Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Types of Outcome Measures
2.4. Data Extraction
2.5. Quality Assessment and Certainty of Evidence
2.6. Data Synthesis
3. Results
3.1. Tongue Pressure in Frailty
3.2. Tongue Pressure in Sarcopenia
3.3. Occlusal Force in Frailty and Sarcopenia
3.4. Suprahyoid Muscle Strength in Frailty and Sarcopenia
3.5. Tongue-Lip Motor Function in Frailty
3.6. Tongue-Lip Motor Function in Sarcopenia
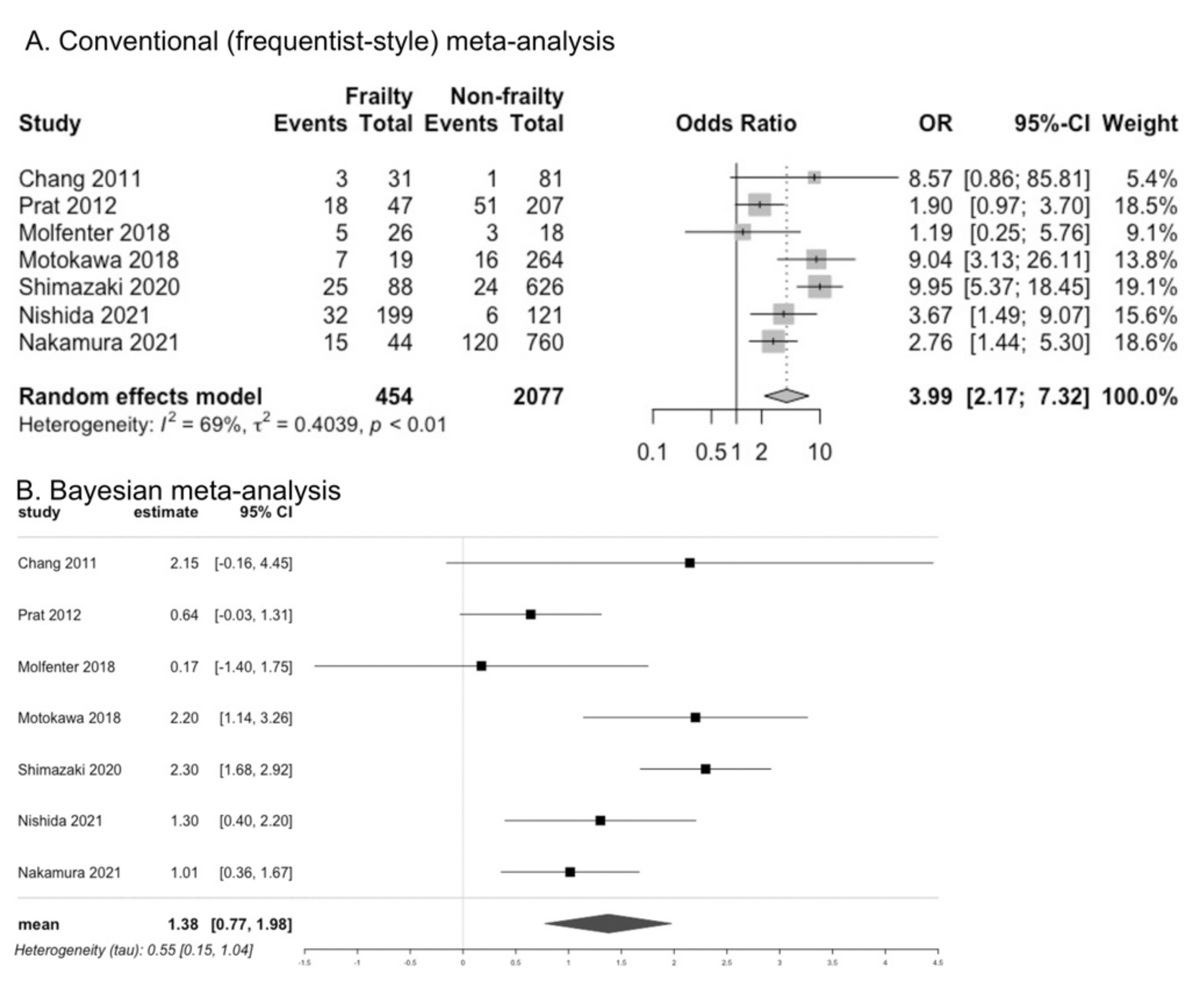
3.7. Presence of Dysphagia in Frailty
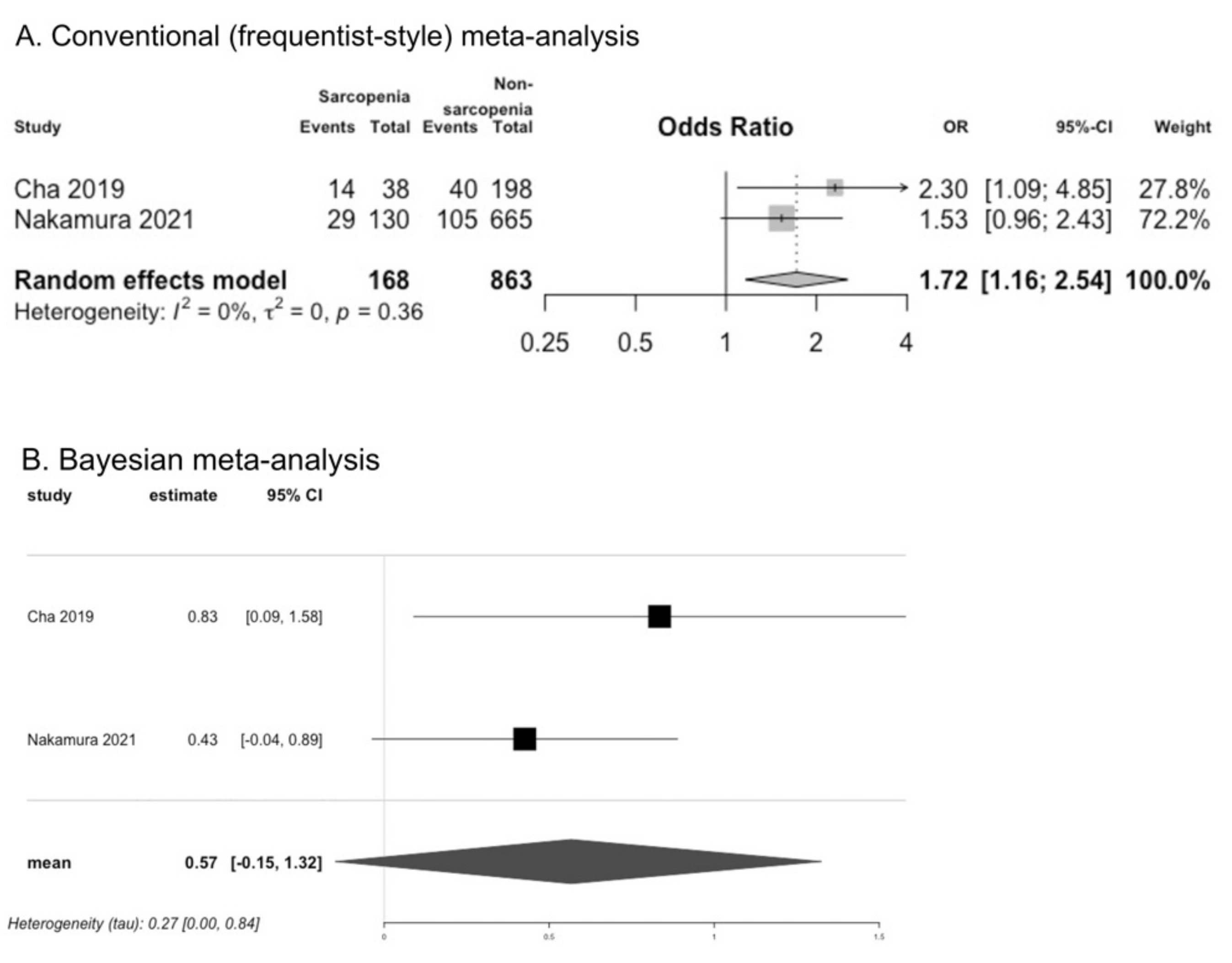
3.8. Presence of Dysphagia in Sarcopenia
3.9. Development of Frailty, Sarcopenia, and Disability
3.9.1. Frailty
3.9.2. Sarcopenia
3.9.3. Disability
3.10. Study Quality and Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekram, A.; Woods, R.L.; Britt, C.; Espinoza, S.; Ernst, M.E.; Ryan, J. The Association between Frailty and All-Cause Mortality in Community-Dwelling Older Individuals: An Umbrella Review. J. Frailty Aging 2021, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Ding, G.; Yu, W.J.; Liu, T.F.; Yan, A.Y.; Chen, H.Y.; Zhang, A.H. Association between frailty and incident risk of disability in community-dwelling elder people: Evidence from a meta-analysis. Public Health 2019, 175, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, P.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 83–90. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Parisius, K.G.H.; Wartewig, E.; Schoonmade, L.J.; Aarab, G.; Gobbens, R.; Lobbezoo, F. Oral frailty dissected and conceptualized: A scoping review. Arch. Gerontol. Geriatr. 2022, 100, 104653. [Google Scholar] [CrossRef]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral frailty and its determinants in older age: A systematic review. Lancet Healthy Longev. 2021, 2, e507–e520. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst. Rev. 2016, 5, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 February 2022).
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, C.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015, 350, h870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1; The Cochrane Collaboration: London, UK, 2008. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Jacqueline, C.; Miranda, C.; Tianjing, L.; Matthew, P.; Vivian, W. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; The Cochrane Collaboration: London, UK, 2022. [Google Scholar]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R (Use-R!); Springer: Cham, Switzerland, 2015. [Google Scholar]
- Röver, C. Bayesian random-effects meta-analysis using the bayesmeta R package. arXiv 2017, arXiv:1711.08683. [Google Scholar] [CrossRef]
