Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment
Abstract
1. Introduction
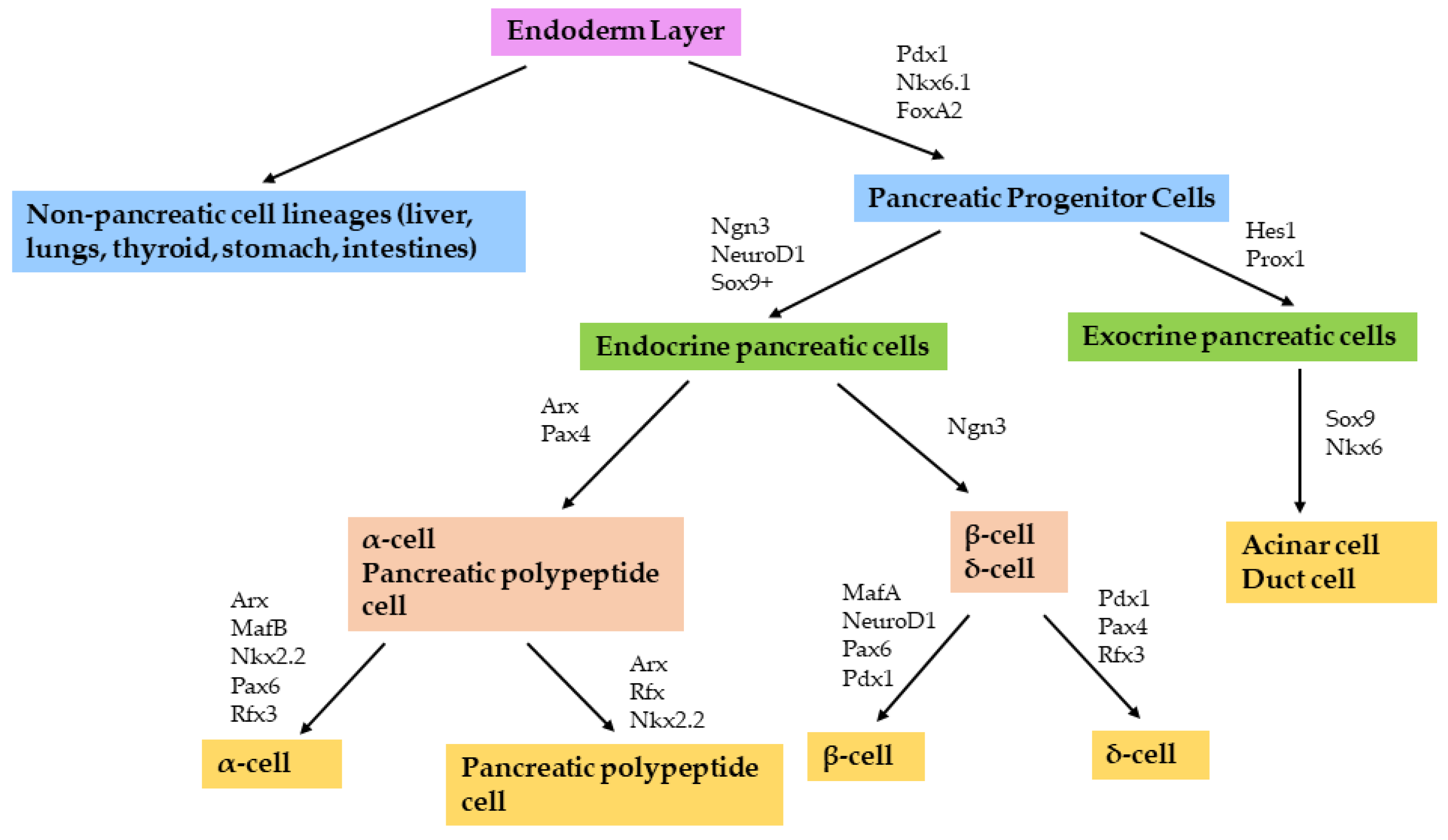
2. Pancreatic Development and Function
3. Transdifferentiation to β-Cells
3.1. Differentiation of Stem Cells
3.2. Transdifferentiation of Somatic Cells
4. Gene Delivery Strategies
4.1. Retroviral Vectors
4.2. Adenoviral Vectors
4.3. Adeno-Associated Viral Vectors
4.4. Lentiviral Vectors
5. Delivery of β-Cell Transcription Factors to Induce Transdifferentiation
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Assembly, U.N.G. World Diabetes Day. In Resolution Adopted by the General Assembly—61/225; UN General Assembly: New York, NY, USA, 2006. [Google Scholar]
- World Health Organization. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 11 May 2022).
