Alpha-Synuclein and Its Role in Melanocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Tissue Samples
2.3. Transfection Experiments
2.4. RNA Isolation, Reverse Transcription, and Quantitative RT-PCR
2.5. Transcriptome Analysis with cDNA Microarray
2.6. Protein Isolation and Western Blot Analysis
2.7. Cell Fractionation
2.8. Melanin Content Determination
2.9. Melanosome Isolation of Cell Culture Supernatant and Staining
2.10. Light Scattering
2.11. Immunofluorescence Analysis
2.12. Fluorescence-Activated-Cell-Sorting (FACS)
2.13. Statistical Analysis
2.14. Accession Numbers
3. Results
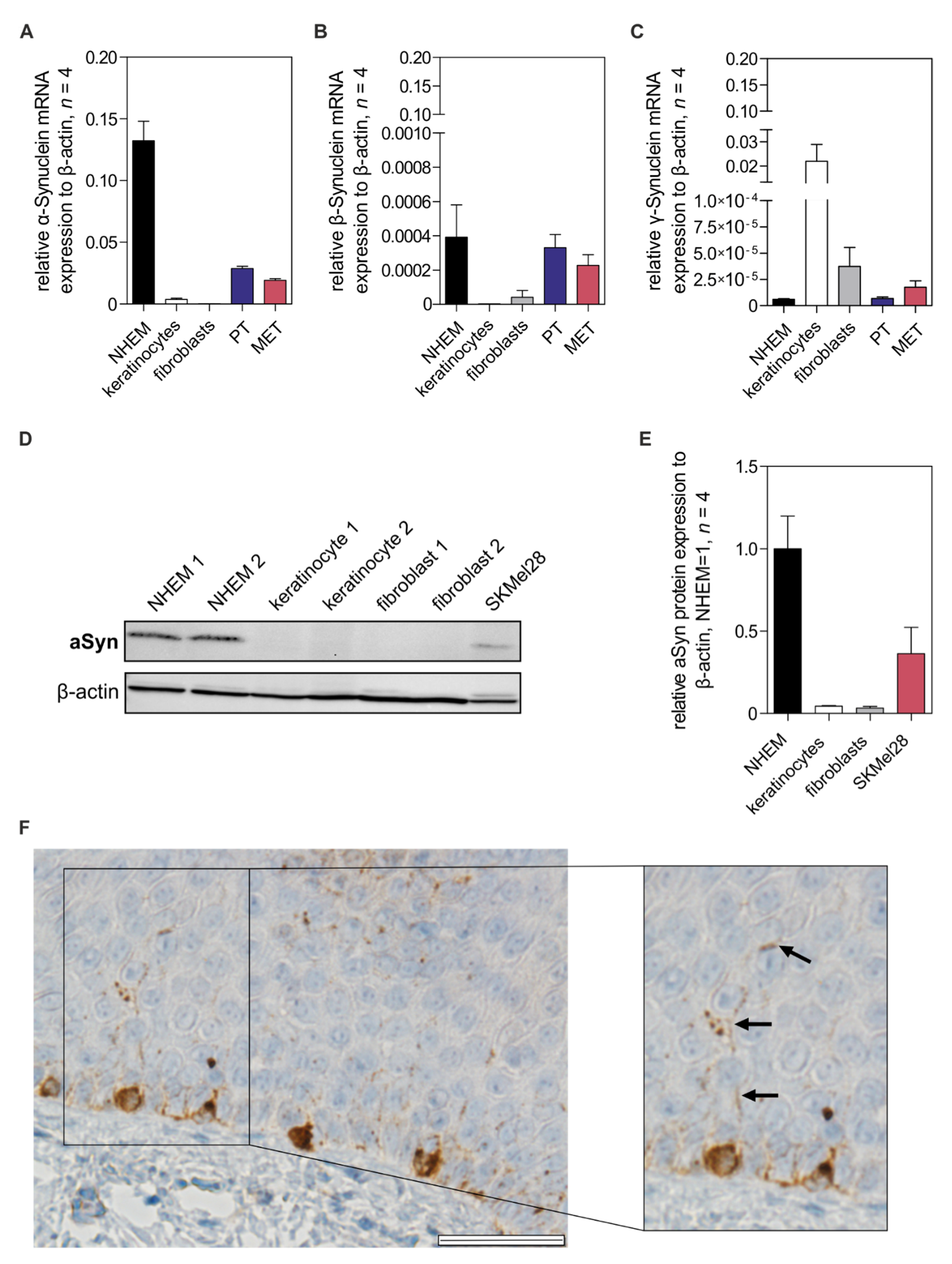
3.1. Alpha-Synuclein Expression in Melanocytes
3.2. Alpha-Synuclein Expression Is Regulated in Melanocytic Differentiation by MITF
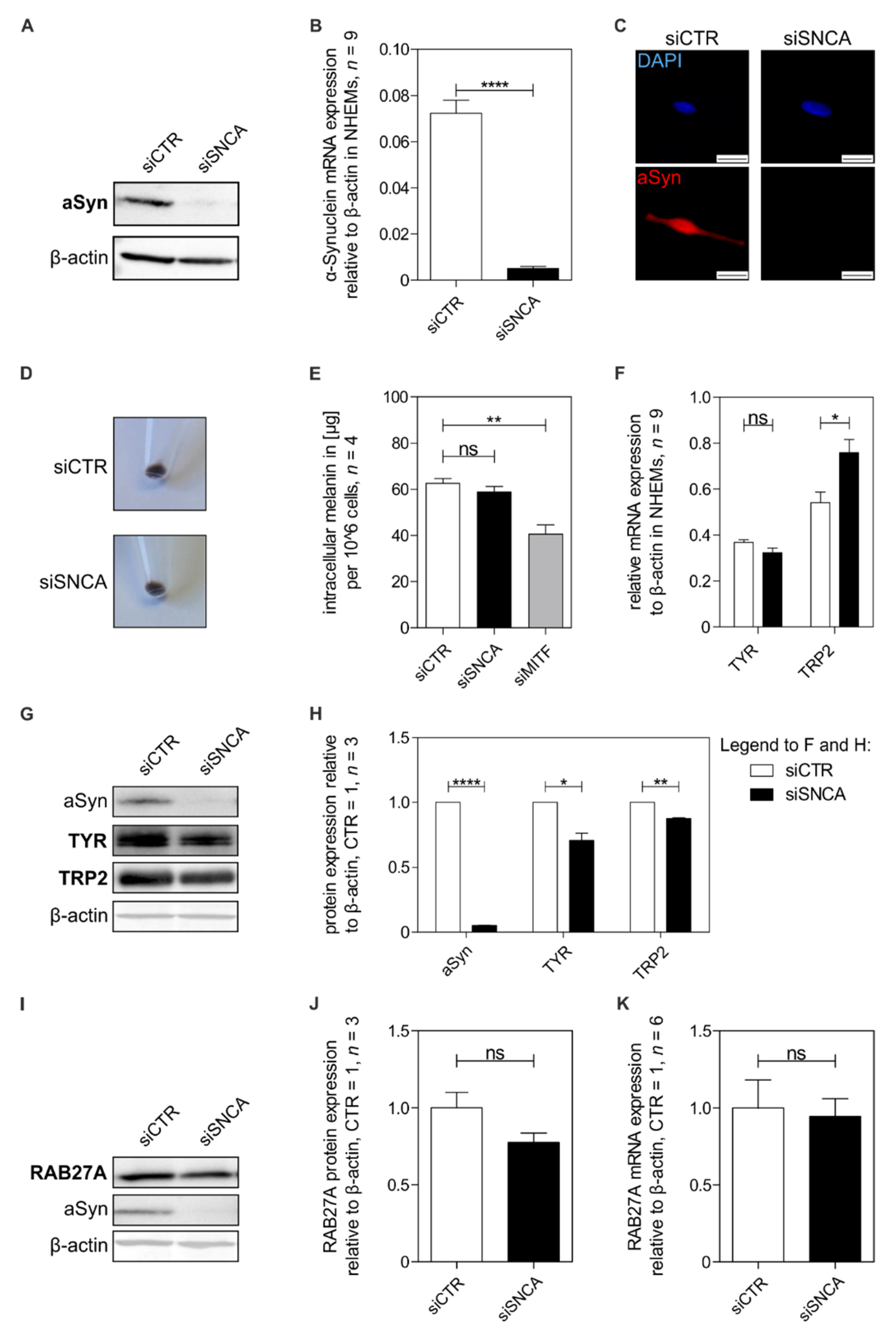
3.3. Influence of Alpha-Synuclein on Melanocytic Differentiation
3.4. Alpha-Synuclein and Mitochondria
3.5. Effect of Alpha-Synuclein on Intracellular Melanosome Distribution and Transport
3.6. Role of Alpha-Synuclein on Melanosome Release and Transfer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergqvist, C.; Ezzedine, K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J. Dermatol. 2021, 48, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Yang, K.; Oak, A.S.W.; Slominski, R.M.; Brożyna, A.A.; Slominski, A.T. Current Molecular Markers of Melanoma and Treatment Targets. Int. J. Mol. Sci. 2020, 21, 3535. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sviderskaya, E.V.; Hill, S.P.; Balachandar, D.; Barsh, G.S.; Bennett, D.C. Agouti signaling protein and other factors modulating differentiation and proliferation of immortal melanoblasts. Dev. Dyn. 2001, 221, 373–379. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. Bras. Derm. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Kinsler, V.A.; Larue, L. The patterns of birthmarks suggest a novel population of melanocyte precursors arising around the time of gastrulation. Pigment Cell Melanoma Res. 2018, 31, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, N.; Berx, G. From neural crest cells to melanocytes: Cellular plasticity during development and beyond. Cell. Mol. Life Sci. 2019, 76, 1919–1934. [Google Scholar] [CrossRef]
- Tatarakis, D.; Cang, Z.; Wu, X.; Sharma, P.P.; Karikomi, M.; MacLean, A.L.; Nie, Q.; Schilling, T.F. Single-cell transcriptomic analysis of zebrafish cranial neural crest reveals spatiotemporal regulation of lineage decisions during development. Cell Rep. 2021, 37, 110140. [Google Scholar] [CrossRef]