- Satake, A.; Kobayashi, W.; Tamura, Y.; Oyama, T.; Fukuta, H.; Inui, A.; Sawada, K.; Ihara, K.; Noguchi, T.; Murashita, K.; et al. Effects of oral environment on frailty: Particular relevance of tongue pressure. Clin. Interv. Aging 2019, 14, 1643–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Hiraoka, A.; Takeda, C.; Mori, T.; Maruyama, M.; Yoshikawa, M.; Tsuga, K. Oral hypofunction and its relation to frailty and sarcopenia in community-dwelling older people. Gerodontology 2022, 39, 26–32. [Google Scholar] [CrossRef]
- Yamanashi, H.; Shimizu, Y.; Higashi, M.; Koyamatsu, J.; Sato, S.; Nagayoshi, M.; Kadota, K.; Kawashiri, S.; Tamai, M.; Takamura, N.; et al. Validity of maximum isometric tongue pressure as a screening test for physical frailty: Cross-sectional study of Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2018, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hamada, T.; Tanaka, A.; Nishi, K.; Kume, K.; Goto, Y.; Beppu, M.; Hijioka, H.; Higashi, Y.; Tabata, H.; et al. Association of Oral Hypofunction with Frailty, Sarcopenia, and Mild Cognitive Impairment: A Cross-Sectional Study of Community-Dwelling Japanese Older Adults. J. Clin. Med. 2021, 10, 1626. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Nonoyama, T.; Tsushita, K.; Arai, H.; Matsushita, K.; Uchibori, N. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2020, 20, 917–926. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, P.Y.; Wang, Y.C.; Wang, T.G.; Han, D.S. Decreased swallowing function in the sarcopenic elderly without clinical dysphagia: A cross-sectional study. BMC Geriatr. 2020, 20, 419. [Google Scholar] [CrossRef]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between Oral Hypofunction and Sarcopenia in Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2021, 18, 6666. [Google Scholar] [CrossRef] [PubMed]
- Machida, N.; Tohara, H.; Hara, K.; Kumakura, A.; Wakasugi, Y.; Nakane, A.; Minakuchi, S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr. Gerontol. Int. 2017, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Koyama, S.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Nishio, N.; Ichikawa, T.; Kunieda, Y.; Ohji, S.; Ito, D.; et al. Relationship between characteristics of skeletal muscle and oral function in community-dwelling older women. Arch. Gerontol. Geriatr. 2018, 79, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hirano, H.; Arai, H.; Morishita, S.; Ohara, Y.; Edahiro, A.; Murakami, M.; Shimada, H.; Kikutani, T.; Suzuki, T. Relationship Between Frailty and Oral Function in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 66–76. [Google Scholar] [CrossRef]
