Magnetopriming Actuates Nitric Oxide Synthesis to Regulate Phytohormones for Improving Germination of Soybean Seeds under Salt Stress
Abstract
:1. Introduction
2. Material and Methods
2.1. Seed Priming with Static Magnetic Field
2.2. Seed Germination under Saline and Non-Saline Conditions
2.3. Salt Tolerance Index (STI)
2.4. In-Vivo Measurement of Na+ and K+ Ions
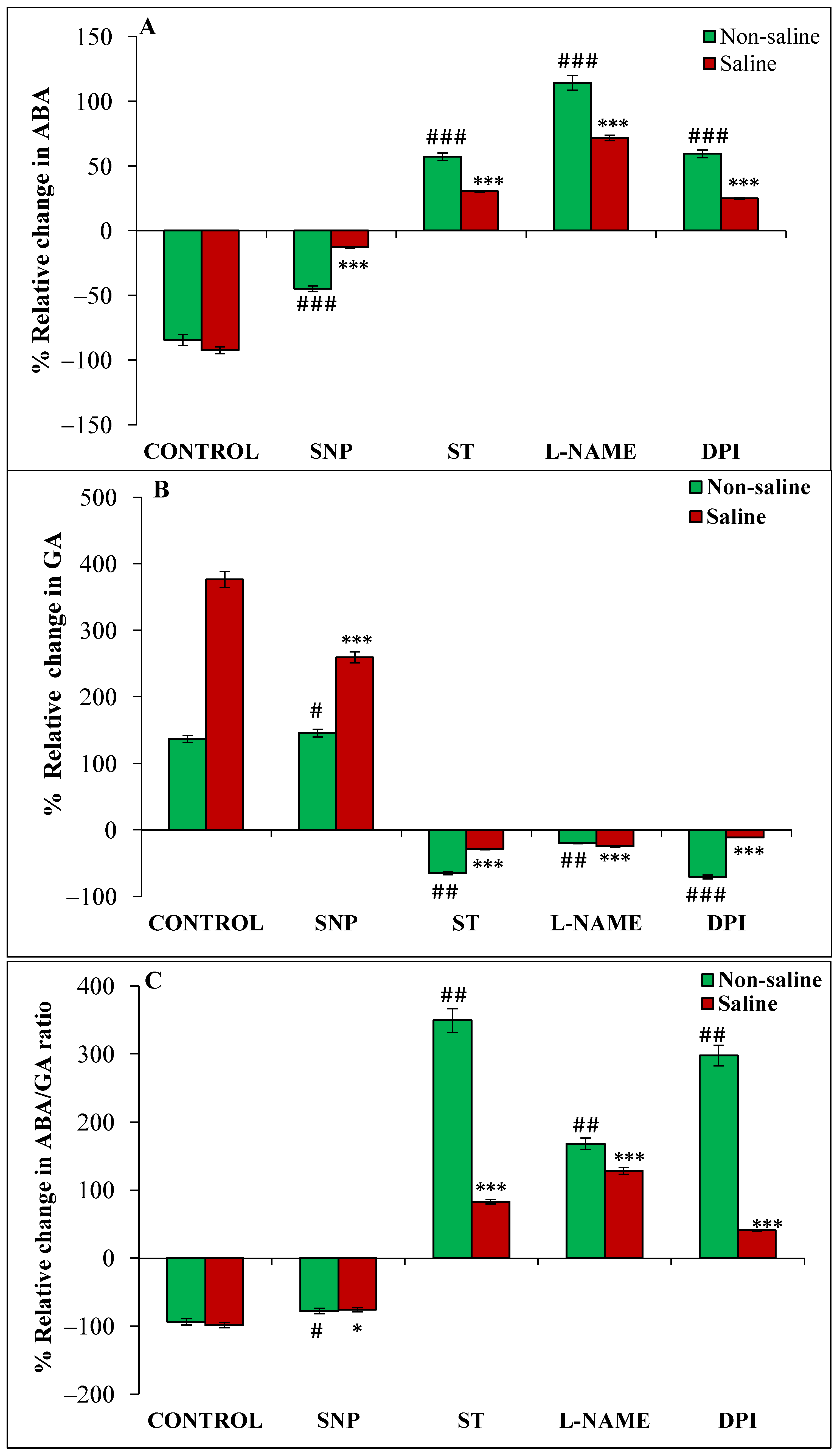
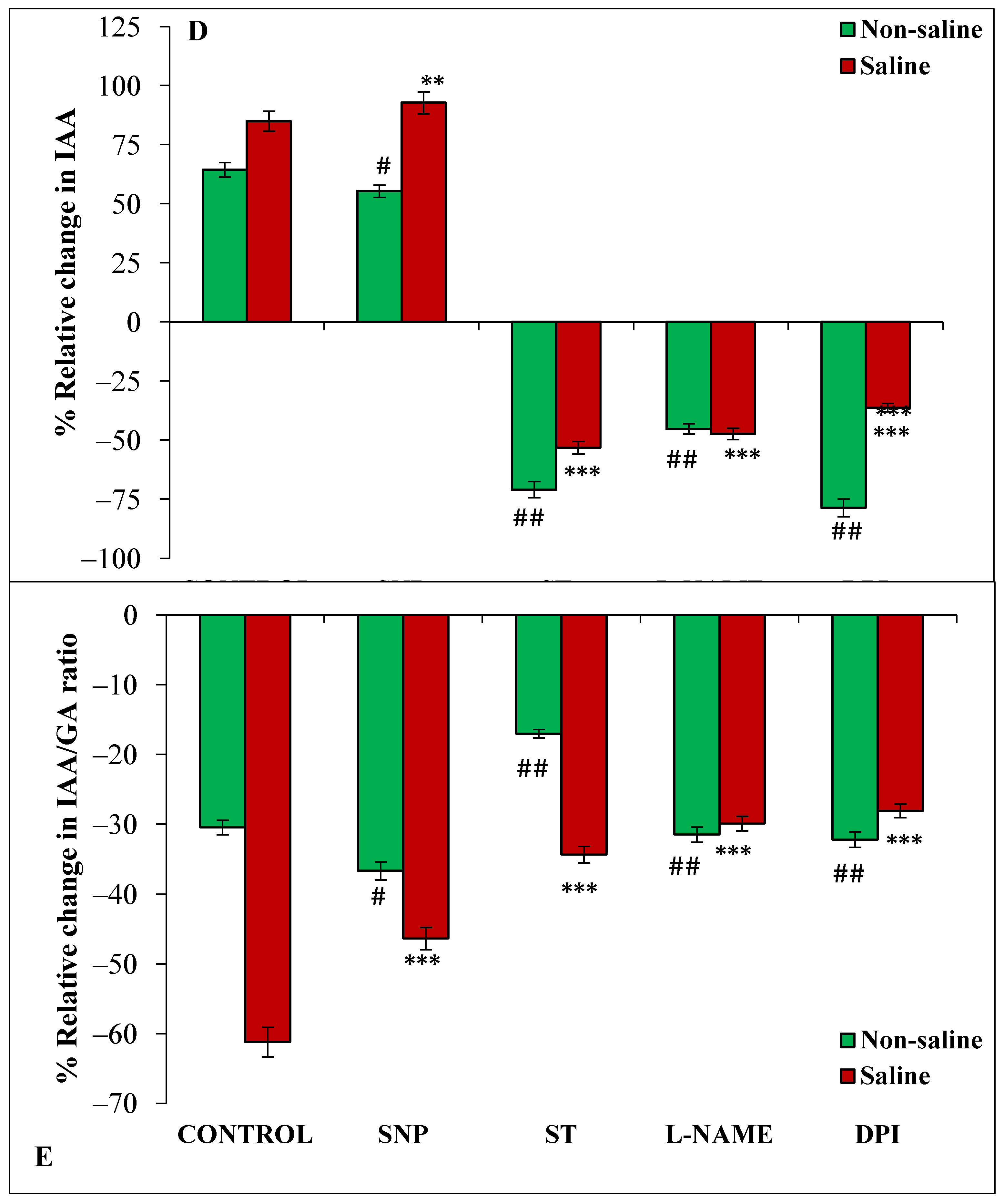
2.5. Estimation of Endogenous ABA, GA and IAA Levels
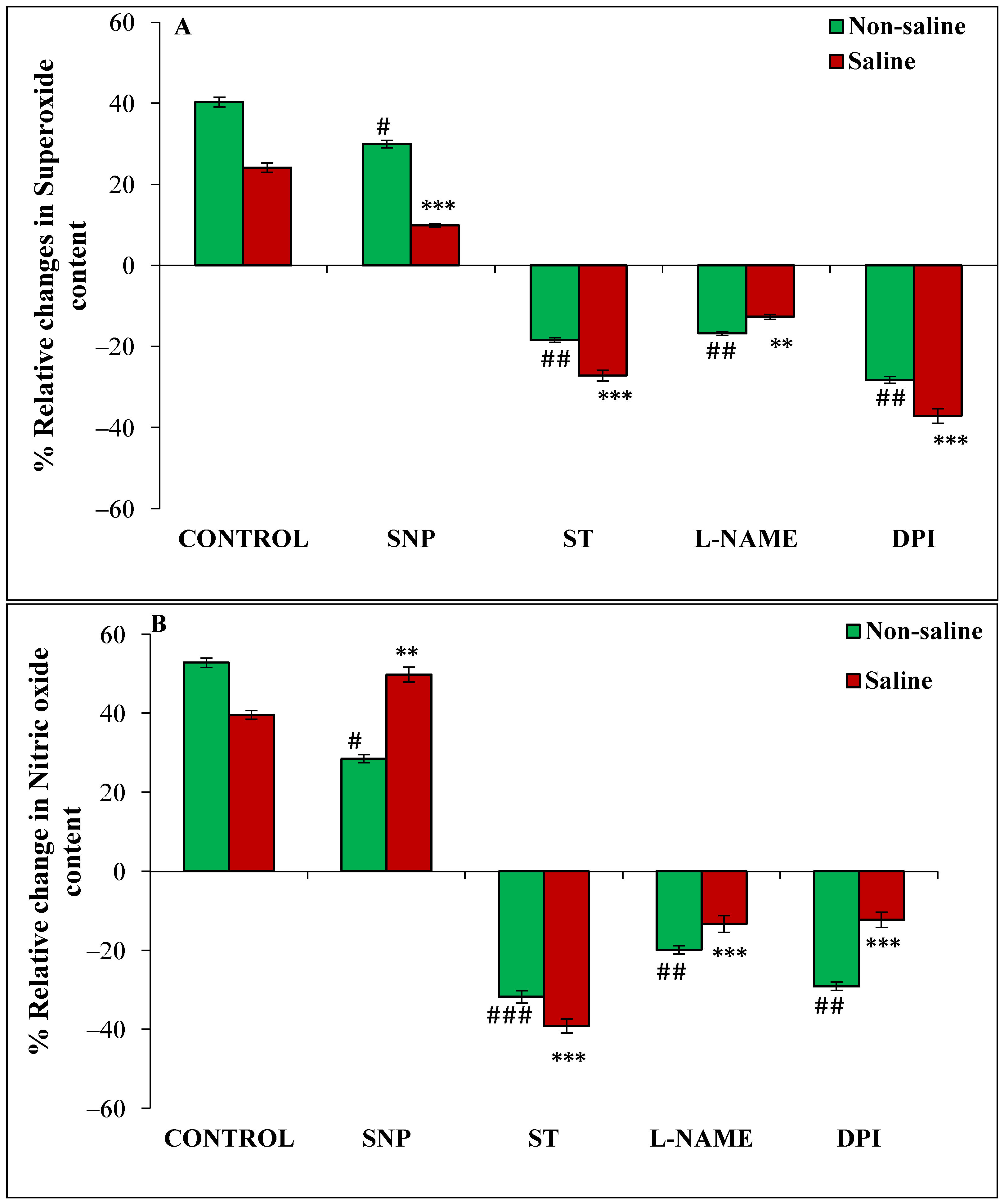
2.6. Estimation of Superoxide (O2•−) and Nitric Oxide (NO) Content
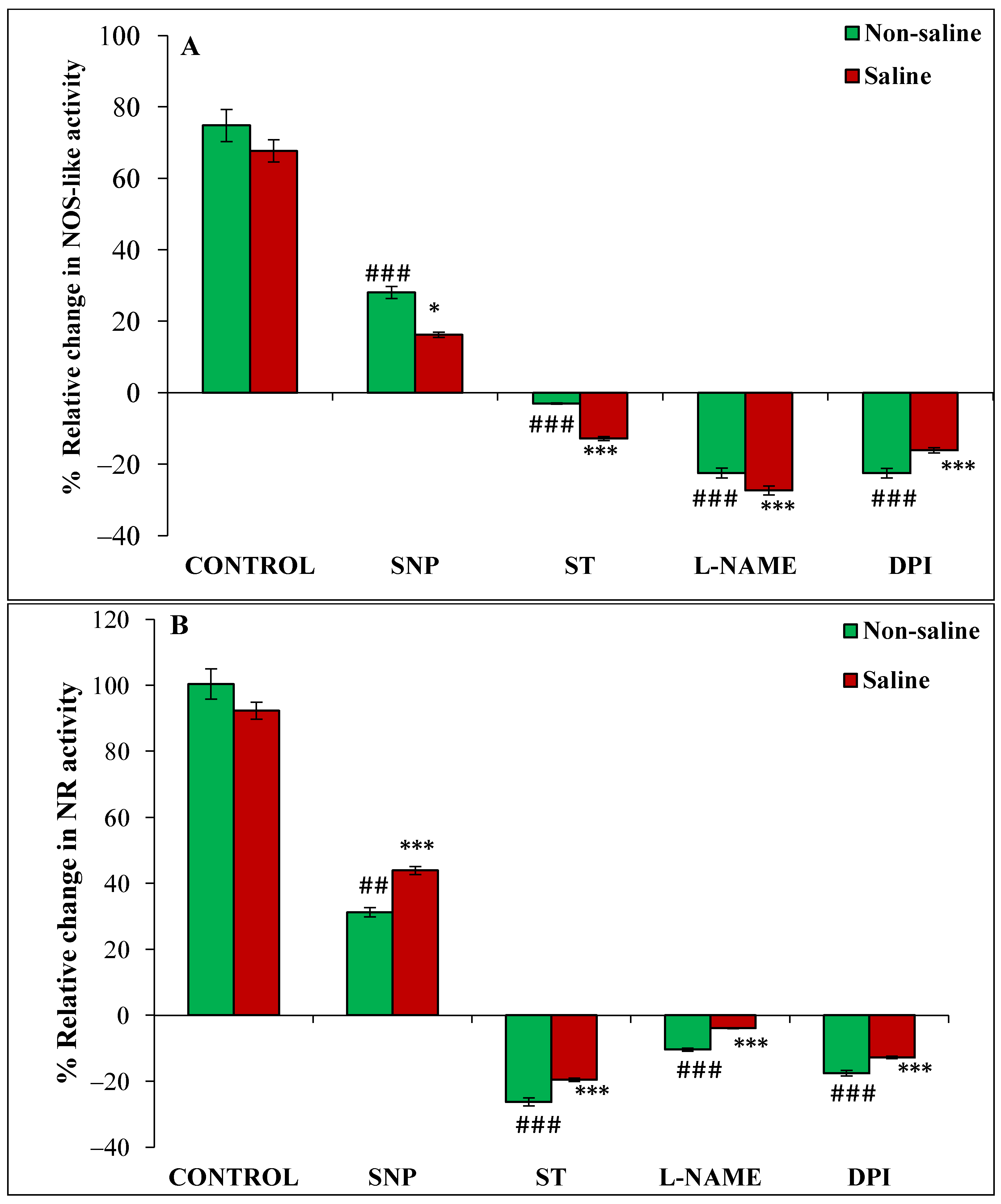
2.7. Nitric Oxide Synthase like (NOS-like) and Nitrate Reductase (NR) Activity
2.8. RNA Extraction and Gene Expression Analysis Using Quantitative Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Salt Tolerance Index (STI)
3.2. Effect of Magnetopriming on Na+/K+ Ratio under Salt Stress
3.3. Effect of Magnetopriming on Phytohormones (ABA, GA and IAA) under Salt Stress
3.4. Effect of Magnetopriming on Superoxide (O2•−), Nitric Oxide (NO) Content, NOS-like and NR Activities under Salt Stress
3.5. Identification of Putative Seed Specific GmNOS-like and GmNR Genes in Soybean Genome
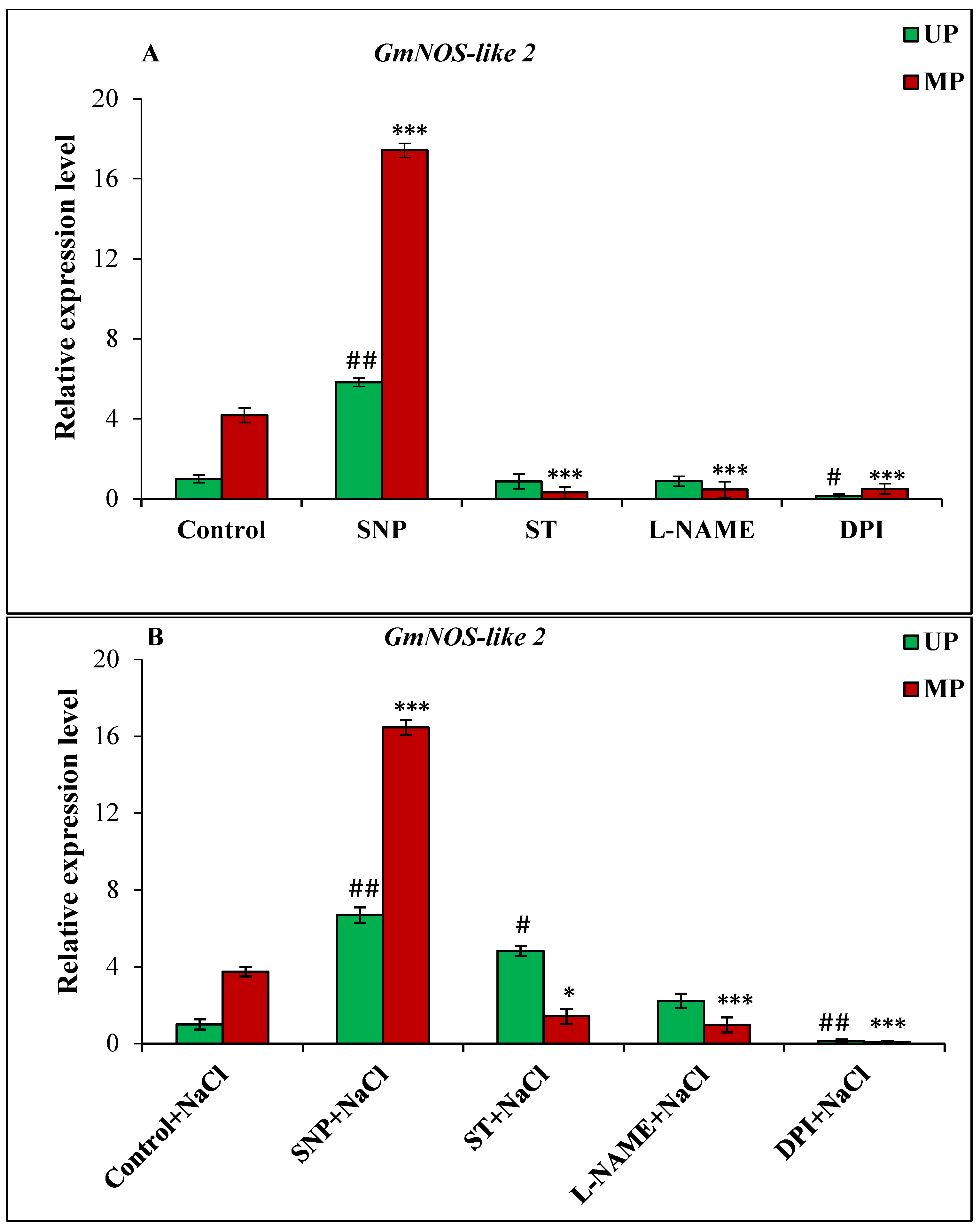
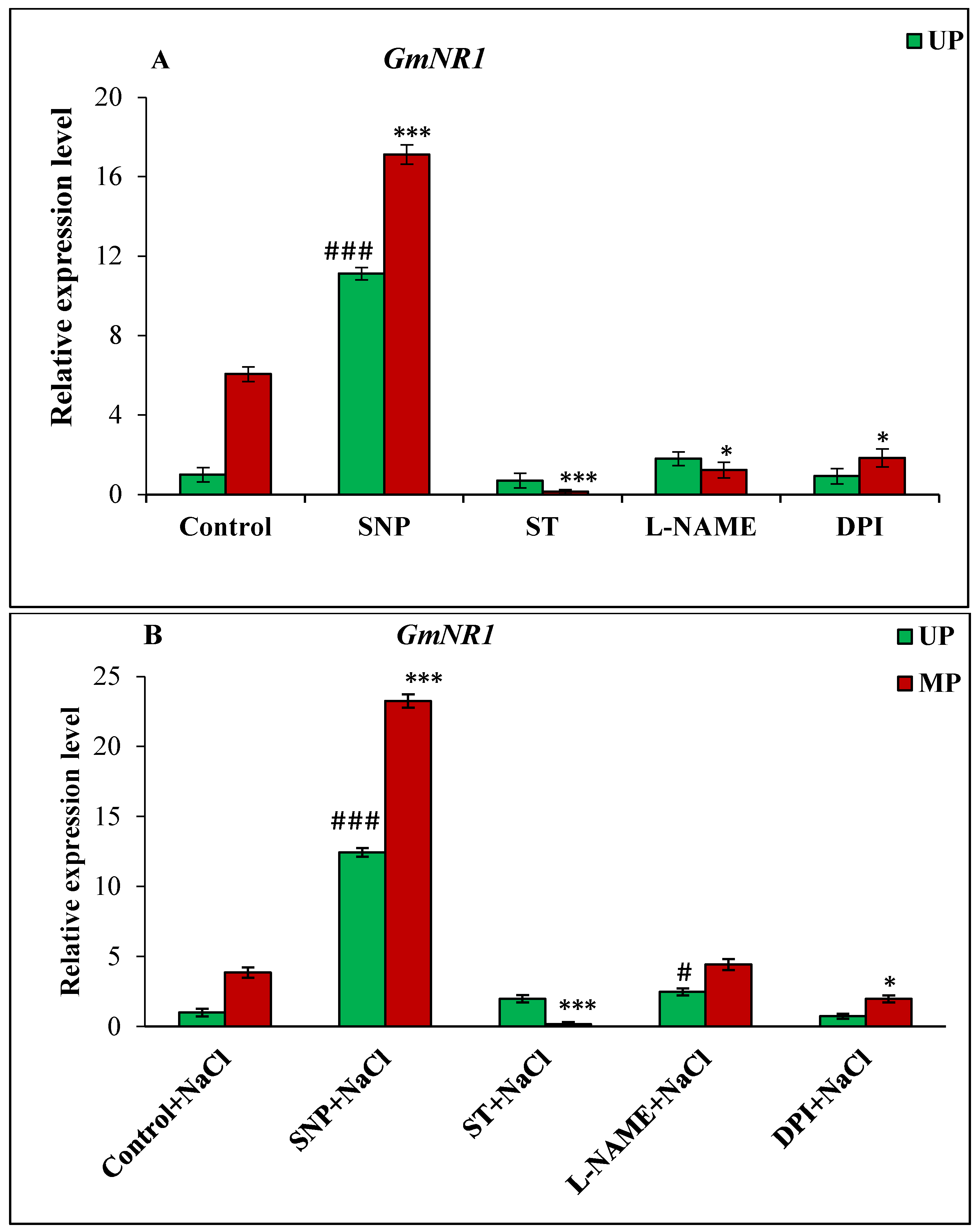
3.6. Effect of Magnetopriming on the Expression of Genes GmNOS-like and GmNR Involved in NO Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Tomar, R.S.; Kataria, S.; Jajoo, A. Behind the scene: Critical role of reactive oxygen species and reactive nitrogen species in salt stress tolerance. J. Agron. Crop Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Kataria, S.; Verma, S.K. Salinity stress responses and adaptive mechanisms in major glycophytic crops: The Story So Far. In Salinity Responses and Tolerance in Plants. Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.P., Eds.; Springer: Cham, Switzerland, 2018; Chapter-1; pp. 1–39. [Google Scholar]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Perez, S.C.