- Colombo, S.; Petit, V.; Wagner, R.Y.; Champeval, D.; Yajima, I.; Gesbert, F.; Aktary, Z.; Davidson, I.; Delmas, V.; Larue, L. Stabilization of β-catenin promotes melanocyte specification at the expense of the Schwann cell lineage. Development 2022, 149, dev194407. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Adameyko, I. Schwann cell precursor: A neural crest cell in disguise? Dev. Biol. 2018, 444, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.M.; Pfeiffer, Z.A.; Ciriello, J.M.; Horrigan, D.M.; Wicks, N.L.; Flaherty, B.; Oancea, E. Melanosomal dynamics assessed with a live-cell fluorescent melanosomal marker. PLoS ONE 2012, 7, e43465. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cui, Z.; Liu, S.; Zhou, J.; Cui, R. Melanosome transport and regulation in development and disease. Pharm. Ther. 2021, 219, 107707. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005, 37, 3–14. [Google Scholar] [CrossRef]
- Bowman, S.L.; Bi-Karchin, J.; Le, L.; Marks, M.S. The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases. Traffic 2019, 20, 404–435. [Google Scholar] [CrossRef] [Green Version]
- Le, L.; Sirés-Campos, J.; Raposo, G.; Delevoye, C.; Marks, M.S. Melanosome Biogenesis in the Pigmentation of Mammalian Skin. Integr. Comp. Biol. 2021, 61, 1517–1545. [Google Scholar] [CrossRef]
- Lee, A.Y. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, R.; Takahashi, Y. Intercellular transfer of organelles during body pigmentation. Curr. Opin. Genet. Dev. 2017, 45, 132–138. [Google Scholar] [CrossRef]
- Fukuda, M. Rab GTPases: Key players in melanosome biogenesis, transport, and transfer. Pigment Cell Melanoma Res. 2021, 34, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R.; van der Brug, M. Cell systems and the toxic mechanism(s) of α-synuclein. Exp. Neurol. 2008, 209, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Rocha, E.M.; de Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Surguchov, A. Intracellular Dynamics of Synucleins: “Here, There and Eyerywhere”. Int. Rev. Cell. Mol. Biol. 2015, 320, 130–169. [Google Scholar] [CrossRef]
