Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond
Abstract
1. Introduction
2. Crossing the Species Barrier—Human Infection with Zoonotic Viruses
3. From Local to Systemic—Disease Course and Immune Responses
4. Biomarkers for the Prediction of Disease Progression in COVID-19 and Infections with HPAIV
5. The Contribution of PRRs to the Innate Immune Responses during COVID-19 and HPAIV Infections
6. Adaptive Immune Response against SARS-CoV-2
7. Importance of Interferons for COVID-19
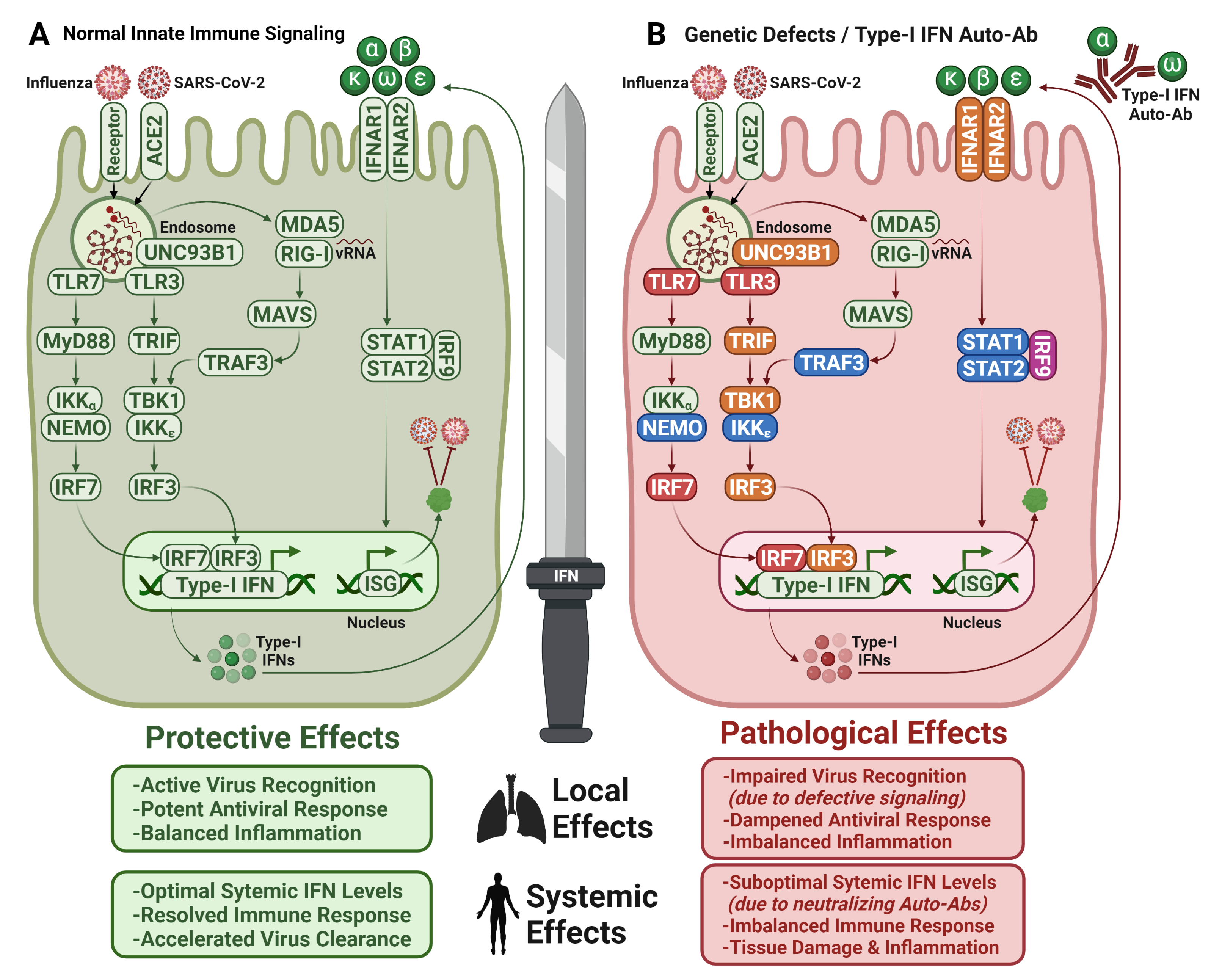
7.1. Type I IFN Autoantibodies in COVID-19
7.2. Therapeutic Application of IFNs for COVID-19 and HPAIV Infections
8. Antiviral and Immunomodulatory Treatments for COVID-19
8.1. Direct-Acting Antivirals for Treatment of COVID-19
8.2. Immunomodulatory Strategies to Alleviate Immunopathology in COVID-19
9. Long-Term Complications of COVID-19
10. COVID-19 and Long COVID in Children
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, H.; Peng, C.; Zhang, C.; Stoian, A.M.M.; Tazi, L.; Brennan, G.; Rothenburg, S. Maladaptation after a virus host switch leads to increased activation of the pro-inflammatory NF-κB pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2115354119. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Kandun, I.N.; Wibisono, H.; Sedyaningsih, E.R.; Yusharmen; Hadisoedarsuno, W.; Purba, W.; Santoso, H.; Septiawati, C.; Tresnaningsih, E.; Heriyanto, B.; et al. Three Indonesian Clusters of H5N1 Virus Infection in 2005. N. Engl. J. Med. 2006, 355, 2186–2194. [Google Scholar] [CrossRef]
- Koopmans, M.; Wilbrink, B.; Conyn, M.; Natrop, G.; van der Nat, H.; Vennema, H.; Meijer, A.; van Steenbergen, J.; Fouchier, R.; Osterhaus, A.; et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004, 363, 587–593. [Google Scholar] [CrossRef]
- Olsen, S.J.; Ungchusak, K.; Sovann, L.; Uyeki, T.M.; Dowell, S.F.; Cox, N.J.; Aldis, W.; Chunsuttiwat, S. Family Clustering of Avian Influenza A (H5N1). Emerg. Infect. Dis. 2005, 11, 1799–1801. [Google Scholar] [CrossRef]
- Ungchusak, K.; Auewarakul, P.; Dowell, S.F.; Kitphati, R.; Auwanit, W.; Puthavathana, P.; Uiprasertkul, M.; Boonnak, K.; Pittayawonganon, C.; Cox, N.J.; et al. Probable Person-to-Person Transmission of Avian Influenza A (H5N1). New Engl. J. Med. 2005, 352, 333–340. [Google Scholar] [CrossRef]
- Hien, T.T.; Liem, N.T.; Dung, N.T.; San, L.T.; Mai, P.P.; Chau, N.V.V.; Suu, P.T.; Dong, V.C.; Mai, L.T.Q.; Thi, N.T.; et al. Avian Influenza A (H5N1) in 10 Patients in Vietnam. New Engl. J. Med. 2004, 350, 1179–1188. [Google Scholar] [CrossRef]
- Rogers, G.N.; Paulson, J.C. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. [Google Scholar] [CrossRef]
- Stevens, J.; Blixt, O.; Glaser, L.; Taubenberger, J.K.; Palese, P.; Paulson, J.C.; Wilson, I.A. Glycan Microarray Analysis of the Hemagglutinins from Modern and Pandemic Influenza Viruses Reveals Different Receptor Specificities. J. Mol. Biol. 2006, 355, 1143–1155. [Google Scholar] [CrossRef]
- Nova, N. Cross-Species Transmission of Coronaviruses in Humans and Domestic Mammals, What Are the Ecological Mechanisms Driving Transmission, Spillover, and Disease Emergence? Front. Public Health 2021, 9, 717941. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Külper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 2021, 26, 2100563. [Google Scholar] [CrossRef]
- Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022, 399, 1941–1953. [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021, 371, 850–854. [Google Scholar] [CrossRef]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 2021, 29, 463–476.e6. [Google Scholar] [CrossRef]
- Murphy, K. SARS CoV-2 Detection From Upper and Lower Respiratory Tract Specimens. Chest 2020, 158, 1804–1805. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Chan, M.C.W.; Chan, W.Y.; Wong, H.K.; Cheung, C.Y.; Kwong, D.L.W.; Wong, M.P.; Chui, W.H.; Poon, L.; Tsao, S.W.; et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat. Med. 2007, 13, 147–149. [Google Scholar] [CrossRef]
- Weinheimer, V.K.; Becher, A.; Tönnies, M.; Holland, G.; Knepper, J.; Bauer, T.T.; Schneider, P.; Neudecker, J.; Rückert, J.C.; Szymanski, K.; et al. Influenza A Viruses Target Type II Pneumocytes in the Human Lung. J. Infect. Dis. 2012, 206, 1685–1694. [Google Scholar] [CrossRef]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Hou, Y.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. eBioMedicine 2020, 60, 102976. [Google Scholar] [CrossRef]
- Lakdawala, S.S.; Jayaraman, A.; Halpin, R.A.; Lamirande, E.W.; Shih, A.R.; Stockwell, T.B.; Lin, X.; Simenauer, A.; Hanson, C.T.; Vogel, L.; et al. The soft palate is an important site of adaptation for transmissible influenza viruses. Nature 2015, 526, 122–125. [Google Scholar] [CrossRef]
- Lee, I.T.; Nakayama, T.; Wu, C.-T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.-K.; Shih, L.-C.; Schürch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Z.; Qu, Y.; Zhu, H.; Zhu, Q.; Tong, W.; Bao, L.; Lv, Q.; Cong, J.; Li, D.; et al. Distinct uptake, amplification, and release of SARS-CoV-2 by M1 and M2 alveolar macrophages. Cell Discov. 2021, 7, 24. [Google Scholar] [CrossRef]
- Chertow, D.; Stein, S.; Ramelli, S.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.; Dickey, J.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence Throughout the Human Body and Brain National Institutes of Health. 2021. Available online: https://www.researchsquare.com/article/rs-1139035/v1 (accessed on 18 May 2022).