- Kera, T.; Kawai, H.; Yoshida, H.; Hirano, H.; Kojima, M.; Fujiwara, Y.; Ihara, K.; Obuchi, S. Classification of frailty using the Kihon checklist: A cluster analysis of older adults in urban areas. Geriatr. Gerontol. Int. 2017, 17, 69–77. [Google Scholar] [CrossRef]
- Horibe, Y.; Watanabe, Y.; Hirano, H.; Edahiro, A.; Ishizaki, K.; Ueda, T.; Sakurai, K. Relationship between masticatory function and frailty in community-dwelling Japanese elderly. Aging Clin. Exp. Res. 2018, 30, 1093–1099. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.I.; Chan, D.C.; Kuo, K.N.; Hsiung, C.A.; Chen, C.Y. Prevalence and correlates of geriatric frailty in a northern Taiwan community. J. Formos Med. Assoc. 2011, 110, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Serra-Prat, M.; Palomera, M.; Gomez, C.; Sar-Shalom, D.; Saiz, A.; Montoya, J.G.; Navajas, M.; Palomera, E.; Clavé, P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: A population-based prospective study. Age Ageing 2012, 41, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Molfenter, S.M.; Brates, D.; Herzberg, E.; Noorani, M.; Lazarus, C. The Swallowing Profile of Healthy Aging Adults: Comparing Noninvasive Swallow Tests to Videofluoroscopic Measures of Safety and Efficiency. J. Speech Lang. Hear. Res. 2018, 61, 1603–1612. [Google Scholar] [CrossRef]
- Motokawa, K. Oral, swallowing functions, and frailty in independent elderly individuals. Hokkaido J. Dent. Sci. 2018, 38, 185–194. (In Japanese) [Google Scholar]
- Nishida, T.; Yamabe, K.; Honda, S. The Influence of Dysphagia on Nutritional and Frailty Status among Community-Dwelling Older Adults. Nutrients 2021, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Sella-Weiss, O. Association between swallowing function, malnutrition and frailty in community dwelling older people. Clin. Nutr. Eepen 2021, 45, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Kim, W.S.; Kim, K.W.; Han, J.W.; Jang, H.C.; Lim, S.; Paik, N.J. Sarcopenia is an Independent Risk Factor for Dysphagia in Community-Dwelling Older Adults. Dysphagia 2019, 34, 692–697. [Google Scholar] [CrossRef]
- Horibe, Y.; Ueda, T.; Watanabe, Y.; Motokawa, K.; Edahiro, A.; Hirano, H.; Shirobe, M.; Ogami, K.; Kawai, H.; Obuchi, S.; et al. A 2-year longitudinal study of the relationship between masticatory function and progression to frailty or pre-frailty among community-dwelling Japanese aged 65 and older. J. Oral Rehabil. 2018, 45, 864–870. [Google Scholar] [CrossRef]