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean—worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Jabeen, Z.; Fayyaz, H.A.; Irshad, F.; Hussain, N.; Hassan, M.N.; Li, J.; Rehman, S.; Haider, W.; Yasmin, H.; Mumtaz, S.; et al. Sodium nitroprusside application improves morphological and physiological attributes of soybean (Glycine max L.) under salinity stress. PLoS ONE 2021, 16, e0248207. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, P.; Anand, A. Seed Priming-Induced Early Vigor in Crops: An Alternate Strategy for Abiotic Stress Tolerance. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 163–180. [Google Scholar] [CrossRef]
- Prajapati, R.; Kataria, S.; Jain, M. Seed priming for alleviation of heavy metal toxicity in plants: An overview. Plant Sci. Today 2020, 7, 308–313. [Google Scholar] [CrossRef]
- Thomas, S.; Anand, A.; Chinnusamy, V.; Dahuja, A.; Basu, S. Magnetopriming circumvents the effect of salinity stress on germination in chickpea seeds. Acta Physiol. Plant. 2013, 35, 3401–3411. [Google Scholar] [CrossRef]
- Mridha, N.; Chattaraj, S.; Chakraborty, D.; Anand, A.; Aggarwal, P.; Nagarajan, S. Pre-sowing static magnetic field treatment for improving water and radiation use efficiency in chickpea (Cicer arietinum L.) under soil moisture stress. Bioelectromagnetics 2016, 37, 400–408. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M. Magnetopriming alleviates adverse effects of abiotic stresses in plants. In Plant Tolerance to Environmental Stress, 1st ed.; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 427–438. [Google Scholar]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.; Menegatti, R.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Sarraf, M.; Deamici, K.M.; Taimourya, H.; Islam, M.; Kataria, S.; Raipuria, R.K.; Abdi, G.; Brestic, M. Effect of Magnetopriming on Photosynthetic Performance of Plants. Int. J. Mol. Sci. 2021, 22, 9353. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K. Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol. Plant. 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Raipuria, R.K.; Kataria, S.; Watts, A.; Jain, M. Magneto-priming promotes nitric oxide via nitric oxide synthase to ameliorate the UV-B stress during germination of soybean seedlings. J. Photochem. Photobiol. B Biol. 2021, 220, 112211. [Google Scholar] [CrossRef]