- Xu, L.; Pu, J. Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application. Parkinsons Dis. 2016, 2016, 1720621. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, O.; Lindquist, N.G. Melanin affinity and its possible role in neurodegeneration. J. Neural Transm. 2013, 120, 1623–1630. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, I.; Chi-Ahumada, E.; Mejía, M.; Castanedo-Cazares, J.P.; Eng, W.; Saikaly, S.K.; Carrizales, J.; Levine, T.D.; Norman, R.A.; Jimenez-Capdeville, M.E. The Presence of Alpha-Synuclein in Skin from Melanoma and Patients with Parkinson’s Disease. Mov. Disord. Clin. Pr. 2017, 4, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Dean, D.N.; Lee, J.C. Linking Parkinson’s Disease and Melanoma: Interplay Between α-Synuclein and Pmel17 Amyloid Formation. Mov. Disord. 2021, 36, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Niemann, N.; Billnitzer, A.; Jankovic, J. Parkinson’s disease and skin. Parkinsonism Relat. Disord. 2021, 82, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wen, Y.; Al-Kuwari, N.; Chen, X. Association Between Parkinson’s Disease and Melanoma: Putting the Pieces Together. Front. Aging Neurosci. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.; Ge, P.; Dawson, V.L.; Dawson, T.M. The cell biology of Parkinson’s disease. J. Cell Biol. 2021, 220, e202012095. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef]
- Rachinger, N.; Fischer, S.; Böhme, I.; Linck-Paulus, L.; Kuphal, S.; Kappelmann-Fenzl, M.; Bosserhoff, A.K. Loss of gene information: Discrepancies between rna sequencing, cdna microarray, and qrt-pcr. Int. J. Mol. Sci. 2021, 22, 9349. [Google Scholar] [CrossRef]
- Mueller, D.W.; Bosserhoff, A.K. MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int. J. Cancer 2011, 129, 1064–1074. [Google Scholar] [CrossRef]
- Jacob, K.; Wach, F.; Holzapfel, U.; Hein, R.; Lengyel, E.; Buettner, R.; Bosserhoff, A.K. In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res. 1998, 8, 211–219. [Google Scholar] [CrossRef]
- Jacob, K.; Bosserhoff, A.K.; Wach, F.; Knüchel, R.; Klein, E.C.; Hein, R.; Buettner, R. Characterization of selected strongly and weakly invasive sublines of a primary human melanoma cell line and isolation of subtractive cDNA clones. Int. J. Cancer 1995, 60, 668–675. [Google Scholar] [CrossRef]
- Völler, D.; Reinders, J.; Meister, G.; Bosserhoff, A.K. Strong reduction of AGO2 expression in melanoma and cellular consequences. Br. J. Cancer 2013, 109, 3116–3124. [Google Scholar] [CrossRef] [Green Version]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linck-Paulus, L.; Lämmerhirt, L.; Völler, D.; Meyer, K.; Engelmann, J.C.; Spang, R.; Eichner, N.; Meister, G.; Kuphal, S.; Bosserhoff, A.K. Learning from Embryogenesis-A Comparative Expression Analysis in Melanoblast Differentiation and Tumorigenesis Reveals miRNAs Driving Melanoma Development. J. Clin. Med. 2021, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.K.; Ellmann, L.; Kuphal, S. Melanoblasts in culture as an in vitro system to determine molecular changes in melanoma. Exp. Dermatol. 2011, 20, 435–440. [Google Scholar] [CrossRef]