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Genet. 2021, 19, 425–441. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van Den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- Burke, R.M.; Killerby, M.E.; Newton, S.; Ashworth, C.E.; Berns, A.L.; Brennan, S.; Bressler, J.M.; Bye, E.; Crawford, R.; Morano, L.H.; et al. Symptom Profiles of a Convenience Sample of Patients with COVID-19—United States, January–April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 904–908. [Google Scholar] [CrossRef]
- Klaser, K.; Molteni, E.; Graham, M.S.; Canas, L.S.; Osterdahl, M.F.; Antonelli, M.; Chen, L.; Deng, J.; Murray, B.; Kerfoot, E.; et al. COVID-19 due to the B.1.617.2 (Delta) variant compared to B.1.1.7 (Alpha) variant of SARS-CoV-2: Two prospective observational cohort studies. medRxiv 2021. [Google Scholar] [CrossRef]
- Hagen, A. How Dangerous Is the Delta Variant (B.1.617.2)? Available online: https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2 (accessed on 20 June 2022).
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Spiteri, G.; Fielding, J.; Diercke, M.; Campese, C.; Enouf, V.; Gaymard, A.; Bella, A.; Sognamiglio, P.; Moros, M.J.S.; Riutort, A.N.; et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Eurosurveillance 2020, 25, 2000178. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- England, J.T.; Abdulla, A.; Biggs, C.; Lee, A.Y.; Hay, K.; Hoiland, R.L.; Wellington, C.L.; Sekhon, M.; Jamal, S.; Shojania, K.; et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021, 45, 100707. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J., Jr.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Robbins-Juarez, S.Y.; Qian, L.; King, K.L.; Stevens, J.S.; Husain, S.A.; Radhakrishnan, J.; Mohan, S. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int. Rep. 2020, 5, 1149–1160. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier-Decrucq, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients With COVID-19. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Mariette, X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Reported Human Infections with Avian Influenza A V. Available online: https://www.cdc.gov/flu/avianflu/reported-human-infections.htm (accessed on 6 May 2022).
- Yang, L.; Zhao, X.; Li, X.; Bo, H.; Li, D.; Liu, J.; Wang, D. Case report for human infection with a highly pathogenic avian influenza A(H5N6) virus in Beijing, China 2019. Biosaf. Health 2020, 2, 49–52. [Google Scholar] [CrossRef]
- Yuen, K.-Y.; Chan, P.; Peiris, J.S.M.; Tsang, D.; Que, T.; Shortridge, K.; Cheung, P.; To, W.; Ho, E.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Yu, H.; Gao, Z.; Feng, Z.; Shu, Y.; Xiang, N.; Zhou, L.; Huai, Y.; Feng, L.; Peng, Z.; Li, Z.; et al. Clinical Characteristics of 26 Human Cases of Highly Pathogenic Avian Influenza A (H5N1) Virus Infection in China. PLoS ONE 2008, 3, e2985. [Google Scholar] [CrossRef] [PubMed]
- Koutsakos, M.; Kedzierska, K.; Subbarao, K. Immune Responses to Avian Influenza Viruses. J. Immunol. 2019, 202, 382–391. [Google Scholar] [CrossRef] [PubMed]
- To, K.-F.; Chan, P.K.; Chan, K.-F.; Lee, W.-K.; Lam, W.-Y.; Wong, K.-F.; Tang, N.L.; Tsang, D.N.; Sung, R.Y.; Buckley, T.A.; et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med Virol. 2001, 63, 242–246. [Google Scholar] [CrossRef]
- Le, M.T.Q.; Wertheim, H.F.L.; Nguyen, H.D.; Taylor, W.; Hoang, P.V.M.; Vuong, C.D.; Nguyen, H.L.K.; Nguyen, H.H.; Nguyen, T.Q.; Nguyen, T.V.; et al. Influenza A H5N1 Clade 2.3.4 Virus with a Different Antiviral Susceptibility Profile Replaced Clade 1 Virus in Humans in Northern Vietnam. PLoS ONE 2008, 3, e3339. [Google Scholar] [CrossRef]
- Kandun, I.N.; Tresnaningsih, E.; Purba, W.H.; Lee, V.; Samaan, G.; Harun, S.; Soni, E.; Septiawati, C.; Setiawati, T.; Sariwati, E.; et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: A case series. Lancet 2008, 372, 744–749. [Google Scholar] [CrossRef]
- Oner, A.F.; Bay, A.; Arslan, S.; Akdeniz, H.; Sahin, H.A.; Cesur, Y.; Epcacan, S.; Yilmaz, N.; Deger, I.; Kizilyildiz, B.; et al. Avian Influenza A (H5N1) Infection in Eastern Turkey in 2006. N. Engl. J. Med. 2006, 355, 2179–2185. [Google Scholar] [CrossRef]
- Chotpitayasunondh, T.; Ungchusak, K.; Hanshaoworakul, W.; Chunsuthiwat, S.; Sawanpanyalert, P.; Kijphati, R.; Lochindarat, S.; Srisan, P.; Suwan, P.; Osotthanakorn, Y.; et al. Human Disease from Influenza A (H5N1), Thailand. Emerg. Infect. Dis. 2005, 11, 201–209. [Google Scholar] [CrossRef]
- Buchy, P.; Mardy, S.; Vong, S.; Toyoda, T.; Aubin, J.-T.; Miller, M.; Touch, S.; Sovann, L.; Dufourcq, J.-B.; Richner, B.; et al. Influenza A/H5N1 virus infection in humans in Cambodia. J. Clin. Virol. 2007, 39, 164–168. [Google Scholar] [CrossRef]
- Uyeki, T.M. Human Infection with Highly Pathogenic Avian Influenza A (H5N1) Virus: Review of Clinical Issues. Clin. Infect. Dis. 2009, 49, 279–290. [Google Scholar] [CrossRef]
- Pan, M.; Gao, R.; Lv, Q.; Huang, S.; Zhou, Z.; Yang, L.; Li, X.; Zhao, X.; Zou, X.; Tong, W.; et al. Human infection with a novel, highly pathogenic avian influenza A (H5N6) virus: Virological and clinical findings. J. Infect. 2016, 72, 52–59. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Peiris, J.; Yu, W.; Leung, C.; Cheung, C.; Ng, W.; Nicholls, J.; Ng, T.; Chan, K.; Lai, S.; Lim, W.; et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 2004, 363, 617–619. [Google Scholar] [CrossRef]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Chau, N.V.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef]
- Chan, M.C.; Cheung, C.Y.; Chui, W.H.; Tsao, S.W.; Nicholls, J.M.; Chan, Y.O.; Chan, R.W.; Long, H.T.; Poon, L.L.; Guan, Y.; et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005, 6, 135. [Google Scholar] [CrossRef]
- Krischuns, T.; Günl, F.; Henschel, L.; Binder, M.; Willemsen, J.; Schloer, S.; Rescher, U.; Gerlt, V.; Zimmer, G.; Nordhoff, C.; et al. Phosphorylation of TRIM28 Enhances the Expression of IFN-β and Proinflammatory Cytokines During HPAIV Infection of Human Lung Epithelial Cells. Front. Immunol. 2018, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Poon, L.; Lau, A.; Luk, W.; Lau, Y.; Shortridge, K.; Gordon, S.; Guan, Y.; Peiris, J. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: A mechanism for the unusual severity of human disease? Lancet 2002, 360, 1831–1837. [Google Scholar] [CrossRef]