- Yaycili, O.; Alikamanoglu, S. The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2005, 83, 109–114. [Google Scholar] [CrossRef]
- Reina, F.G.; Pascual, L.A.; Fundora, I.A. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics 2001, 22, 596–602. [Google Scholar] [CrossRef]
- Zafra, A.; Rodríguez-García, M.I.; Alché, J.D.D. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, Y.; Wang, J.; Babla, M.; Zhao, C.; García-Mata, C.; Sani, E.; Differ, C.; Mak, M.; Hills, A.; et al. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol. 2015, 209, 1456–1469. [Google Scholar] [CrossRef] [Green Version]
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide 2019, 85, 17–27. [Google Scholar] [CrossRef]
- Verleysen, K.; Coppitters, D.; Parente, A.; De Paepe, W.; Contino, F. How can power-to-ammonia be robust? Optimization of an ammonia synthesis plant powered by a wind turbine considering operational uncertainties. Fuel 2020, 266, 117049. [Google Scholar] [CrossRef]
- Lau, S.-E.; Hamdan, M.; Pua, T.-L.; Saidi, N.; Tan, B. Plant Nitric Oxide Signaling under Drought Stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2019, 187, 109785. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Landi, M.; Zheng, B.; Yan, D.; Yuan, H. Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biol. Plant. 2020, 64, 512–518. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Rastogi, A.; Brestic, M. Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth. Res. 2021, 150, 263–278. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination. Arab. Book 2008, 6, e0119. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Meng, Y.F.; Chen, H.; Shuai, X.; Luo, J.; Ding, S.; Tang, S.; Xu, J.; Liu, W.L.; Du, J. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci. Rep. 2016, 6, 22073. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.D.; Zhang, H.; Zhao, Y.; Feng, Z.Y.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.M.