- Decean, H.; Perde-Schrepler, M.; Tatomir, C.; Fischer-Fodor, E.; Brie, I.; Virag, P. Modulation of the pro-inflammatory cytokines and matrix metalloproteinases production in co-cultivated human keratinocytes and melanocytes. Arch. Dermatol. Res. 2013, 305, 705–714. [Google Scholar] [CrossRef]
- Kumar, R.; Parsad, D.; Kanwar, A.; Kaul, D. Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 599–604. [Google Scholar] [CrossRef]
- Ma, H.J.; Zhao, G.; Zi, S.X.; Li, D.G.; Liu, W.; Yang, Q.Q. Efficacy of quantifying melanosome transfer with flow cytometry in a human melanocyte-HaCaT keratinocyte co-culture system in vitro. Exp. Dermatol. 2010, 19, e282–e285. [Google Scholar] [CrossRef]
- Hannus, M.; Beitzinger, M.; Engelmann, J.C.; Weickert, M.-T.; Spang, R.; Hannus, S.; Meister, G. SiPools: Highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res. 2014, 42, 8049–8061. [Google Scholar] [CrossRef] [Green Version]
- Pommer, M.; Kuphal, S.; Bosserhoff, A.K. Amphiregulin Regulates Melanocytic Senescence. Cells 2021, 10, 326. [Google Scholar] [CrossRef]
- Schiffner, S.; Braunger, B.M.; de Jel, M.M.; Coupland, S.E.; Tamm, E.R.; Bosserhoff, A.K. Tg(Grm1) transgenic mice: A murine model that mimics spontaneous uveal melanoma in humans? Exp. Eye Res. 2014, 127, 59–68. [Google Scholar] [CrossRef]
- Kappelmann, M.; Kuphal, S.; Meister, G.; Vardimon, L.; Bosserhoff, A.-K. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene 2013, 35, 2984–2991. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.Y.; Choi, N.; Lee, J.U.; Lee, E.J.; Kim, J.Y.; Choi, W.J.; Oh, S.H.; Sung, J.-H. Marliolide derivative induces melanosome degradation via nrf2/p62-mediated autophagy. Int. J. Mol. Sci. 2021, 22, 3995. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Matamá, T.; Guimarães, D.; Gomes, A.; Cavaco-Paulo, A. Fluorescent quantification of melanin. Pigment Cell Melanoma Res. 2016, 29, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, T.; Zhu, J.; Hwu, W.J.; Jankovic, J. The Role of Alpha-Synuclein in Melanin Synthesis in Melanoma and Dopaminergic Neuronal Cells. PLoS ONE 2012, 7, e45183. [Google Scholar] [CrossRef] [PubMed]