- Lipatov, A.S.; Andreansky, S.; Webby, R.J.; Hulse, D.J.; Rehg, J.E.; Krauss, S.; Perez, D.R.; Doherty, P.C.; Webster, R.G.; Sangster, M.Y. Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortants in mice: The role of cytokines and B- and T-cell responses. J. Gen. Virol. 2005, 86, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Iwatsuki-Horimoto, K.; Takano, R.; Nidom, C.A.; Le, M.T.Q.; Nagamura-Inoue, T.; Horimoto, T.; Yamashita, N.; Kawaoka, Y. Cytokine production by primary human macrophages infected with highly pathogenic H5N1 or pandemic H1N1 2009 influenza viruses. J. Gen. Virol. 2011, 92, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.P.; Wong, C.H.K.; Cheung, C.Y.; Chan, M.C.W.; Lee, S.M.Y.; Nicholls, J.M.; Guan, Y.; Peiris, J.S.M. Viral Genetic Determinants of H5N1 Influenza Viruses That Contribute to Cytokine Dysregulation. J. Infect. Dis. 2009, 200, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Hoffmann, E.; Webster, R.G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. USA 2007, 104, 12479–12481. [Google Scholar] [CrossRef]
- Xu, T.; Qiao, J.; Zhao, L.; He, G.; Li, K.; Wang, J.; Tian, Y.; Wang, H. Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice. Eur. Respir. J. 2009, 33, 852–860. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rosenheim, J.; Bell, L.C.; Chandran, A.; Guerra-Assuncao, J.A.; Pollara, G.; Whelan, M.; Artico, J.; Joy, G.; Kurdi, H.; et al. Blood transcriptional biomarkers of acute viral infection for detection of pre-symptomatic SARS-CoV-2 infection: A nested, case-control diagnostic accuracy study. Lancet Microbe 2021, 2, e508–e517. [Google Scholar] [CrossRef]
- Tang, B.M.; Shojaei, M.; Parnell, G.P.; Huang, S.; Nalos, M.; Teoh, S.; O’Connor, K.; Schibeci, S.; Phu, A.L.; Kumar, A.; et al. A novel immune biomarker IFI27 discriminates between influenza and bacteria in patients with suspected respiratory infection. Eur. Respir. J. 2017, 49, 1602098. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Wöll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R.; Lim, M.A.; Oehadian, A.; Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: A meta-analysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937175. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Hearth J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Yang, Y.; Ma, H.; Li, Z.; Zhang, J.; Cheng, J.; Zhang, X.; Zhao, Y.; Xia, Z.; et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol. Med. 2020, 12, e12421. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Su, N.; Bao, X.; Li, Y.; Jin, J. Simplified immune-dysregulation index: A novel marker predicts 28-day mortality of intensive care patients with COVID-19. Intensiv. Care Med. 2020, 46, 1645–1647. [Google Scholar] [CrossRef]
- Giannakodimos, I.; Gkountana, G.-V.; Lykouras, D.; Karkoulias, K.; Tsakas, S. The Role of Interleukin-6 in the Pathogenesis, Prognosis and Treatment of Severe COVID-19. Curr. Med. Chem. 2020, 28, 5328–5338. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cao, J.; Wang, Q.; Shi, Q.; Liu, K.; Luo, Z.; Chen, X.; Chen, S.; Yu, K.; Huang, Z.; et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J. Intensiv. Care 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.-R.; Fobker, M.; Kühn, J.; Braune, S.; Göbel, U.; Thölking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, D.; Badawi, A. C-reactive protein as a biomarker of severe H1N1 influenza. Agents Actions 2019, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Farrar, J.; Han, A.M.; Hayden, F.G.; Hyer, R.; de Jong, M.D.; Lochindarat, S.; Nguyen, T.K.T.; Nguyen, T.H.; Tran, T.H.; et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005, 353, 1374–1385. [Google Scholar]
- Soepandi, P.Z.; Burhan, E.; Mangunnegoro, H.; Nawas, A.; Aditama, T.Y.; Partakusuma, L.; Isbaniah, F.; Ikhsan, M.; Swidarmoko, B.; Sutiyoso, A.; et al. Clinical Course of Avian Influenza A(H5N1) in Patients at the Persahabatan Hospital, Jakarta, Indonesia, 2005. Chest 2010, 138, 665–673. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Ramos, I.; Fernandez-Sesma, A. Innate Immunity to H5N1 Influenza Viruses in Humans. Viruses 2012, 4, 3363–3388. [Google Scholar] [CrossRef]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern Recognition Receptors and the Innate Immune Response to Viral Infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Kanneganti, T. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, K.; Zhang, Z.; Duan, F.; Guo, L.; Luo, W.; Mok, B.W.-Y.; Thakur, A.; Ke, X.; Motallebnejad, P.; et al. Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nat. Commun. 2022, 13, 2028. [Google Scholar] [CrossRef]
- Dalskov, L.; Møhlenberg, M.; Thyrsted, J.; Blay-Cadanet, J.; Poulsen, E.T.; Folkersen, B.H.; Skaarup, S.H.; Olagnier, D.; Reinert, L.; Enghild, J.J.; et al. SARS-CoV-2 evades immune detection in alveolar macrophages. EMBO Rep. 2020, 21, e51252. [Google Scholar] [CrossRef]
- Wang, J.; Nikrad, M.P.; Travanty, E.A.; Zhou, B.; Phang, T.; Gao, B.; Alford, T.; Ito, Y.; Nahreini, P.; Hartshorn, K.; et al. Innate Immune Response of Human Alveolar Macrophages during Influenza A Infection. PLoS ONE 2012, 7, e29879. [Google Scholar] [CrossRef]
- Cuevas, A.M.; Clark, J.M.; Potter, J.J. Increased TLR/MyD88 signaling in patients with obesity: Is there a link to COVID-19 disease severity? Int. J. Obes. 2021, 45, 1152–1154. [Google Scholar] [CrossRef]
- Sohn, K.M.; Lee, S.-G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Paik, S.; et al. COVID-19 Patients Upregulate Toll-like Receptor 4-mediated Inflammatory Signaling That Mimics Bacterial Sepsis. J. Korean Med Sci. 2020, 35, e343. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; You, F. Publisher Correction: SARS-CoV-2 spike protein interacts with and activates TLR4 (Cell Research, (2021), 31, 7, (818-820), 10.1038/s41422-021-00495-9). Cell Res. 2021, 31, 825. [Google Scholar] [CrossRef]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Lee, S.M.Y.; Cheung, C.-Y.; Mao, H.; Lai, A.K.W.; Chan, R.W.Y.; Chan, M.C.W.; Tu, W.; Guan, Y.; Lau, Y.-L.; et al. H5N1 Influenza Virus–Induced Mediators Upregulate RIG-I in Uninfected Cells by Paracrine Effects Contributing to Amplified Cytokine Cascades. J. Infect. Dis. 2011, 204, 1866–1878. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Sakai, K.; Gotoh, S.; Miorin, L.; De Jesus, P.D.; et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.; Long, J.C.; Bauer, D.L.V.; Fan, R.L.Y.; Yen, H.-L.; Sharps, J.; Siegers, J.Y.; Killip, M.J.; French, H.; Oliva-Martín, M.J.; et al. Mini viral RNAs act as innate immune agonists during influenza virus infection. Nat. Microbiol. 2018, 3, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Arai, Y.; Yamanaka, I.; Okomato, T.; Isobe, A.; Nakai, N.; Kamimura, N.; Suzuki, T.; Daidoji, T.; Ono, T.; et al. Stimulation of Dysregulated IFN- β Responses by Aberrant SARS-CoV-2 Small Viral RNAs Acting as RIG-I Agonists. 2022. Available online: https://assets.researchsquare.com/files/rs-1271053/v2/b9e39a7d-71a8-46e9-b293-e1334e366176.pdf?c=1647456052 (accessed on 6 May 2022).