- Katharine, F.; Hunt, B.C.W.; Gayle, C. Gestational Diabetes. Obstet. Gynaecol. Reprod. Med. 2014, 24, 238–244. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Li, X.; Zheng, P.; Hu, F.; Zhou, Z. Vaccination with a co-expression DNA plasmid containing GAD65 fragment gene and IL-10 gene induces regulatory CD4+ T cells that prevent experimental autoimmune diabetes. Diabetes Metab. Res. Rev. 2016, 32, 522–533. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar]
- Control, D.; Group, C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Hovorka, R. Closed-loop insulin delivery: From bench to clinical practice. Nat. Rev. Endocrinol. 2011, 7, 385–395. [Google Scholar] [CrossRef]
- Ryan, E.; Lakey, J.; Paty, B.; Imes, S.; Korbutt, G.S.; Kneteman, N.M.; Bigam, D.; Rajotte, R.V.; Shapiro, A.M. Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes 2002, 51, 2148–2157. [Google Scholar] [CrossRef]
- Meivar-Levy, I.; Ferber, S. New organs from our own tissues: Liver-to-pancreas transdifferentiation. Trends Endocrinol. Metab. 2003, 14, 460–466. [Google Scholar] [CrossRef]
- Mali, S. Delivery systems for gene therapy. Indian J. Hum. Genet. 2013, 19, 3. [Google Scholar] [CrossRef]
- Da Silva Xavier, G. The cells of the islets of langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef]
- Ang, S.-L.; Wierda, A.; Wong, D.; Stevens, K.A.; Cascio, S.; Rossant, J.; Zaret, K.S. The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF3/forkhead proteins. Development 1993, 119, 1301–1315. [Google Scholar] [CrossRef]
- Gao, N.; LeLay, J.; Vatamaniuk, M.Z.; Rieck, S.; Friedman, J.R.; Kaestner, K.H. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008, 22, 3435–3448. [Google Scholar] [CrossRef]
- Offield, M.F.; Jetton, T.L.; Labosky, P.A.; Ray, M.; Stein, R.W.; Magnuson, M.A.; Hogan, B.; Wright, C. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996, 122, 983–995. [Google Scholar] [CrossRef]
- Schwitzgebel, V.M.; Scheel, D.W.; Conners, J.R.; Kalamaras, J.; Lee, J.E.; Anderson, D.J.; Sussel, L.; Johnson, J.D.; German, M.S. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 2000, 127, 3533–3542. [Google Scholar] [CrossRef]
- Naya, F.; Huang, H.; Qiu, Y.; Mutoh, H.; Demayo, F.; Leiter, A.; Tsai, M.; Naya, F. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997, 11, 2323–2334. [Google Scholar] [CrossRef]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef]
- Qu, X.; Afelik, S.; Jensen, J.N.; Bukys, M.A.; Kobberup, S.; Schmerr, M.; Xiao, F.; Nyeng, P.; Albertoni, M.V.; Grapin-Botton, A.J.D.B. Notch-mediated post-translational control of Ngn3 protein stability regulates pancreatic patterning and cell fate commitment. Dev. Biol. 2013, 376, 1–12. [Google Scholar] [CrossRef]
- Apelqvist, Å.; Li, H.; Sommer, L.; Beatus, P.; Anderson, D.J.; Honjo, T.; de Angelis, M.H.; Lendahl, U.; Edlund, H.J.N. Notch signalling controls pancreatic cell differentiation. Nature 1999, 400, 877–881. [Google Scholar] [CrossRef]
- Jensen, J.; Pedersen, E.E.; Galante, P.; Hald, J.; Heller, R.S.; Ishibashi, M.; Kageyama, R.; Guillemot, F.; Serup, P.; Madsen, O.D.J.N.G. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000, 24, 36–44. [Google Scholar] [CrossRef]