- Flori, E.; Mastrofrancesco, A.; Kovacs, D.; Ramot, Y.; Briganti, S.; Bellei, B.; Paus, R.; Picardo, M. 2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation. Pigment Cell Melanoma Res. 2011, 24, 618–630. [Google Scholar] [CrossRef]
- Hu, D.N. Methodology for evaluation of melanin content and production of pigment cells in vitro. Photochem. Photobiol. 2008, 84, 645–649. [Google Scholar] [CrossRef]
- Shimasaki, T.; Yamamoto, S.; Arisawa, T. Exosome research and co-culture study. Biol. Pharm. Bull. 2018, 41, 1311–1321. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Wäster, P.; Eriksson, I.; Vainikka, L.; Rosdahl, I.; Öllinger, K. Extracellular vesicles are transferred from melanocytes to keratinocytes after UVA irradiation. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Wang, A.; Chan Miller, C.; Szostak, J.W. Core-Shell Modeling of Light Scattering by Vesicles: Effect of Size, Contents, and Lamellarity. Biophys. J. 2019, 116, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Kappelmann-Fenzl, M.; Kuphal, S.; Krupar, R.; Schadendorf, D.; Umansky, V.; Vardimon, L.; Hellerbrand, C.; Bosserhoff, A.-K. Complex formation with monomeric α-tubulin and importin 13 fosters c-jun protein stability and is required for c-jun’s nuclear translocation and activity. Cancers 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Böhme, I.; Bosserhoff, A. Extracellular acidosis triggers a senescence-like phenotype in human melanoma cells. Pigment Cell Melanoma Res. 2020, 33, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohi, Y.; Qin, H.; Hong, C.; Blouin, L.; Polo, J.M.; Guo, T.; Qi, Z.; Downey, S.L.; Manos, P.D.; Rossi, D.J.; et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 2011, 13, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Kamitani, T. Parkinson’s disease-related protein, α-synuclein, in Malignant Melanoma. PLoS ONE 2010, 5, e10481. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef]
- Valdinocci, D.; Simões, R.F.; Kovarova, J.; Cunha-Oliveira, T.; Neuzil, J.; Pountney, D.L. Intracellular and Intercellular Mitochondrial Dynamics in Parkinson’s Disease. Front. Neurosci 2019, 13, 930. [Google Scholar] [CrossRef] [Green Version]