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef]
- Dey, M.; Cao, C.; Dar, A.C.; Tamura, T.; Ozato, K.; Sicheri, F.; Dever, T.E. Mechanistic Link between PKR Dimerization, Autophosphorylation, and eIF2α Substrate Recognition. Cell 2005, 122, 901–913. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Oja, A.E.; Saris, A.; Ghandour, C.A.; Kragten, N.A.; Hogema, B.M.; Nossent, E.J.; Heunks, L.M.; Cuvalay, S.; Slot, E.; Linty, F.; et al. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur. J. Immunol. 2020, 50, 1998–2012. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, 6508. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Kinning, K.T.; Sullivan, K.D.; Araya, P.; Smith, K.P.; Granrath, R.E.; Shaw, J.R.; Baxter, R.; Jordan, K.R.; Russell, S.; et al. Specialized interferon action in COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2116730119. [Google Scholar] [CrossRef]
- Meyts, I.; Casanova, J. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur. J. Immunol. 2021, 51, 1039–1061. [Google Scholar] [CrossRef]
- Ciancanelli, M.J.; Huang, S.X.L.; Luthra, P.; Garner, H.; Itan, Y.; Volpi, S.; Lafaille, F.G.; Trouillet, C.; Schmolke, M.; Albrecht, R.A.; et al. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 2015, 348, 448–453. [Google Scholar] [CrossRef]
- Hernandez, N.; Melki, I.; Jing, H.; Habib, T.; Huang, S.S.; Danielson, J.; Kula, T.; Drutman, S.; Belkaya, S.; Rattina, V.; et al. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J. Exp. Med. 2018, 215, 2567–2585. [Google Scholar] [CrossRef]
- Lim, H.K.; Huang, S.X.; Chen, J.; Kerner, G.; Gilliaux, O.; Bastard, P.; Dobbs, K.; Hernandez, N.; Goudin, N.; Hasek, M.L.; et al. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 2019, 216, 2038–2056. [Google Scholar] [CrossRef]
- Bastard, P.; Zhang, Q.; Zhang, S.-Y.; Jouanguy, E.; Casanova, J.-L. Type I interferons and SARS-CoV-2: From cells to organisms. Curr. Opin. Immunol. 2022, 74, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, Q.; Casanova, J.-L.; Su, H.C.; Abel, L.; Bastard, P.; Cobat, A.; Jouanguy, E.; Notarangelo, L.; Covid The COVID Team. Severe COVID-19 in the young and healthy: Monogenic inborn errors of immunity? Nat. Rev. Immunol. 2020, 20, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Puel, A.; Zhang, S.; Eidenschenk, C.; Ku, C.-L.; Casrouge, A.; Picard, C.; von Bernuth, H.; Senechal, B.; Plancoulaine, S.; et al. Human TLR-7-, -8-, and -9-Mediated Induction of IFN-α/β and -λ Is IRAK-4 Dependent and Redundant for Protective Immunity to Viruses. Immunity 2005, 23, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, L.B.; Ben-Ali, M.; Quach, H.; Laval, G.; Patin, E.; Pickrell, J.K.; Bouchier, C.; Tichit, M.; Neyrolles, O.; Gicquel, B.; et al. Evolutionary Dynamics of Human Toll-Like Receptors and Their Different Contributions to Host Defense. PLoS Genet. 2009, 5, e1000562. [Google Scholar] [CrossRef]
- Puel, A.; Bastard, P.; Bustamante, J.; Casanova, J.L. Human autoantibodies underlying infectious diseases. J. Exp. Med. 2022, 219, e20211387. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Abers, M.S.; Rosen, L.B.; Delmonte, O.M.; Shaw, E.; Bastard, P.; Imberti, L.; Quaresima, V.; Biondi, A.; Bonfanti, P.; Castagnoli, R.; et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 2021, 99, 917–921. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodríguez, Y.; Gallo, J.E.; Salazar-Uribe, J.C.; Santander, M.J.; Cala, M.P.; Zapata, W.; Zapata, M.I.; et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 2021, 118, 102598. [Google Scholar] [CrossRef]
- Vazquez, S.E.; Bastard, P.; Kelly, K.; Gervais, A.; Norris, P.J.; Dumont, L.J.; Casanova, J.-L.; Anderson, M.S.; DeRisi, J.L. Neutralizing Autoantibodies to Type I Interferons in COVID-19 Convalescent Donor Plasma. J. Clin. Immunol. 2021, 41, 1169–1171. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Miao, V.N.; Owings, A.H.; Navia, A.W.; Tang, Y.; Bromley, J.D.; Lotfy, P.; Sloan, M.; Laird, H.; Williams, H.B.; et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021, 184, 4713–4733.e22. [Google Scholar] [CrossRef]
- Troya, J.; Bastard, P.; Planas-Serra, L.; Ryan, P.; Ruiz, M.; de Carranza, M.; Torres, J.; Martínez, A.; Abel, L.; Casanova, J.-L.; et al. Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J. Clin. Immunol. 2021, 41, 914–922. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Karbuz, A.; Gervais, A.; Tayoun, A.A.; Aiuti, A.; Belot, A.; Bolze, A.; Gaudet, A.; Bondarenko, A.; et al. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- Carapito, R.; Li, R.; Helms, J.; Carapito, C.; Gujja, S.; Rolli, V.; Guimaraes, R.; Malagon-Lopez, J.; Spinnhirny, P.; Lederle, A.; et al. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl. Med. 2022, 14, abj7521. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Chauvineau-Grenier, A.; Bastard, P.; Servajean, A.; Gervais, A.; Rosain, J.; Jouanguy, E.; Cobat, A.; Casanova, J.-L.; Rossi, B. Autoantibodies Neutralizing Type I Interferons in 20% of COVID-19 Deaths in a French Hospital. J. Clin. Immunol. 2022, 42, 459–470. [Google Scholar] [CrossRef]
- Goncalves, D.; Mezidi, M.; Bastard, P.; Perret, M.; Saker, K.; Fabien, N.; Pescarmona, R.; Lombard, C.; Walzer, T.; Casanova, J.; et al. Antibodies against type I interferon: Detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl. Immunol. 2021, 10, e1327. [Google Scholar] [CrossRef]
- Koning, R.; Bastard, P.; Casanova, J.-L.; Brouwer, M.C.; van de Beek, D.; van Agtmael, M.; Algera, A.G.; Appelman, B.; van Baarle, F.; Bax, D.; et al. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensiv. Care Med. 2021, 47, 704–706. [Google Scholar] [CrossRef]
- Raadsen, M.P.; Gharbharan, A.; Jordans, C.C.E.; Mykytyn, A.Z.; Lamers, M.M.; van den Doel, P.B.; Endeman, H.; van den Akker, J.P.C.; Geurtsvan Kessel, C.H.; Koopmans, M.P.G.; et al. Interferon-α2 Auto-antibodies in Convalescent Plasma Therapy for COVID-19. J. Clin. Immunol. 2022, 42, 232–239. [Google Scholar] [CrossRef]