- Takeuchi, N.; Sawada, N.; Ekuni, D.; Morita, M. Oral Factors as Predictors of Frailty in Community-Dwelling Older People: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 1145. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yoshihara, A.; Sato, N.; Sato, M.; Minagawa, K.; Shimada, M.; Nishimuta, M.; Ansai, T.; Yoshitake, Y.; Ono, T.; et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. J. Oral Rehabil. 2018, 45, 17–24. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Yang, A.-Y.; Chen, Y.-C.; Lee, S.-D.; Lee, S.-H.; Chen, J.-W. Association between Dysphagia and Frailty in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1812. [Google Scholar] [CrossRef]
- Zhao, W.T.; Yang, M.; Wu, H.M.; Yang, L.; Zhang, X.M.; Huang, Y. Systematic Review and Meta-Analysis of the Association between Sarcopenia and Dysphagia. J. Nutr. Health Aging 2018, 22, 1003–1009. [Google Scholar] [CrossRef]
- Albani, V.; Nishio, K.; Ito, T.; Kotronia, E.; Moynihan, P.; Robinson, L.; Hanratty, B.; Kingston, A.; Abe, Y.; Takayama, M.; et al. Associations of poor oral health with frailty and physical functioning in the oldest old: Results from two studies in England and Japan. BMC Geriatr. 2021, 21, 187. [Google Scholar] [CrossRef]
- Baba, H.; Watanabe, Y.; Miura, K.; Ozaki, K.; Matsushita, T.; Kondoh, M.; Okada, K.; Hasebe, A.; Ayabe, T.; Nakamura, K.; et al. Oral frailty and carriage of oral Candida in community-dwelling older adults (Check-up to discover Health with Energy for senior Residents in Iwamizawa; CHEER Iwamizawa). Gerodontology 2022, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Yilmaz, O.; Durmazoglu, S.; Kilic, C.; Tascioglu, C.; Karan, M.A. Association between Dysphagia and Frailty in Community Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Buehring, B.; Hind, J.; Fidler, E.; Krueger, D.; Binkley, N.; Robbins, J. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J. Am. Geriatr. Soc. 2013, 61, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Chen, L.R.; Wang, H.I.; Wang, T.G.; Han, D.S. Suboptimal Tongue Pressure Is Associated with Risk of Malnutrition in Community-Dwelling Older Individuals. Nutrients 2021, 13, 1821. [Google Scholar] [CrossRef]
- Chaleekrua, S.; Janpol, K.; Wattanapan, P. Swallowing Problems among Community-Dwelling Elderly in Northeastern Thailand. J. Prim. Care Community Health 2021, 12, 21501327211019596. [Google Scholar] [CrossRef]
- González-Fernández, M.; Humbert, I.; Winegrad, H.; Cappola, A.R.; Fried, L.P. Dysphagia in old-old women: Prevalence as determined according to self-report and the 3-ounce water swallowing test. J. Am. Geriatr. Soc. 2014, 62, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Hironaka, S.; Kugimiya, Y.; Watanabe, Y.; Motokawa, K.; Hirano, H.; Kawai, H.; Kera, T.; Kojima, M.; Fujiwara, Y.; Ihara, K.; et al. Association between oral, social, and physical frailty in community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 89, 104105. [Google Scholar] [CrossRef]