- Lee, J.C.; Smith, S.B.; Watada, H.; Lin, J.; Scheel, D.; Wang, J.; Mirmira, R.G.; German, M.S.J.D. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes 2001, 50, 928–936. [Google Scholar] [CrossRef]
- Shih, H.P.; Kopp, J.L.; Sandhu, M.; Dubois, C.L.; Seymour, P.A.; Grapin-Botton, A.; Sander, M.J.D. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 2012, 139, 2488–2499. [Google Scholar] [CrossRef] [PubMed]
- Peshavaria, M.; Henderson, E.; Sharma, A.; Wright, C.; Stein, R. Functional characterization of the transactivation properties of the PDX-1 homeodomain protein. Mol. Cell. Biol. 1997, 17, 3987–3996. [Google Scholar] [CrossRef]
- Zhang, C.; Moriguchi, T.; Kajihara, M.; Esaki, R.; Harada, A.; Shimohata, H.; Oishi, H.; Hamada, M.; Morito, N.; Hasegawa, K. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 2005, 25, 4969–4976. [Google Scholar] [CrossRef]
- Du, A.; Hunter, C.S.; Murray, J.; Noble, D.; Cai, C.-L.; Evans, S.M.; Stein, R.; May, C.L. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes 2009, 58, 2059–2069. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.M. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef]
- Johnson, J.H.; Newgard, C.B.; Milburn, J.L.; Lodish, H.F.; Thorens, B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J. Biol. Chem. 1990, 265, 6548–6551. [Google Scholar] [CrossRef]
- Matschinsky, F.M. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 1996, 45, 223–241. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Shiota, C.; Prasadan, K.; Guo, P.; El-Gohary, Y.; Paredes, J.; Welsh, C.; Wiersch, J.; Gittes, G.K. No evidence for β cell neogenesis in murine adult pancreas. J. Clin. Investig. 2013, 123, 2207–2217. [Google Scholar] [CrossRef]
- Menge, B.A.; Tannapfel, A.; Belyaev, O.; Drescher, R.; Müller, C.; Uhl, W.; Schmidt, W.E.; Meier, J.J. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 2008, 57, 142–149. [Google Scholar] [CrossRef]
- Tosh, D.; Slack, J.M. How cells change their phenotype. Nat. Rev. Mol. Cell Biol. 2002, 3, 187–194. [Google Scholar] [CrossRef]
- Mollinari, C.; Zhao, J.; Lupacchini, L.; Garaci, E.; Merlo, D.; Pei, G. Transdifferentiation: A new promise for neurodegenerative diseases. Cell Death Dis. 2018, 9, 830. [Google Scholar] [CrossRef]
- Cieślar-Pobuda, A.; Rafat, M.; Knoflach, V.; Skonieczna, M.; Hudecki, A.; Małecki, A.; Urasińska, E.; Ghavami, S.; Łos, M.J. Human induced pluripotent stem cell differentiation and direct transdifferentiation into corneal epithelial-like cells. Oncotarget 2016, 7, 42314. [Google Scholar] [CrossRef]
- Marigo, I.; Dazzi, F. The immunomodulatory properties of mesenchymal stem cells. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 593–602. [Google Scholar]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Thompson, D.; Donner, T.W.; Bellin, M.D.; Hsueh, W.; Pettus, J.; Wilensky, J.; Daniels, M.; Wang, R.M.; Brandon, E.P. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2021, 2, 100466. [Google Scholar] [CrossRef]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.; Warnock, G.L.; Levings, M.K. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef]
- Katuchova, J.; Harvanova, D.; Spakova, T.; Kalanin, R.; Farkas, D.; Durny, P.; Rosocha, J.; Radonak, J.; Petrovic, D.; Siniscalco, D. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr. Pathol. 2015, 26, 95–103. [Google Scholar] [CrossRef]