- Solanich, X.; Rigo-Bonnin, R.; Gumucio, V.-D.; Bastard, P.; Rosain, J.; Philippot, Q.; Perez-Fernandez, X.-L.; Fuset-Cabanes, M.-P.; Gordillo-Benitez, M.; Suarez-Cuartin, G.; et al. Pre-existing Autoantibodies Neutralizing High Concentrations of Type I Interferons in Almost 10% of COVID-19 Patients Admitted to Intensive Care in Barcelona. J. Clin. Immunol. 2021, 41, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Van der Wijst, M.G.P.; Vazquez, S.E.; Hartoularos, G.C.; Bastard, P.; Grant, T.; Bueno, R.; Lee, D.S.; Greenland, J.R.; Sun, Y.; Perez, R.; et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021, 13, eabh2624. [Google Scholar] [CrossRef] [PubMed]
- Trouillet-Assant, S.; Viel, S.; Gaymard, A.; Pons, S.; Richard, J.-C.; Perret, M.; Villard, M.; Brengel-Pesce, K.; Lina, B.; Mezidi, M.; et al. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020, 146, 206–208.e2. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Credle, J.J.; Gunn, J.; Sangkhapreecha, P.; Monaco, D.R.; Zheng, X.A.; Tsai, H.J.; Wilbon, A.; Morgenlander, W.R.; Dong, Y.; Jayaraman, S.; et al. Neutralizing IFNL3 Autoantibodies in Severe COVID-19 Identified Using Molecular Indexing of Proteins by Self-Assembly. bioRxiv 2021. [Google Scholar] [CrossRef]
- Beccuti, G.; Ghizzoni, L.; Cambria, V.; Codullo, V.; Sacchi, P.; Lovati, E.; Mongodi, S.; Iotti, G.A.; Mojoli, F. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: Letter to the editor. J. Endocrinol. Investig. 2020, 43, 1175–1177. [Google Scholar] [CrossRef]
- Carpino, A.; Buganza, R.; Matarazzo, P.; Tuli, G.; Pinon, M.; Calvo, P.L.; Montin, D.; Licciardi, F.; De Sanctis, L. Autoimmune Polyendocrinopathy–Candidiasis–Ectodermal Dystrophy in Two Siblings: Same Mutations but Very Different Phenotypes. Genes 2021, 12, 169. [Google Scholar] [CrossRef]
- Bastard, P.; Orlova, E.; Sozaeva, L.; Lévy, R.; James, A.; Schmitt, M.M.; Ochoa, S.; Kareva, M.; Rodina, Y.; Gervais, A.; et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021, 218, e20210554. [Google Scholar] [CrossRef]
- Meisel, C.; Akbil, B.; Meyer, T.; Lankes, E.; Corman, V.M.; Staudacher, O.; Unterwalder, N.; Kölsch, U.; Drosten, C.; Mall, M.A.; et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type. J. Clin. Investig. 2021, 131, e150867. [Google Scholar] [CrossRef]
- Lopez, J.; Mommert, M.; Mouton, W.; Pizzorno, A.; Brengel-Pesce, K.; Mezidi, M.; Villard, M.; Lina, B.; Richard, J.-C.; Fassier, J.-B.; et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 2021, 218, e20211211. [Google Scholar] [CrossRef]
- Bastard, P.; Michailidis, E.; Hoffmann, H.-H.; Chbihi, M.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Seeleuthner, Y.; Gervais, A.; Materna, M.; et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021, 218, e20202486. [Google Scholar] [CrossRef]
- Klein, S.L.; Pekosz, A.; Park, H.-S.; Ursin, R.L.; Shapiro, J.R.; Benner, S.E.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.J.; et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef]
- Wang, T.T.; Ravetch, J.V. Functional diversification of IgGs through Fc glycosylation. J. Clin. Investig. 2019, 129, 3492–3498. [Google Scholar] [CrossRef]
- Beck, D.B.; Aksentijevich, I. Susceptibility to severe COVID-19. Science 2020, 370, 404–405. [Google Scholar] [CrossRef]
- Paul, F.; Pellegrini, S.; Uzé, G. IFNA2: The prototypic human alpha interferon. Gene 2015, 567, 132–137. [Google Scholar] [CrossRef]
- Severa, M.; Farina, C.; Salvetti, M.; Coccia, E.M. Three Decades of Interferon-β in Multiple Sclerosis: Can We Repurpose This Information for the Management of SARS-CoV2 Infection? Front. Immunol. 2020, 11, 1459. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Reder, A.T.; Feng, X. How Type I Interferons Work in Multiple Sclerosis and Other Diseases: Some Unexpected Mechanisms. J. Interf. Cytokine Res. 2014, 34, 589–599. [Google Scholar] [CrossRef]
- Mantlo, E.; Bukreyeva, N.; Maruyama, J.; Paessler, S.; Huang, C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020, 179, 104811. [Google Scholar] [CrossRef]
- Kumar, S.; Çalışkan, D.M.; Janowski, J.; Faist, A.; Conrad, B.C.G.; Lange, J.; Ludwig, S.; Brunotte, L. Beyond Vaccines: Clinical Status of Prospective COVID-19 Therapeutics. Front. Immunol. 2021, 12, 752227. [Google Scholar] [CrossRef]
- Stanifer, M.L.; Guo, C.; Doldan, P.; Boulant, S. Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces. Front. Immunol. 2020, 11, 608645. [Google Scholar] [CrossRef] [PubMed]
- Vanderheiden, A.; Ralfs, P.; Chirkova, T.; Upadhyay, A.A.; Zimmerman, M.G.; Bedoya, S.; Aoued, H.; Tharp, G.M.; Pellegrini, K.L.; Manfredi, C.; et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J. Virol. 2020, 94, e00985-20. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef]
- Metz-Zumaran, C.; Kee, C.; Doldan, P.; Guo, C.; Stanifer, M.L.; Boulant, S. Increased Sensitivity of SARS-CoV-2 to Type III Interferon in Human Intestinal Epithelial Cells. J. Virol. 2022, 96, e0170521. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, M.L.; Kee, C.; Cortese, M.; Zumaran, C.M.; Triana, S.; Mukenhirn, M.; Kraeusslich, H.-G.; Alexandrov, T.; Bartenschlager, R.; Boulant, S. Critical Role of Type III Interferon in Controlling SARS-CoV-2 Infection in Human Intestinal Epithelial Cells. Cell Rep. 2020, 32, 107863. [Google Scholar] [CrossRef] [PubMed]
- EIGER Biopharmaceuticals. Eiger’s Single-Dose Peginterferon Lambda for COVID-19 Reduced Risk of Hospitalization or ER Visits by 50% in a Predominantly Vaccinated Population in Phase 3 TOGETHER Study. Available online: https://www.prnewswire.com/news-releases/eigers-single-dose-peginterferon-lambda-for-covid-19-reduced-risk-of-hospitalization-or-er-visits-by-50-in-a-predominantly-vaccinated-population-in-phase-3-together-study-301504827.html (accessed on 4 May 2022).