- Hoshino, D.; Hirano, H.; Edahiro, A.; Motokawa, K.; Shirobe, M.; Watanabe, Y.; Motohashi, Y.; Ohara, Y.; Iwasaki, M.; Maruoka, Y.; et al. Association between Oral Frailty and Dietary Variety among Community-Dwelling Older Persons: A Cross-Sectional Study. J. Nutr. Health Aging 2021, 25, 361–368. [Google Scholar] [CrossRef]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Edahiro, A.; Ohara, Y.; Hirano, H.; Shinkai, S.; Awata, S. Association between Oral Frailty and Nutritional Status among Community-Dwelling Older Adults: The Takashimadaira Study. J. Nutr. Health Aging 2020, 24, 1003–1010. [Google Scholar] [CrossRef]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Edahiro, A.; Ohara, Y.; Hirano, H.; Shinkai, S.; Awata, S. A Two-Year Longitudinal Study of the Association between Oral Frailty and Deteriorating Nutritional Status among Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2020, 18, 213. [Google Scholar] [CrossRef]
- Iwasaki, M.; Watanabe, Y.; Motokawa, K.; Shirobe, M.; Inagaki, H.; Motohashi, Y.; Mikami, Y.; Taniguchi, Y.; Osuka, Y.; Seino, S.; et al. Oral frailty and gait performance in community-dwelling older adults: Findings from the Takashimadaira study. J. Prosthodont. Res. 2021, 65, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Sakai, R.; Okamura, T.; Miki, A.; Hamaguchi, M.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2019, 19, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, Y.; Motokawa, K.; Yamamoto, K.; Hayakawa, M.; Mikami, Y.; Iwasaki, M.; Ohara, Y.; Shirobe, M.; Edahiro, A.; Watanabe, Y.; et al. Relationship between the rate of a decreased oral function and the nutrient intake in community-dwelling older persons: An examination using oral function-related items in a questionnaire for latter-stage elderly people. Nihon Ronen Igakkai Zasshi 2021, 58, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Otobe, Y.; Suzuki, M.; Kimura, Y.; Ishiyama, D.; Kojima, I.; Masuda, H.; Kusumi, H.; Yamada, M. Relationship between the Kihon Checklist and all-cause hospitalization among community-dwelling older adults. Geriatr. Gerontol. Int. 2022, 22, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Molfenter, S.M.; Lenell, C.; Lazarus, C.L. Volumetric Changes to the Pharynx in Healthy Aging: Consequence for Pharyngeal Swallow Mechanics and Function. Dysphagia 2019, 34, 129–137. [Google Scholar] [CrossRef]