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Kim, S.H.; Bahk, S.; Ali, A.; Nguyen, X.C.; Yun, D.J.; Chung, W.S. The auxin signaling repressor IAA8 promotes seed germination through down-regulation of ABI3 transcription in Arabidopsis. Front. Plant Sci. 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Bialek, K.; Michalczuk, L.; Cohen, J.D. Auxin biosynthesis during seed germination in Phaseolusvulgaris. Plant Physiol. 1992, 100, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Kataria, S.; Rastogi, A.; Bele, A.; Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol. Mol. Biol. Plants 2020, 26, 931–945. [Google Scholar] [CrossRef]
- Goudarzi, M.; Pakniyat, H. Evaluation of wheat cultivars under salinity stress based on some agronomic and physiological traits. J. Agric. Soc. Sci. 2008, 4, 35–38. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef] [Green Version]
- Kelen, M.; Demiralay, E.Ç.; Sen, S.; Alsancak, G.Ö. Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri × Vitis rupestris) and rose oil (Rosa damascene Mill.) by reversed phase liquid chromatography. Turk. J. Chem. 2004, 28, 603–610. [Google Scholar]
- Chaitanya, K.K.; Naithani, S.C. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn. f. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid induced antioxidant activities in Stylosan-thesguianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Yang, F.; Loake, G.J.; Chu, C. Nitric Oxide and Protein S-Nitrosylation Are Integral to Hydrogen Peroxide-Induced Leaf Cell Death in Rice. Plant Physiol. 2011, 158, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; de los Angeles Cabrera, M.; Henrquez, M.J.; Contreras, R.A.; Morales, B.; Moenne, A. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol. 2012, 158, 1451–1462. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2017, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Zhao, S.; Dong, H.; Zhang, H.; Sun, L.; Miao, C. Nia1 and Nia2 are Involved in Exogenous Salicylic Acid-induced Nitric Oxide Generation and Stomatal Closure in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.K.; Powell, A.A.; Bingham, I.J. Comparison