- Tang, D.-Q.; Cao, L.-Z.; Burkhardt, B.R.; Xia, C.-Q.; Litherland, S.A.; Atkinson, M.A.; Yang, L.-J. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 2004, 53, 1721–1732. [Google Scholar] [CrossRef]
- Lechner, A.; Yang, Y.-G.; Blacken, R.A.; Wang, L.; Nolan, A.L.; Habener, J.F. No evidence for significant transdifferentiation of bone marrow into pancreatic β-cells in vivo. Diabetes 2004, 53, 616–623. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Gamble, A.; Pawlick, R.; Pepper, A.R.; Bruni, A.; Adesida, A.; Senior, P.A.; Korbutt, G.S.; Shapiro, A.J. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PLoS ONE 2018, 13, e0206449. [Google Scholar] [CrossRef] [PubMed]
- Kerby, A.; Jones, E.S.; Jones, P.M.; King, A.J. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy 2013, 15, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Swingen, C.; Zhang, J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr. Cardiol. Rev. 2013, 9, 63–72. [Google Scholar] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, W.-J.; Sun, C.; Hou, C.-Z.; Yang, X.-M.; Gao, J.-G. Induced pluripotent stem cells: Origins, applications, and future perspectives. J. Zhejiang Univ. Sci. B 2013, 14, 1059–1069. [Google Scholar] [CrossRef]
- Mallon, B.S.; Hamilton, R.S.; Kozhich, O.A.; Johnson, K.R.; Fann, Y.C.; Rao, M.S.; Robey, P.G. Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res. 2014, 12, 376–386. [Google Scholar] [CrossRef]
- Shahjalal, H.M.; Abdal Dayem, A.; Lim, K.M.; Jeon, T.-I.; Cho, S.-G. Generation of pancreatic β cells for treatment of diabetes: Advances and challenges. Stem Cell Res. Ther. 2018, 9, 355. [Google Scholar] [CrossRef]
- Tuch, B.E.; Beynon, S.; Tabiin, M.T.; Sassoon, R.; Goodman, R.J.; Simpson, A.M. Effect of β-cell toxins on genetically engineered insulin-secreting cells. J. Autoimmun. 1997, 10, 239–244. [Google Scholar] [CrossRef]
- Tabiin, M.; Tuch, B.; Bai, L.; Han, X.; Simpson, A. Resistance of insulin-secreting hepatocytes to the toxicity of human autoimmune cytokines. Autoimmunity 2001, 17, 229–242. [Google Scholar] [CrossRef]
- Tabiin, M.T.; White, C.P.; Morahan, G.; Tuch, B.E. Insulin expressing hepatocytes not destroyed in transgenic NOD mice. J. Autoimmune Dis. 2004, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Lipes, M.A.; Cooper, E.M.; Skelly, R.; Rhodes, C.J.; Boschetti, E.; Weir, G.C.; Davalli, A.M. Insulin-secreting non-islet cells are resistant to autoimmune destruction. Proc. Natl. Acad. Sci. USA 1996, 93, 8595–8600. [Google Scholar] [CrossRef] [PubMed]
- Bramswig, N.; Kaestner, K. Transcriptional regulation of α-cell differentiation. Diabetes Obes. Metab. 2011, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.L. Adult insulin-and glucagon-producing cells differentiate from two independent cell lineages. Development 2000, 127, 2317–2322. [Google Scholar] [CrossRef]
- Bonal, C.; Herrera, P.L. Genes controlling pancreas ontogeny. Int. J. Dev. Biol. 2004, 52, 823–835. [Google Scholar] [CrossRef]
- Ren, B.; O’Brien, B.A.; Byrne, M.R.; Ch’ng, E.; Gatt, P.N.; Swan, M.A.; Nassif, N.T.; Wei, M.Q.; Gijsbers, R.; Debyser, Z. Long-term reversal of diabetes in non-obese diabetic mice by liver-directed gene therapy. J. Gene Med. 2013, 15, 28–41. [Google Scholar] [CrossRef]