- Morishita, M.; Ikeda, T.; Saito, N.; Sanou, M.; Yasuda, M.; Takao, S. Relationship between oral function and life-space mobility or social networks in community-dwelling older people: A cross-sectional study. Clin. Exp. Dent. Res. 2021, 7, 552–560. [Google Scholar] [CrossRef]
- Murotani, Y.; Hatta, K.; Takahashi, T.; Gondo, Y.; Kamide, K.; Kabayama, M.; Masui, Y.; Ishizaki, T.; Matsuda, K.I.; Mihara, Y.; et al. Oral Functions Are Associated with Muscle Strength and Physical Performance in Old-Old Japanese. Int. J. Environ. Res. Public Health 2021, 18, 13199. [Google Scholar] [CrossRef]
- Nakao, Y.; Yamashita, T.; Honda, K.; Katsuura, T.; Hama, Y.; Nakamura, Y.; Ando, K.; Ishikura, R.; Kodama, N.; Uchiyama, Y.; et al. Association Among Age-Related Tongue Muscle Abnormality, Tongue Pressure, and Presbyphagia: A 3D MRI Study. Dysphagia 2021, 36, 483–491. [Google Scholar] [CrossRef]
- Nishida, T.; Yamabe, K.; Honda, S. Dysphagia is associated with oral, physical, cognitive and psychological frailty in Japanese community-dwelling elderly persons. Gerodontology 2020, 37, 185–190. [Google Scholar] [CrossRef]
- Nishimoto, M.; Tanaka, T.; Takahashi, K.; Unyaporn, S.; Fujisaki-Sueda-Sakai, M.; Yoshizawa, Y.; Iijima, K. Oral frailty is associated with food satisfaction in community-dwelling older adults. Nihon Ronen Igakkai Zasshi 2020, 57, 273–281. [Google Scholar] [CrossRef]
- Iwai-Saito, K.; Shobugawa, Y.; Aida, J.; Kondo, K. Frailty is associated with susceptibility and severity of pneumonia in older adults (A JAGES multilevel cross-sectional study). Sci. Rep. 2021, 11, 7966. [Google Scholar] [CrossRef]
- Suzuki, H.; Ayukawa, Y.; Ueno, Y.; Atsuta, I.; Jinnouchi, A.; Koyano, K. Relationship between Maximum Tongue Pressure Value and Age, Occlusal Status, or Body Mass Index among the Community-Dwelling Elderly. Medicina 2020, 56, 623. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Sawada, N.; Ekuni, D.; Morita, M. Oral diadochokinesis is related to decline in swallowing function among community-dwelling Japanese elderly: A cross-sectional study. Aging Clin. Exp. Res. 2021, 33, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Takehara, S.; Hirani, V.; Wright, F.A.C.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Cumming, R.G. Appetite, oral health and weight loss in community-dwelling older men: An observational study from the Concord Health and Ageing in Men Project (CHAMP). BMC Geriatr. 2021, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hirano, H.; Ohara, Y.; Nishimoto, M.; Iijima, K. Oral Frailty Index-8 in the risk assessment of new-onset oral frailty and functional disability among community-dwelling older adults. Arch. Gerontol. Geriatr. 