of the seed germination and early seedling growth of soybean in saline conditions. Seed Sci. Res. 2002, 12, 165–172. [Google Scholar] [CrossRef]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the ger-mination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A “green” approach for yield enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Effect of sodium nitroprusside on physiological and anatomical features of salt-stressed Raphanus sativus. Plant Physiol. Biochem. 2021, 169, 160–170. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- de Souza Miranda, R.; Gomes-Filho, E.; Prisco, J.T.; Alvarez-Pizarro, J.C. Ammonium improves tolerance to salinity stress in Sorghum bicolor plants. Plant Growth Regul. 2016, 78, 121–131. [Google Scholar] [CrossRef]
- Rathod, G.R.; Anand, A. Effect of seed magneto-priming on growth, yield and Na/K ratio in wheat (Triticumaestivum L.) under salt stress. Ind. J. Plant Physiol. 2016, 21, 15–22. [Google Scholar] [CrossRef]
- Gadelha, C.G.; De Souza Miranda, R.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gom-esilho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatrophacurcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017, 212, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, Y.; Zhang, P.; Liu, H.; Zhang, X.; Zhang, R. Atmospheric application of trace amounts of nitric oxide enhances tolerance to salt stress and improves nutritional quality in spinach (Spinacia oleracea L.). Food Chem. 2015, 173, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Yadav, S.; Wani, A.S.; Irfan, M.; Alyemini, M.N.; Ahmad, A. Impact of sodium nitroprusside on nitrate reductase, prolineand antioxidant systemin Solanumlycopersicum under salinity stress. Hort. Environ. Biotechnol. 2012, 53, 362–367. [Google Scholar] [CrossRef]
- Kong, X.; Wang, T.; Li, W.; Tang, W.; Zhang, D.; Dong, H. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 61. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Wei, Q.; Zhang, W. Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 2006, 224, 545–555. [Google Scholar] [CrossRef]
