J. Am. Heart Assoc. 2019, 8, e011824. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Geng, J.; Qu, M.; Yuan, F.; Wang, Y.; Pan, J.; Li, Y.; Ma, Y.; Zhou, P.; Zhang, Z.; et al. Oligodendrocyte precursor cells transplantation protects blood-brain barrier in a mouse model of brain ischemia via Wnt/beta-catenin signaling. Cell Death Dis. 2020, 11, 9. [Google Scholar] [CrossRef]
- Guerit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Liebner, S.; Plate, K.H. Differentiation of the brain vasculature: The answer came blowing by the Wnt. J. Angiogenes Res. 2010, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl Acad Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef] [Green Version]
- Funa, K.; Sasahara, M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J. Neuroimmune Pharm. 2014, 9, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Arjunan, P.; Lee, C.; Li, Y.; Kumar, A.; Hou, X.; Wang, B.; Wardega, P.; Zhang, F.; Dong, L.; et al. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J. Exp. Med. 2010, 207, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.L.; Okuno, N.; Hamashima, T.; Dang, S.T.; Fujikawa, M.; Ishii, Y.; Enomoto, A.; Maki, T.; Nguyen, H.N.; Nguyen, V.T.; et al. Vascular PDGFR-alpha protects against BBB dysfunction after stroke in mice. Angiogenesis 2021, 24, 35–46. [Google Scholar] [CrossRef]
- Baron-Van Evercooren, A.; Avellana-Adalid, V.; Ben Younes-Chennoufi, A.; Gansmuller, A.; Nait-Oumesmar, B.; Vignais, L. Cell-cell interactions during the migration of myelin-forming cells transplanted in the demyelinated spinal cord. Glia 1996, 16, 147–164. [Google Scholar] [CrossRef]
- Errede, M.; Girolamo, F.; Ferrara, G.; Strippoli, M.; Morando, S.; Boldrin, V.; Rizzi, M.; Uccelli, A.; Perris, R.; Bendotti, C.; et al. Blood-brain barrier alterations in the cerebral cortex in experimental autoimmune encephalomyelitis. J. Neuropathol. Exp. Neurol. 2012, 71, 840–854. [Google Scholar] [CrossRef] [Green Version]
- Girolamo, F.; Errede, M.; Longo, G.; Annese, T.; Alias, C.; Ferrara, G.; Morando, S.; Trojano, M.; Kerlero de Rosbo, N.; Uccelli, A.; et al. Defining the role of NG2-expressing cells in experimental models of multiple sclerosis. A biofunctional analysis of the neurovascular unit in wild type and NG2 null mice. PLoS ONE 2019, 14, e0213508. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb Perspect Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [Green Version]
- Stadelmann, C.; Albert, M.; Wegner, C.; Bruck, W. Cortical pathology in multiple sclerosis. Curr. Opin Neurol. 2008, 21, 229–234. [Google Scholar] [CrossRef]
- Huitema, M.J.D.; Strijbis, E.M.M.; Luchicchi, A.; Bol, J.; Plemel, J.R.; Geurts, J.J.G.; Schenk, G.J. Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination. Int. J. Mol. Sci. 2021, 22, 12634. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Bo, L.; Mork, S.; Chang, A.; Trapp, B.D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001, 50, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Schul, E.; Geurts, J.; van der Valk, P.; Drukarch, B.; van Dam, A.M. Pathological differences between white and grey matter multiple sclerosis lesions. Ann. NY. Acad Sci. 2015, 1351, 99–113. [Google Scholar] [CrossRef]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2018, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Strijbis, E.M.M.; Kooi, E.J.; van der Valk, P.; Geurts, J.J.G. Cortical Remyelination Is Heterogeneous in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2017, 76, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.; Antel, J.; Bruck, W.; Stadelmann, C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [Green Version]
- Guttmann, C.R.; Rousset, M.; Roch, J.A.; Hannoun, S.; Durand-Dubief, F.; Belaroussi, B.; Cavallari, M.; Rabilloud, M.; Sappey-Marinier, D.; Vukusic, S.; et al. Multiple sclerosis lesion formation and early evolution revisited: A weekly high-resolution magnetic resonance imaging study. Mult. Scler. 2016, 22, 761–769. [Google Scholar] [CrossRef]