- Haasbach, E.; Droebner, K.; Vogel, A.B.; Planz, O. Low-Dose Interferon Type I Treatment Is Effective Against H5N1 and Swine-Origin H1N1 Influenza A Viruses In Vitro and In Vivo. J. Interf. Cytokine Res. 2011, 31, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.L.; Smith, D.W.; Cummins, M.J.; Jacoby, P.A.; Cummins, J.M.; Beilharz, M.W. Low-dose oral interferon alpha as prophylaxis against viral respiratory illness: A double-blind, parallel controlled trial during an influenza pandemic year. Influ. Other Respir. Viruses 2013, 7, 854–862. [Google Scholar] [CrossRef]
- Sutter, K.; Dickow, J.; Dittmer, U. Interferon α subtypes in HIV infection. Cytokine Growth Factor Rev. 2018, 40, 13–18. [Google Scholar] [CrossRef]
- Matos, A.D.R.; Wunderlich, K.; Schloer, S.; Schughart, K.; Geffers, R.; Seders, M.; De Witt, M.; Christersson, A.; Wiewrodt, R.; Wiebe, K.; et al. Antiviral potential of human IFN-α subtypes against influenza A H3N2 infection in human lung explants reveals subtype-specific activities. Emerg. Microbes Infect. 2019, 8, 1763–1776. [Google Scholar] [CrossRef]
- Guo, K.; Shen, G.; Kibbie, J.; Gonzalez, T.; Dillon, S.M.; Smith, H.A.; Cooper, E.H.; Lavender, K.; Hasenkrug, K.J.; Sutter, K.; et al. Qualitative Differences Between the IFNα subtypes and IFNβ Influence Chronic Mucosal HIV-1 Pathogenesis. PLOS Pathog. 2020, 16, e1008986. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Lai, F.; Wang, Y.; Sutter, K.; Dittmer, U.; Ye, J.; Zai, W.; Liu, M.; Shen, F.; et al. Functional Comparison of Interferon-α Subtypes Reveals Potent Hepatitis B Virus Suppression by a Concerted Action of Interferon-α and Interferon-γ Signaling. Hepatology 2021, 73, 486–502. [Google Scholar] [CrossRef]
- Schuhenn, J.; Meister, T.L.; Todt, D.; Bracht, T.; Schork, K.; Billaud, J.-N.; Elsner, C.; Heinen, N.; Karakoese, Z.; Haid, S.; et al. Differential interferon-α subtype induced immune signatures are associated with suppression of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2111600119. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.N.; To, K.; Lee, C.-K.; Lee, K.-L.; Chan, K.K.C.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; et al. Convalescent Plasma Treatment Reduced Mortality in Patients With Severe Pandemic Influenza A (H1N1) 2009 Virus Infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, J.; Huang, Y.; Liu, X.; Xu, Y.; Chen, S.; Liu, D.; Lin, Z.; Liu, X.; Li, Y. Efficacy of convalescent plasma for the treatment of severe influenza. Crit. Care 2020, 24, 469. [Google Scholar] [CrossRef]
- Marano, G.; Vaglio, S.; Pupella, S.; Facco, G.; Catalano, L.; Liumbruno, G.M.; Grazzini, G. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016, 14, 152–157. [Google Scholar] [CrossRef][Green Version]
- Nitesh, J.; Kashyap, R.; Surani, S.R. What we learned in the past year in managing our COVID-19 patients in intensive care units? World J. Crit. Care Med. 2021, 10, 81–101. [Google Scholar] [CrossRef]
- Brown, B.L.; McCullough, J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus. Apher. Sci. 2020, 59, 102790. [Google Scholar] [CrossRef]
- Bertoglio, F.; Meier, D.; Langreder, N.; Steinke, S.; Rand, U.; Simonelli, L.; Heine, P.A.; Ballmann, R.; Schneider, K.-T.; Roth, K.D.R.; et al. SARS-CoV-2 neutralizing human recombinant antibodies selected from pre-pandemic healthy donors binding at RBD-ACE2 interface. Nat. Commun. 2021, 12, 1577. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. COVID-19 Treatment Guidelines; Anti-SARS-CoV-2 Antibody Products. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 4 May 2022).
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; Van Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L.; et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef]
- Tanni, S.E.; Silvinato, A.; Floriano, I.; Bacha, H.A.; Barbosa, A.N.; Bernardo, W.M. Use of remdesivir in patients with COVID-19: A systematic review and meta-analysis. J. Bras. Pneumol. 2022, 48, e20210393. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic Med. Chem. Lett. 2012, 22, 2705–2707. [Google Scholar] [CrossRef]
- Lo, M.K.; Jordan, R.; Arvey, A.; Sudhamsu, J.; Shrivastava-Ranjan, P.; Hotard, A.L.; Flint, M.; McMullan, L.; Siegel, D.; Clarke, M.O.; et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017, 7, srep43395. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19 (accessed on 4 May 2022).