2021, 94, 104340. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nanri, H.; Watanabe, Y.; Yoshida, T.; Yokoyama, K.; Itoi, A.; Date, H.; Yamaguchi, M.; Miyake, M.; Yamagata, E.; et al. Prevalence of Frailty Assessed by Fried and Kihon Checklist Indexes in a Prospective Cohort Study: Design and Demographics of the Kyoto-Kameoka Longitudinal Study. J. Am. Med. Dir. Assoc. 2017, 18, e733–e737. [Google Scholar] [CrossRef] [PubMed]
| Country | Study Year | Study Design | Mean Age (SD) of All Participants | Number of Participants (Men, %) | Prevalence of Frailty and Sarcopenia, n (%) | |
|---|---|---|---|---|---|---|
| Cha et al., 2019 | Seongnam, Korea | 2005–2006 | Cross-sectional | 76.6 (5.8) | 236 (114, 48.3) | Pre-frailty: NR Frailty: NR Sarcopenia: 38 (16.1) |
| Chang et al., 2011 | Taipei, Taiwan | NR | Cross-sectional | 71.1 (3.8) | 275 (127, 46.2) | Pre-frailty: 161 (58.5) Frailty: 31 (11.3) Sarcopenia: NR |
| Chen et al., 2020 | Taipei, Taiwan | NR | Cross-sectional | 75.1 (5.8) | 94 (26, 27.7) | Pre-frailty: NR Frailty: NR Sarcopenia: 47 (50) |
| Horibe et al., 2018 | Tokyo, Japan | 2014 | Cross-sectional | 72.7 (5.2) | 659 (264, 40.1) | Pre-frailty: 220 (33.4) Frailty: 92 (14.0) Sarcopenia: NR |
| Horibe et al., 2018 | Tokyo, Japan | 2013–2015 | Prospective cohort | NR | 418 (175, 41.9) | Pre-frailty: NR Frailty: baseline 62 (7.9%), follow-up 113 (14.1) Sarcopenia: NR |
| Iwasaki et al., 2018 | Niigata, Japan | 2003–2008 | Prospective cohort | 75 (0) | 322 (181, 56.2) | Pre-frailty: NR Frailty: baseline 0, follow-up 49 (15.2) Sarcopenia: NR |
| Kera et al., 2017 | Tokyo, Japan | 2011–2013 | Cross-sectional | NR | 1380 (592, 42.9) | Pre-frailty: NR Frailty: 369 (26.7) Sarcopenia: NR |
| Kugimiya et al., 2021 | Tokyo, Japan | 2018 | Cross-sectional | 76.7 (8.4) | 871 (268, 30.8) | Pre-frailty: NR Frailty: NR Sarcopenia: 163 (18.7) |
| Machida et al., 2017 | NR, Japan | NR | Cross-sectional | NR | 197 (97, 49.2) | Pre-frailty: NR Frailty: NR Sarcopenia: 68 (34.5) |
| Molfenter et al., 2018 | New York, U.S. | NR | Cross-sectional | 76.9 (7.1) | 44 (21, 47.7) | Pre-frailty or frailty: 26 (59.1) Sarcopenia: NR |
| Motokawa 2018 | Saitama, Japan | 2014 | Cross-sectional | 69.6 (NR) | 283 (121, 42.8) | Pre-frailty: 165 (58.3) Frailty: 19 (6.7) Sarcopenia: NR |
| Murakami et al., 2014 | Tokyo, Japan | 2012 | Cross-sectional | 73.0 (5.1) | 761 (314, 41.3) | Pre-frailty: NR Frailty: NR Sarcopenia: 116 (15.2) |
| Nishida et al., 2021 | Niigata, Japan | 2018–2019 | Cross-sectional | 77.3 (6.6) | 320 (52, 16.3) | Pre-frailty: 154 (48.1) Frailty: 45 (14.1) Sarcopenia: NR |