- Shi, Q.; Ding, F.; Wang, X.; Wei, M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol. Biochem. 2007, 45, 542–550. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2010, 248, 447–455. [Google Scholar] [CrossRef]
- Shine, M.; Guruprasad, K.; Anand, A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 2012, 33, 428–437. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant Systems are Regulated by Nitric Oxide-Mediated Post-translational Modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Shan, C.; Yang, T. Nitric oxide acts downstream of hydrogen peroxide in the regulation of ascorbate and glutathione metabo-lism by jasmonic acid in Agropyron cristatum leaves. Biol. Plant. 2017, 61, 779–784. [Google Scholar] [CrossRef]
- Corpas, F.J.; del Rio, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J. Integr. Plant Biol. 2019, 61, 803–816. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Igamberdiev, A.U.; Mur, L.A.J.; Gupta, K.J. Current approaches to measure nitric oxide in plants. J. Exp. Bot. 2019, 70, 4333–4343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.; Sharma, P.; Anand, A.; Pandita, V.K.; Bhatia, A.; Pushkar, S. Raffinose and Hexose Sugar Content During Germination Are Related to Infrared Thermal Fingerprints of Primed Onion (Allium cepa L.) Seeds. Front. Plant Sci. 2020, 11, 579037. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Liu, H.; Zhang, L.; Wang, W. Enhancement in rice seed germination via improved respiratory metabolism under chilling stress. Food Energy Secur. 2020, 9. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and Biochemical Mechanisms of Seed Priming-Induced Chilling Tolerance in Rice Cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Planchet, E. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006, 57, 3043–3055. [Google Scholar] [CrossRef]
- Efreschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.-C.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef]