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef]
- Duwe, S. Influenza viruses—Antiviral therapy and resistance. GMS Infect. Dis. 2017, 5, Doc04. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Barnard, D.L.; Day, C.W.; Smee, D.F.; Bailey, K.W.; Wong, M.-H.; Morrey, J.D.; Furuta, Y. Efficacy of Orally Administered T-705 on Lethal Avian Influenza A (H5N1) Virus Infections in Mice. Antimicrob. Agents Chemother. 2007, 51, 845–851. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Huang, J.; Yin, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial. Front. Pharmacol. 2021, 12, 683296. [Google Scholar] [CrossRef] [PubMed]
- Bosaeed, M.; Alharbi, A.; Mahmoud, E.; Alrehily, S.; Bahlaq, M.; Gaifer, Z.; Alturkistani, H.; Alhagan, K.; Alshahrani, S.; Tolbah, A.; et al. Efficacy of favipiravir in adults with mild COVID-19: A randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2022, 28, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.O.; Jin, P.; Rahman, K.M. Strategies for drug repurposing against coronavirus targets. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100072. [Google Scholar] [CrossRef] [PubMed]
- Extance, A. Covid-19: What is the evidence for the antiviral Paxlovid? BMJ 2022, 377, o1037. [Google Scholar] [CrossRef]
- Lamb, Y.N. Nirmatrelvir Plus Ritonavir: First Approval. Drugs 2022, 82, 585–591. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Lee, J.-C.; Chiu, C.-W.; Lee, C.-C.; Tsai, P.-J.; Hsu, I.-L.; Ko, W.-C. Oral Nirmatrelvir/Ritonavir Therapy for COVID-19: The Dawn in the Dark? Antibiotics 2022, 11, 220. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Van De Veen, W.; Brüggen, M.-C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Rizk, J.G.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Forthal, D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs 2020, 80, 1267–1292. [Google Scholar] [CrossRef]
- Kennedy, G.A.; Varelias, A.; Vuckovic, S.; Le Texier, L.; Gartlan, K.H.; Zhang, P.; Thomas, G.; Anderson, L.; Boyle, G.; Cloonan, N.; et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014, 15, 1451–1459. [Google Scholar] [CrossRef]
- Sheng, F.S.F.; Han, M.H.M.; Huang, Z.H.Z.; Zhang, L.Z.L. Interleukin 6 receptor inhibitor tocilizumab suppresses cytokine expression, inflammasome activation and phagocytosis in a cell model of sepsis. Pharmazie 2016, 71, 636–639. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Alzghari, S.K.; Acuña, V.S. Supportive Treatment with Tocilizumab for COVID-19: A Systematic Review. J. Clin. Virol. 2020, 127, 104380. [Google Scholar] [CrossRef]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Marx, W.; O’Neil, A.; Athan, E.; Walder, K.; Berk, M.; Olive, L.; Carvalho, A.F.; et al. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine 2021, 144, 155593. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G.; et al. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- Okeke, F.; Mone, A.; Swaminath, A. The Course of SARS-COV2 Infection Was Not Severe in a Crohn’s Patient Who Administered Maintenance Anti-TNF Therapy Overlapping the Early Pre-Symptomatic Period of Infection. Antibodies 2020, 9, 42. [Google Scholar] [CrossRef]
- Duret, P.-M.; Sebbag, E.; Mallick, A.; Gravier, S.; Spielmann, L.; Messer, L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann. Rheum. Dis. 2020, 79, 1251–1252. [Google Scholar] [CrossRef]
- Abdullah, A.; Neurath, M.F.; Atreya, R. Mild COVID-19 Symptoms in an Infliximab-Treated Ulcerative Colitis Patient: Can Ongoing Anti-TNF Therapy Protect against the Viral Hyperinflammatory Response and Avoid Aggravated Outcomes? Visc. Med. 2020, 36, 338–342. [Google Scholar] [CrossRef]
- Darwish, I.; Mubareka, S.; Liles, W.C. Immunomodulatory therapy for severe influenza. Expert Rev. Anti-infective Ther. 2011, 9, 807–822. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miro-Mur, F.A. Immunomodulatory therapy for the management of severe COVID-Beyond the anti-viral therapy: A comprehensive review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Safaei, S.; Karimi-Googheri, M. Letter to the Editor: Toll-Like Receptor Antagonists as a Potential Therapeutic Strategy Against Cytokine Storm in COVID-19-Infected Patients. Viral Immunol. 2021, 34, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Das, N.C.; Mukherjee, S. Targeting human TLRs to combat COVID-19: A solution? J. Med Virol. 2021, 93, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; André, P.; Blanco, J.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, srep40791. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C. Focus shifts to antibody cocktails for COVID-19 cytokine storm. Nat. Biotechnol. 2020, 38, 905–908. [Google Scholar] [CrossRef]
- Feuillet, V.; Canard, B.; Trautmann, A. Combining Antivirals and Immunomodulators to Fight COVID-19. Trends Immunol. 2021, 42, 31–44. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.-H.; Yang, Z.-Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, J.; Shi, D.; Chen, W.; Li, J.; Yan, R.; Bi, Y.; Hu, W.; Zhu, Z.; Yu, Y.; et al. Glucocorticoids improve severe or critical COVID-19 by activating ACE2 and reducing IL-6 levels. Int. J. Biol. Sci. 2020, 16, 2382–2391. [Google Scholar] [CrossRef]
- Keller, M.J.; Kitsis, E.A.; Arora, S.; Chen, J.-T.; Agarwal, S.; Ross, M.J.; Tomer, Y.; Southern, W. Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J. Hosp. Med. 2020, 15, 489–493. [Google Scholar] [CrossRef]
- Honigsbaum, M.; Krishnan, L. Taking pandemic sequelae seriously: From the Russian influenza to COVID-19 long-haulers. Lancet 2020, 396, 1389–1391. [Google Scholar] [CrossRef]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influ. Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef]
- Kumar, K.; Guirgis, M.; Zieroth, S.; Lo, E.; Menkis, A.H.; Arora, R.C.; Freed, D.H. Influenza Myocarditis and Myositis: Case Presentation and Review of the Literature. Can. J. Cardiol. 2011, 27, 514–522. [Google Scholar] [CrossRef]
- Aranda, J.; Oriol, I.; Martín, M.; Feria, L.; Vázquez, N.; Rhyman, N.; Vall-Llosera, E.; Pallarés, N.; Coloma, A.; Pestaña, M.; et al. Long-term impact of COVID-19 associated acute respiratory distress syndrome. J. Infect. 2021, 83, 581–588. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Martin-Villares, C.; Molina-Ramirez, C.P.; Bartolome-Benito, M.; Bernal-Sprekelsen, M.; Perez-Fernandez, A.; Alcantara-Armenteros, S.; Monjas-Cánovas, I.; Sancho-Mestre, M.; Alemán-Lopez, O.; Deola-Trasserra, M.D.; et al. Outcome of 1890 tracheostomies for critical COVID-19 patients: A national cohort study in Spain. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1605–1612. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Shaw, B.; Daskareh, M.; Gholamrezanezhad, A. The lingering manifestations of COVID-19 during and after convalescence: Update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). La Radiol. Med. 2021, 126, 40–46. [Google Scholar] [CrossRef]
- Van Gassel, R.J.J.; Bels, J.L.M.; Raafs, A.; van Bussel, B.C.T.; van de Poll, M.C.G.; Simons, S.O.; van der Meer, L.W.L.; Gietema, H.A.; Posthuma, R.; van Santen, S. High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated Survivors of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 371–374. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Méndez, R.; Latorre, A.; González-Jiménez, P.; Feced, L.; Bouzas, L.; Yépez, K.; Ferrando, A.; Zaldívar-Olmeda, E.; Reyes, S.; Menéndez, R. Reduced Diffusion Capacity in COVID-19 Survivors. Ann. Am. Thorac. Soc. 2021, 18, 1253–1255. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Nordvig, A.S.; Fong, K.T.; Willey, J.Z.; Thakur, K.T.; Boehme, A.K.; Vargas, W.S.; Smith, C.J.; Elkind, M.S. Potential Neurologic Manifestations of COVID-19. Neurol. Clin. Pr. 2021, 11, e135–e146. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef]
- Kaseda, E.T.; Levine, A.J. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin. Neuropsychol. 2020, 34, 1498–1514. [Google Scholar] [CrossRef]