- Ren, B.; O’Brien, B.; Swan, M.; Koina, M.; Nassif, N.; Wei, M.; Simpson, A. Long-term correction of diabetes in rats after lentiviral hepatic insulin gene therapy. Diabetologia 2007, 50, 1910–1920. [Google Scholar] [CrossRef]
- Lawandi, J.; Tao, C.; Ren, B.; Williams, P.; Ling, D.; Swan, M.A.; Nassif, N.T.; Torpy, F.R.; O’brien, B.A.; Simpson, A.M. Reversal of diabetes following transplantation of an insulin-secreting human liver cell line: Melligen cells. Mol. Ther. Methods Clin. Dev. 2015, 2, 15011. [Google Scholar] [CrossRef]
- La, Q.T.; Ren, B.; Logan, G.J.; Cunningham, S.C.; Khandekar, N.; Nassif, N.T.; O’Brien, B.A.; Alexander, I.E.; Simpson, A.M. Use of a Hybrid Adeno-Associated Viral Vector Transposon System to Deliver the Insulin Gene to Diabetic NOD Mice. Cells 2020, 9, 2227. [Google Scholar] [CrossRef]
- Hsu, P.Y.-J.; Kotin, R.M.; Yang, Y.-W. Glucose-and metabolically regulated hepatic insulin gene therapy for diabetes. Pharm. Res. 2008, 25, 1460–1468. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, Y.; Nagasawa, M.; Hara, A.; Kodera, T.; Kojima, I. In vitro transdifferentiation of mature hepatocytes into insulin-producing cells. Endocr. J. 2006, 53, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.-S.; Kim, J.-W.; Park, H.-S.; Sun, C.-L.; Lee, S.-H.; Cho, J.-H.; Oh, J.-A.; Song, K.-H.; Son, H.-Y.; Hideaki, K. Generation of insulin producing cells from the mouse primary hepatocytes. Tissue Eng. Reg. Med. 2011, 8, 564–573. [Google Scholar]
- Alam, T.; Wai, P.; Held, D.; Vakili, S.T.T.; Forsberg, E.; Sollinger, H. Correction of diabetic hyperglycemia and amelioration of metabolic anomalies by minicircle DNA mediated glucose-dependent hepatic insulin production. PLoS ONE 2013, 8, e67515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.; Halkin, A.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Doi, R.; Toyoda, E.; Tulachan, S.S.; Kami, K.; Mori, T.; Ito, D.; Kawaguchi, Y.; Fujimoto, K.; Gittes, G.K. Hepatic regeneration and enforced PDX-1 expression accelerate transdifferentiation in liver. Surgery 2004, 136, 449–457. [Google Scholar] [CrossRef]
- Ber, I.; Shternhall, K.; Perl, S.; Ohanuna, Z.; Goldberg, I.; Barshack, I.; Benvenisti-Zarum, L.; Meivar-Levy, I.; Ferber, S. Functional, persistent, and extended liver to pancreas transdifferentiation. J. Biol. Chem. 2003, 278, 31950–31957. [Google Scholar] [CrossRef]
- Imai, J.; Katagiri, H.; Yamada, T.; Ishigaki, Y.; Ogihara, T.; Uno, K.; Hasegawa, Y.; Gao, J.; Ishihara, H.; Sasano, H. Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem. Biophys. Res. Commun. 2005, 326, 402–409. [Google Scholar]
- Jung, Y.; Zhou, R.; Kato, T.; Usui, J.K.; Muratani, M.; Oishi, H.; Heck, M.M.; Takahashi, S. Isl1 β overexpression with key β cell transcription factors enhances glucose-responsive hepatic insulin production and secretion. Endocrinology 2018, 159, 869–882. [Google Scholar] [CrossRef]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008, 455, 627–632. [Google Scholar]