- Signorelli, S.; Considine, M.J. Nitric Oxide Enables Germination by a Four-Pronged Attack on ABA-Induced Seed Dormancy. Front. Plant Sci. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Née, G.; Xiang, Y.; Soppe, W.J.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Shu, K.; Meng, Y.; Shuai, H.; Liu, W.; Du, J.B.; Liu, J.; Yang, W.Y. Dormancy and germination: How does the crop seed decide? Plant Biol. 2015, 17, 1104–1112. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Bethke, P.C.; Libourel, I.; Aoyama, N.; Chung, Y.-Y.; Still, D.W.; Jones, R.L. The Arabidopsis Aleurone Layer Responds to Nitric Oxide, Gibberellin, and Abscisic Acid and Is Sufficient and Necessary for Seed Dormancy. Plant Physiol. 2007, 143, 1173–1188. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Belfield, E.J.; Cao, Y.; Smith, J.A.C.; Harberd, N.P. An Arabidopsis Soil-Salinity–Tolerance Mutation Confers Ethylene-Mediated Enhancement of Sodium/Potassium Homeostasis. Plant Cell 2013, 25, 3535–3552. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yamaguchi, S.; Khan, M.A.; An, P.; Liu, X.; Tran, L.-S.P. Roles of Gibberellins and Abscisic Acid in Regulating Germination of Suaeda salsa Dimorphic Seeds Under Salt Stress. Front. Plant Sci. 2016, 6, 1235. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Podleśna, A.; Bojarszczuk, J.; Podleśny, J. Effect of Pre-sowing Magnetic Field Treatment on Some Biochemical and Physiological Processes in Faba Bean (Vicia faba L. spp. Minor). J. Plant Growth Regul. 2019, 38, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Jakubowska, A. The Mechanism of IAA Level Regulation in Plants; Mikołaj Kopernik University: Toruń, Poland, 2004; pp. 1–116. (In Polish) [Google Scholar]
- Belin, C.; Megies, C.; Hauserová, E.; Lopez-Molina, L. Abscisic Acid Represses Growth of the Arabidopsis Embryonic Axis after Germination by Enhancing Auxin Signaling. Plant Cell 2009, 21, 2253–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, M.; Lee, G.I.; Wang, Y.; Crane, B.R.; Klessig, D.F. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 2008, 283, 32957–32967. [Google Scholar] [CrossRef] [Green Version]



| S. No. | Treatment | Description |
|---|---|---|
| 1. | Control | Unprimed and magnetoprimed seeds kept in distilled water under non-saline condition |