- Ritchie, K.; Chan, D.; Watermeyer, T. The cognitive consequences of the COVID-19 epidemic: Collateral damage? Brain Commun. 2020, 2, fcaa069. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Bogue, T.; Koshy, A.; Bindal, P.; Merrill, M.; Aird, W.C.; Bauer, K.A.; Zwicker, J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020, 136, 1342–1346. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Li, J.; Shen, L.; Zhu, L.; Liang, Y.; Lin, X.; Jiao, N.; Cheng, S.; Huang, Y.; et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol. Hepatol. 2021, 6, 344–346. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in Covid-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; King, K.L.; Robbins-Juarez, S.Y.; Khairallah, P.; Toma, K.; Verduzco, H.A.; Daniel, E.; Douglas, D.; Moses, A.A.; Peleg, Y.; et al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS ONE 2020, 15, e0244131. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Combes, A.; Becquemin, M.-H.; Beigelman-Aubry, C.; Hatem, S.; Brun, A.-L.; Zraik, N.; Carrat, F.; Grenier, P.A.; Richard, J.-C.M.; et al. Long-term Outcomes of Pandemic 2009 Influenza A(H1N1)-Associated Severe ARDS. Chest 2012, 142, 583–592. [Google Scholar] [CrossRef]
- Melms, J.C.; Biermann, J.; Huang, H.; Wang, Y.; Nair, A.; Tagore, S.; Katsyv, I.; Rendeiro, A.F.; Amin, A.D.; Schapiro, D.; et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 2021, 595, 114–119. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Kurrer, M.O.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.; Silverwood, R.J.; Di Gessa, G.; et al. Risk factors for ongoing symptomatic COVID-19 and post-COVID-19 syndrome: Analyses of 10 lon-gitudinal studies and electronic health records in the UK. medRxiv 2021. [Google Scholar] [CrossRef]
- Strain, W.D.; Sherwood, O.; Banerjee, A.; Van der Togt, V.; Hishmeh, L.; Rossman, J. The Impact of COVID Vaccination on Symptoms of Long COVID: An International Survey of People with Lived Experience of Long COVID. Vaccines 2021, 10, 652. [Google Scholar] [CrossRef]
- Kuodi, P.; Gorelik, Y.; Zayyad, H.; Wertheim, O.; Wiegler, K.B.; Jabal, K.A.; Dror, A.A.; Nazzal, S.; Glikman, D.; Edelstein, M. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: A cross-sectional study of patients tested between March 2020 and November 2021. medRxiv 2022. [Google Scholar] [CrossRef]
- Rahman, M.; Imam, H.; Nahar, N.; Chowdhury, F.U.H. Third dose vaccine With BNT162b2 and its response on Long COVID after Breakthrough infections. medRxiv 2021. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 1–7. [Google Scholar] [CrossRef]
- Yasuhara, J.; Kuno, T.; Takagi, H.; Sumitomo, N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr. Pulmonol. 2020, 55, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents. JAMA Pediatr. 2020, 174, 882. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Worlock, K.B.; Huang, N.; Lindeboom, R.G.H.; Butler, C.R.; Kumasaka, N.; Conde, C.D.; Mamanova, L.; Bolt, L.; Richardson, L.; et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 2022, 602, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Viner, R.M.; Whittaker, E. Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet 2020, 395, 1741–1743. [Google Scholar] [CrossRef]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020, 26, 100527. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Nakamura, A.; Ikeda, K.; Hamaoka, K. Aetiological Significance of Infectious Stimuli in Kawasaki Disease. Front. Pediatr. 2019, 7, 244. [Google Scholar] [CrossRef]
- Banday, A.Z.; Arul, A.; Vignesh, P.; Singh, M.P.; Goyal, K.; Singh, S. Kawasaki disease and influenza—New lessons from old associations. Clin. Rheumatol. 2021, 40, 2991–2999. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. New Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef]
- Stephenson, T.; Pereira, S.M.P.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef]
- Schreiber, A.; Viemann, D.; Schöning, J.; Schloer, S.; Zambrano, A.M.; Brunotte, L.; Faist, A.; Schöfbänker, M.; Hrincius, E.; Hoffmann, H.; et al. The MEK1/2-inhibitor ATR-002 efficiently blocks SARS-CoV-2 propagation and alleviates pro-inflammatory cytokine/chemokine responses. Cell. Mol. Life Sci. 2022, 79, 65. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Günl, F.; Mecate-Zambrano, A.; Rehländer, S.; Hinse, S.; Ludwig, S.; Brunotte, L. Shooting at a Moving Target—Effectiveness and Emerging Challenges for SARS-CoV-2 Vaccine Development. Vaccines 2021, 9, 1052. [Google Scholar] [CrossRef]
- Alwan, N.A. The teachings of Long COVID. Commun. Med. 2021, 1, 15. [Google Scholar] [CrossRef]
- Alwan, N.A. Lessons from Long COVID: Working with patients to design better research. Nat. Rev. Immunol. 2022, 22, 201–202. [Google Scholar] [CrossRef]
| COVID-19 | HPAIV (H5N1, H7N9 etc.) | |
|---|---|---|
| Human-to-human transmission | Yes, very efficient via aerosols Transmission before symptoms onset possible | No (only rare reports) Until today, persistent incompatibility to the human receptor |
| Cell entry | Primary target cell: Type II pneumocytes, ciliated cells Human receptor: ACE2 Spike processing protease: TMPRSS2, Catepsin L | Primary target cell: Type II pneumocytes Human receptor: α-2,6-Sialic acid HA processing protease: TMPRSS2, Furin |
| Clinical manifestations | Anosmia, fatigue, cough, dyspnea, sore throat, diarrhea, nausea, pneumonia, ARDS, organ failure | Hospitalized cases: Pneumonia, ARDS, organ failure, leukopenia, lymphopenia, decreased platelets, encephalitis, septic shock |
| Disease characteristics in severe cases | Target organ: URT, lungs Organ tropism: lung, heart, kidneys, brain, gut Immune response: excessive/dysregulated cytokine levels | Target organ: URT, lungs Organ tropism: lungs, brain, heart, kidneys Immune response: excessive/dysregulated cytokine levels |
| Biomarkers and laboratory parameters for severe disease | High cytokine levels of IL-6, IL-1β, IL-2, IL-8, IL-17, G-CSF, GMCSF, IP-10, MCP-1, TNF-α Increased levels of CRP, Procalcitonin, LDH, and D-Dimers High ferritin/transferrin ratio Glycocalyx damage | High cytokine levels of IL-6, IL-8, TNF-α, CXCL9, IP-10, MCP-1 Increased levels of CRP, LDH, and D-Dimers High creatinine and aminotransferases |
| Involved immune receptors | MDA5, TLR1/2/4/5/8/9 | RIG-I, TLR3/7, PKR |
| Monoclonal antibodies approved by FDA/EMA | Sotrovimab, Bebtelovimab, Tocilizumab | |
| Direct-acting anti-viral drugs (DAA), approved by FDA/EMA (incl. emergency use) | Nucleoside analogs: Remdesivir, Molnupiravir Protease inhibitor: Paxlovid | NA inhibitor: Oseltamivir Endonuclease inhibitor: Baloxavir |
| Anti-inflammatory treatments, approved by FDA/EMA (for emergency use) | JAK1/2 inhibitor: Baricitinib | |
| Long-term symptoms | Long COVID: ‘Brain fog‘, restricted pulmonary function, cardiac symptoms, myocardial inflammation, neurological and neuropsychiatric symptoms, venous thromboembolism, gastrointestinal symptoms, new-onset diabetes, diabetic ketoacidosis, acute kidney injury, hair loss | Chronic fatigue syndrome, myocarditis, encephalopathy or encephalitis, restricted pulmonary function, multi-organ dysfunction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faist, A.; Janowski, J.; Kumar, S.; Hinse, S.; Çalışkan, D.M.; Lange, J.; Ludwig, S.; Brunotte, L. Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond. Cells 2022, 11, 2198. https://doi.org/10.3390/cells11142198
Faist A, Janowski J, Kumar S, Hinse S, Çalışkan DM, Lange J, Ludwig S, Brunotte L. Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond. Cells. 2022; 11(14):2198. https://doi.org/10.3390/cells11142198
Chicago/Turabian StyleFaist, Aileen, Josua Janowski, Sriram Kumar, Saskia Hinse, Duygu Merve Çalışkan, Julius Lange, Stephan Ludwig, and Linda Brunotte. 2022. "Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond" Cells 11, no. 14: 2198. https://doi.org/10.3390/cells11142198
APA StyleFaist, A., Janowski, J., Kumar, S., Hinse, S., Çalışkan, D. M., Lange, J., Ludwig, S., & Brunotte, L. (2022). Virus Infection and Systemic Inflammation: Lessons Learnt from COVID-19 and Beyond. Cells, 11(14), 2198. https://doi.org/10.3390/cells11142198