- Menges, S.; Minakaki, G.; Schaefer, P.M.; Meixner, H.; Prots, I.; Schlötzer-Schrehardt, U.; Friedland, K.; Winner, B.; Outeiro, T.F.; Winklhofer, K.F.; et al. Alpha-Synuclein prevents the formation of spherical mitochondria and apoptosis under oxidative stress. Sci. Rep. 2017, 7, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Nahacka, Z.; Zobalova, R.; Dubisova, M.; Rohlena, J.; Neuzil, J. Miro proteins connect mitochondrial function and intercellular transport. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 401–425. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Hou, K.; Wu, Y.; Chen, H.; Ning, Z. Melanoma and Nanotechnology-Based Treatment. Front. Oncol. 2022, 12, 858185. [Google Scholar] [CrossRef]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef]
- Goding, C.R.; Arnheiter, H. Mitf—The first 25 years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand, M.I.; Terzioglu, M.; Galter, D.; Zhu, S.; Hofstetter, C.; Lindqvist, E.; Thams, S.; Bergstrand, A.; Hansson, F.S.; Trifunovic, A.; et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Cabin, D.E.; Shimazu, K.; Murphy, D.; Cole, N.B.; Gottschalk, W.; MclIwani, K.L.; Orrison, B.; Chen, A.; Ellis, C.E.; Paylor, R.; et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002, 22, 8797–8807. [Google Scholar] [CrossRef] [Green Version]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Barceló-Coblijn, G.; Golovko, M.Y.; Weinhofer, I.; Berger, J.; Murphy, E.J. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J. Neurochem. 2007, 101, 132–141. [Google Scholar] [CrossRef]
- Ellis, C.E.; Murphy, E.J.; Mitchell, D.C.; Golovko, M.Y.; Scaglia, F.; Barceló-Coblijn, G.C.; Nussbaum, R.L. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol. Cell Biol. 2005, 25, 10190–10201. [Google Scholar] [CrossRef] [Green Version]
- Koch, J.C.; Bitow, F.; Haack, J.; d’Hedouville, Z.; Zhang, J.-N.; Tönges, L.; Michel, U.; Oliveira, L.M.A.; Jovin, T.M.; Liman, J.; et al. Alpha-Synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons. Cell Death Dis. 2015, 6, e1811. [Google Scholar] [CrossRef] [Green Version]
- Seebauer, L.; Schneider, Y.; Drobny, A.; Plötz, S.; Koudelka, T.; Tholey, A.; Prots, I.; Winner, B.; Zunke, F.; Winkler, J.; et al. Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson’s Patient-Derived Neuronal Cells. Int. J. Mol. Sci. 2022, 23, 1812. [Google Scholar] [CrossRef]
- Cookson, M.R. alpha-Synuclein and neuronal cell death. Mol. Neurodegener. 2009, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Hume, A.N.; Seabra, M.C. Melanosomes on the move: A model to understand organelle dynamics. Biochem. Soc. Trans. 2011, 39, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef]