- Banga, A.; Akinci, E.; Greder, L.V.; Dutton, J.R.; Slack, J.M. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc. Natl. Acad. Sci. USA 2012, 109, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nakanishi, M.; Zumsteg, A.; Shear, M.; Wright, C.; Melton, D.A.; Zhou, Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife 2014, 3, e01846. [Google Scholar] [CrossRef]
- Akinci, E.; Banga, A.; Tungatt, K.; Segal, J.; Eberhard, D.; Dutton, J.R.; Slack, J.M. Reprogramming of various cell types to a beta-like state by Pdx1, Ngn3 and MafA. PLoS ONE 2013, 8, e82424. [Google Scholar] [CrossRef] [PubMed]
- Akinci, E.; Banga, A.; Greder, L.V.; Dutton, J.R.; Slack, J.M. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem. J. 2012, 442, 539–550. [Google Scholar] [CrossRef]
- Xu, H.; Tsang, K.S.; Chan, J.C.; Yuan, P.; Fan, R.; Kaneto, H.; Xu, G. The combined expression of Pdx1 and MafA with either Ngn3 or NeuroD improves the differentiation efficiency of mouse embryonic stem cells into insulin-producing cells. Cell Transplant. 2013, 22, 147–158. [Google Scholar] [CrossRef]
- Tang, D.-Q.; Lu, S.; Sun, Y.-P.; Rodrigues, E.; Chou, W.; Yang, C.; Cao, L.-Z.; Chang, L.-J.; Yang, L.-J. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1-and Pdx1-VP16 mediated by lentiviral vectors. Lab. Investig. 2006, 86, 83–93. [Google Scholar] [CrossRef]
- Fodor, A.; Harel, C.; Fodor, L.; Armoni, M.; Salmon, P.; Trono, D.; Karnieli, E. Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: An ex vivo gene therapy approach. Diabetologia 2007, 50, 121–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgan, R.A.; Anderson, W.F. Human Gene Therapy. Annu. Rev. Biochem. 1993, 62, 191–217. [Google Scholar] [CrossRef]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; de Saint Basile, G.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.-L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.-L.; Fraser, C.C.; Cavazzana-Calvo, M. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Randrianarison-Jewtoukoff, V.; Perricaudet, M.J.B. Recombinant adenoviruses as vaccines. Biologicals 1995, 23, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.S.M.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Volpers, C.; Kochanek, S. Adenoviral vectors for gene transfer and therapy. J. Gene Med. 2004, 6, S164–S171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S.; Liu, D.P.; Liang, C.C. Challenges and strategies: The immune responses in gene therapy. Med. Res. Rev. 2004, 24, 748–761. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Lai-Yin, T.; Hsiang-Fu, K.; Lu, H.; Lam, K. Diabetes gene therapy: Potential and challenges. Curr. Gene Ther. 2003, 3, 65–82. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Zaiss, A.; Muruve, D. Immunity to adeno-associated virus vectors in animals and humans: A continued challenge. Gene Ther. 2008, 15, 808–816. [Google Scholar] [CrossRef]
- Ronen, K.; Negre, O.; Roth, S.; Colomb, C.; Malani, N.; Denaro, M.; Brady, T.; Fusil, F.; Gillet-Legrand, B.; Hehir, K. Distribution of lentiviral vector integration sites in mice following therapeutic gene transfer to treat β-thalassemia. Mol. Ther. 2011, 19, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Buchschacher, G.L., Jr.; Wong-Staal, F. Development of lentiviral vectors for gene therapy for human diseases. Blood J. Am. Soc. Hematol. 2000, 95, 2499–2504. [Google Scholar]