| 2. | Control + NaCl | Unprimed and magnetoprimed seeds kept in 50 mM NaCl for saline condition |
| 3. | ST, L-NAME, and DPI | Unprimed and magnetoprimed seeds kept in NO (SNP, ST and L-NAME) and ROS (DPI) modulators under non-saline condition |
| 4. | ST + NaCl, L-NAME + NaCl, and DPI + NaCl | Unprimed and magnetoprimed seeds kept in NO (SNP, ST and L-NAME) and ROS (DPI) modulators under saline condition |
| S. No. | Gene Locus | Primer Name | Primer Sequence (5′ to 3′) |
|---|---|---|---|
| 1. | Glyma.09G224600 (GmNOS-like 1) | GmNOS1-F | AATAAGAAGAAAAAGAAGAAA |
| GmNOS1-R | TTCGAAGCTGGTGGTGTTTCT | ||
| 2. | Glyma.12G012400 (GmNOS-like 2) | GmNOS2-F | TGTGGACAGTTATGATCCCAA |
| GmNOS2-R | AACAGCCTTGGGGACGTGCAC | ||
| 3. | Glyma.13G083800 (GmNR1) | GmNR1-F | GACCGGTTCAAGCTATGGTAC |
| GmNR1-R | TTTCTCCAAATTAGGCTGCAC | ||
| 4. | Glyma.06G109200 (GmNR2) | GmNR2-F | GCTACCCCAGCCGCCGCCGCC |
| GmNR2-R | AAACGGACAAGGGAAGAGTTC | ||
| 5. | Glyma.19G052400 (GmEF1A) | GmEF1A-F | TGAAGCTGGTATTTCTAAGGA |
| GmEF1A-R | GTAACCAACCTTCTTCAAGTAG | ||
| 6. | Glyma.20G136000 (GmTUA4) | GmTUA4-F | CGTGCAGTGTTTGTAGATCTT |
| GmTUA4-R | GATCAACAATCTCTTTCCCAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataria, S.; Anand, A.; Raipuria, R.K.; Kumar, S.; Jain, M.; Watts, A.; Brestic, M. Magnetopriming Actuates Nitric Oxide Synthesis to Regulate Phytohormones for Improving Germination of Soybean Seeds under Salt Stress. Cells 2022, 11, 2174. https://doi.org/10.3390/cells11142174
Kataria S, Anand A, Raipuria RK, Kumar S, Jain M, Watts A, Brestic M. Magnetopriming Actuates Nitric Oxide Synthesis to Regulate Phytohormones for Improving Germination of Soybean Seeds under Salt Stress. Cells. 2022; 11(14):2174. https://doi.org/10.3390/cells11142174
Chicago/Turabian StyleKataria, Sunita, Anjali Anand, Ritesh Kumar Raipuria, Sunil Kumar, Meeta Jain, Anshul Watts, and Marian Brestic. 2022. "Magnetopriming Actuates Nitric Oxide Synthesis to Regulate Phytohormones for Improving Germination of Soybean Seeds under Salt Stress" Cells 11, no. 14: 2174. https://doi.org/10.3390/cells11142174