- Hawk, B.J.D.; Khounlo, R.; Shin, Y.K. Alpha-Synuclein Continues to Enhance SNARE-Dependent Vesicle Docking at Exorbitant Concentrations. Front. Neurosci. 2019, 13, 216. [Google Scholar] [CrossRef] [Green Version]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [Green Version]
- Yoo, G.; Yeou, S.; Son, J.B.; Shin, Y.K.; Lee, N.K. Cooperative inhibition of SNARE-mediated vesicle fusion by α-synuclein monomers and oligomers. Sci. Rep. 2021, 11, 10955. [Google Scholar] [CrossRef]
- Scott, G.; Zhao, Q. Rab3a and SNARE proteins: Potential regulators of melanosome movement. J. Invest. Derm. 2021, 116, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Ohbayashi, N.; Fukuda, M. SNARE dynamics during melanosome maturation. Biochem. Soc. Trans. 2018, 46, 911–917. [Google Scholar] [CrossRef]
- Benito-Martínez, S.; Salavessa, L.; Raposo, G.; Marks, M.S.; Delevoye, C. Melanin Transfer and Fate within Keratinocytes in Human Skin Pigmentation. Integr. Comp. Biol. 2021, 61, 1546–1555. [Google Scholar] [CrossRef]





| Primer | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | Product Size in bp | Tm in °C |
|---|---|---|---|---|
| ACTB | CTACGTCGCCCTGGACTTCGAGC | GATGGAGCCGCCGATCCACACGG | 384 | 88 |
| MITF | TCTACCGTCTCTCACTGGATTGG | GCTTTACCTGCTGCCGTTGG | 141 | 83 |
| RAB27A | TGATGGAGCGAACTGCTTTTC | CCCTACACCAGAGTCTCCCAA | 296 | 78 |
| SNCA | GCAGAAGCAGCAGGAAAGAC | TTCCTGTGGGGCTCCTTCTT | 232 | 86 |
| SNCB | CGTGTTCATGAAGGGCCTGT | GTGAGGCCTGTTCCTTGGTT | 187 | 88 |
| SNCG | GACCTCAGTGGCCGAGAAGAC | CTCTTCAGGTCATCCACGCT | 261 | 89 |
| TRP2 | TGGAGTGGTCCCTACATCCTA | TCACTGGTGGTTTCTTCCG | 165 | 85 |
| TYR | CTCAAAGCAGCATGCACAAT | GCCCAGATCTTTGGATGAAA | 265 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachinger, N.; Mittag, N.; Böhme-Schäfer, I.; Xiang, W.; Kuphal, S.; Bosserhoff, A.K. Alpha-Synuclein and Its Role in Melanocytes. Cells 2022, 11, 2087. https://doi.org/10.3390/cells11132087
Rachinger N, Mittag N, Böhme-Schäfer I, Xiang W, Kuphal S, Bosserhoff AK. Alpha-Synuclein and Its Role in Melanocytes. Cells. 2022; 11(13):2087. https://doi.org/10.3390/cells11132087
Chicago/Turabian StyleRachinger, Nicole, Nora Mittag, Ines Böhme-Schäfer, Wei Xiang, Silke Kuphal, and Anja K. Bosserhoff. 2022. "Alpha-Synuclein and Its Role in Melanocytes" Cells 11, no. 13: 2087. https://doi.org/10.3390/cells11132087
APA StyleRachinger, N., Mittag, N., Böhme-Schäfer, I., Xiang, W., Kuphal, S., & Bosserhoff, A. K. (2022). Alpha-Synuclein and Its Role in Melanocytes. Cells, 11(13), 2087. https://doi.org/10.3390/cells11132087