- Matsuoka, T.-A.; Kawashima, S.; Miyatsuka, T.; Sasaki, S.; Shimo, N.; Katakami, N.; Kawamori, D.; Takebe, S.; Herrera, P.L.; Kaneto, H. Mafa enables Pdx1 to effectively convert pancreatic islet progenitors and committed islet α-cells into β-cells in vivo. Diabetes 2017, 66, 1293–1300. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Thorel, F.; Boyer, D.F.; Herrera, P.L.; Wright, C.V. Context-specific α-to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011, 25, 1680–1685. [Google Scholar] [CrossRef]
- Yechoor, V.; Liu, V.; Espiritu, C.; Paul, A.; Oka, K.; Kojima, H.; Chan, L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev. Cell 2009, 16, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Guo, P.; Shiota, C.; Zhang, T.; Coudriet, G.M.; Fischbach, S.; Prasadan, K.; Fusco, J.; Ramachandran, S.; Witkowski, P. Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 2018, 22, 78–90.e4. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, H.; Katsumata, T.; Oishi, H.; Tai, P.-H.; Sekiguchi, Y.; Koshida, R.; Jung, Y.; Kudo, T.; Takahashi, S. Generation of insulin-producing cells from the mouse liver using β cell-related gene transfer including Mafa and Mafb. PLoS ONE 2014, 9, e113022. [Google Scholar] [CrossRef] [PubMed]
| Vector | Advantages | Disadvantages | Delivery of Transcription Factors | References |
|---|---|---|---|---|
| Adenoviral | Transfers genes to dividing and non-dividing cells Large packaging capacity (~8 kB) Produce high virus titres Gene delivery at high multiplicity of infection | Transient expression Highly immunogenic | Pdx1 only Pdx1, Ngn3 and MafA Pdx1, NeuroD1 and MafA Pdx1, NeuroD1, MafA and Isl1 | [64,65,68,69,70,71,72,73,74,75,76,77,78] |
| Adeno-Associated | Transfers genes to dividing and non-dividing cells Integrates into host genome with stable gene expression Low immunogenicity | Low packaging capacity (~5 kB) Neutralising antibodies can be generated Requires helper virus for effective use | Pdx1 only | [75] |
| Lentiviral | Transfers genes to dividing and non-dividing cells Integrates into host genome with stable gene expression Large packaging capacity (~10 kB) Third-generation vectors are self-inactivating No known immunogenic proteins created Produce high virus titre (108 TU/mL)Simple system for vector manipulation and production | Possibility of insertional mutagenesis in host genome in second-generation LV Contains three HIV-1 genes (gag, pol, rev) | Pdx1/Pdx1-VP16 only | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahoney, A.L.G.; Nassif, N.T.; O’Brien, B.A.; Simpson, A.M. Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells 2022, 11, 2145. https://doi.org/10.3390/cells11142145
Mahoney ALG, Nassif NT, O’Brien BA, Simpson AM. Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells. 2022; 11(14):2145. https://doi.org/10.3390/cells11142145
Chicago/Turabian StyleMahoney, Alexandra L. G., Najah T. Nassif, Bronwyn A. O’Brien, and Ann M. Simpson. 2022. "Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment" Cells 11, no. 14: 2145. https://doi.org/10.3390/cells11142145
APA StyleMahoney, A. L. G., Nassif, N. T., O’Brien, B. A., & Simpson, A. M. (2022). Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells, 11(14), 2145. https://doi.org/10.3390/cells11142145