- Petersen, M.A.; Ryu, J.K.; Chang, K.J.; Etxeberria, A.; Bardehle, S.; Mendiola, A.S.; Kamau-Devers, W.; Fancy, S.P.J.; Thor, A.; Bushong, E.A.; et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron 2017, 96, 1003–1012 e1007. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.K.; Rafalski, V.A.; Meyer-Franke, A.; Adams, R.A.; Poda, S.B.; Rios Coronado, P.E.; Pedersen, L.O.; Menon, V.; Baeten, K.M.; Sikorski, S.L.; et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol 2018, 19, 1212–1223. [Google Scholar] [CrossRef]
- Petersen, M.A.; Tognatta, R.; Meyer-Franke, A.; Bushong, E.A.; Mendiola, A.S.; Yan, Z.; Muthusamy, A.; Merlini, M.; Meza-Acevedo, R.; Cabriga, B.; et al. BMP receptor blockade overcomes extrinsic inhibition of remyelination and restores neurovascular homeostasis. Brain 2021, 144, 2291–2301. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Spieth, L.; Saher, G. Local cholesterol metabolism orchestrates remyelination. Trends Neurosci. 2022, 45, 272–283. [Google Scholar] [CrossRef]
- Nait-Oumesmar, B.; Picard-Riera, N.; Kerninon, C.; Decker, L.; Seilhean, D.; Hoglinger, G.U.; Hirsch, E.C.; Reynolds, R.; Baron-Van Evercooren, A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 4694–4699. [Google Scholar] [CrossRef] [Green Version]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.J.M.; Frisen, J.; Lyons, D.A. Revisiting remyelination: Towards a consensus on the regeneration of CNS myelin. Semin Cell Dev. Biol. 2021, 116, 3–9. [Google Scholar] [CrossRef]
- Jakel, S.; Agirre, E.; Mendanha Falcao, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Stallcup, W.B. The NG2 proteoglycan: Past insights and future prospects. J. Neurocytol. 2002, 31, 423–435. [Google Scholar] [CrossRef]
- Nishiyama, A.; Dahlin, K.J.; Prince, J.T.; Johnstone, S.R.; Stallcup, W.B. The primary structure of NG2, a novel membrane-spanning proteoglycan. J. Cell Biol. 1991, 114, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Stegmuller, J.; Schneider, S.; Hellwig, A.; Garwood, J.; Trotter, J. AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J. Neurocytol. 2002, 31, 497–505. [Google Scholar] [CrossRef]
- Stallcup, W.B.; Huang, F.J. A role for the NG2 proteoglycan in glioma progression. Cell Adh. Migr. 2008, 2, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Tillet, E.; Ruggiero, F.; Nishiyama, A.; Stallcup, W.B. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J. Biol. Chem. 1997, 272, 10769–10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favor, J.; Gloeckner, C.J.; Janik, D.; Klempt, M.; Neuhauser-Klaus, A.; Pretsch, W.; Schmahl, W.; Quintanilla-Fend, L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: An extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics 2007, 175, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, A.; Stallcup, W.B. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol. Biol Cell 1993, 4, 1097–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushi, J.; Makagiansar, I.T.; Stallcup, W.B. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell 2004, 15, 3580–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgintino, D.; Girolamo, F.; Errede, M.; Capobianco, C.; Robertson, D.; Stallcup, W.B.; Perris, R.; Roncali, L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 2007, 10, 35–45. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Finocchiaro, G.; Pizzi, G.; Nunnari, M.; Gitto, G.; Fabiano, V.; Parisi, A.; Venuti, A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res. 2009, 29, 449–453. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeiffer, F. Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease. Cells 2022, 11, 1954. https://doi.org/10.3390/cells11121954
Pfeiffer F. Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease. Cells. 2022; 11(12):1954. https://doi.org/10.3390/cells11121954
Chicago/Turabian StylePfeiffer, Friederike. 2022. "Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease" Cells 11, no. 12: 1954. https://doi.org/10.3390/cells11121954
APA StylePfeiffer, F. (2022). Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease. Cells, 11(12), 1954. https://doi.org/10.3390/cells11121954