| Nakamura et al., 2021 | Kagoshima, Japan | 2018 | Cross-sectional | 74.9 (6.29) | 832 (303, 36.4) | Pre-frailty: NR Frailty: 44 (5.5) Sarcopenia: 130 (16.4) |
| Serra-Prat et al., 2012 | Barcelona, Spain | NR | Cross-sectional | 78.2 (5.6) | 254 (136, 53.5) | Pre-frailty: NR Frailty: 46 (18.1) Sarcopenia: NR |
| Suzuki et al., 2018 | NR, Japan | NR | Cross-sectional | (Median [IQR]: 81.0 [75.0–85.0]) | 245 (0, 0) | Pre-frailty: NR Frailty: NR Sarcopenia: 29 (11.8) |
| Satake et al., 2019 | Aomori, Japan | 2016 | Cross-sectional | 74.4 (7.8) | 467 (173, 37.0) | Pre-frailty: NR Frailty: 47 (10.1) Sarcopenia: NR |
| Shimazaki et al., 2020 | Aichi, Japan | 2018 | Cross-sectional | NR | 978 (463, 47.3) | Pre-frailty: 295 (30.2) Frailty: 81 (8.3) Sarcopenia: NR |
| Takeuchi et al., 2022 | Okayama, Japan | 2017–2021 | Prospective cohort | 71.9 (5.4) | 97 (34, 35.1) | Pre-frailty: NR Frailty: baseline 0 (0), follow-up 34 (35.1) Sarcopenia: NR |
| Tanaka et al., 2018 | Chiba, Japan | 2012–2016 | Prospective cohort | 73.0 (5.5) | 2011 (NR, 50) | Pre-frailty: NR Frailty: baseline 0 (0), follow-up 83 (7.2) Sarcopenia: baseline 0 (0%), follow-up 63 (5.2) |
| Watanabe et al., 2017 | Aichi, Japan | 2011–2012 | Cross-sectional | 72.1 (5.6) | 4720 (2274, 48.2) | Pre-frailty: 2691 (57.0) Frailty: 535 (11.3) Sarcopenia: NR |
| Weiss 2021 | Israel | NR | Cross-sectional | NR | 180 (74, 41.1) | Pre-frailty: 67 (37.2) Frailty: 20 (11.2) Sarcopenia: NR |
| Yoshida et al., 2022 | Kyoto, Japan | 2019 | Cross-sectional | 75.0 (NR) | 340 (69, 20.3) | Pre-frailty: 155 (46.8) Frailty: 9 (2.7) Sarcopenia: 35 (10.3) |
| Yamanashi et al., 2018 | Nagasaki, Japan | 2014–2015 | Cross-sectional | 72.8 (7) | 1603 (650, 40.5) | Pre-frailty: 605 (37.7) Frailty: 30 (1.9) Sarcopenia: NR |
| Frailty | Sarcopenia | Tongue Pressure | Occlusal Force | Suprahyoid Muscle Strength | Tongue-Lip Motor Function “pa” | Tongue-Lip Motor Function “ta” | Tongue-Lip Motor Function “ka” | Overall Tongue-Lip Motor Function | Dysphagia | |
| Cha et al., 2019 | ◯ AWGS 2014 | ◯ SSA | ||||||||
| Chang et al., 2011 | ◯ CHS | ◯ Self-reported | ||||||||
| Chen et al., 2020 | ◯ AWGS 2014 | ◯ | ◯ VF, EAT-10, WST | |||||||
| Horibe et al., 2018 a | ◯ KCL | ◯ | ||||||||
| Horibe et al., 2018 b | ◯ KCL | ◯ | ||||||||
| Iwasaki et al., 2018 | ◯ CHS | ◯ | ||||||||
| Kera 2016 | ◯ KCL | ◯ | ||||||||
| Kugimiya et al., 2021 | ◯ AWGS 2019 | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ EAT-10 | |||
| Machida et al., 2017 | ◯ AWGS 2014 | ◯ | ◯ | |||||||
| Molfenter et al., 2018 | ◯ CHS | ◯ VF | ||||||||
| Motokawa 2018 | ◯ J-CHS | ◯ Seirei-Questionnaire | ||||||||
| Murakami et al., 2014 | ◯ AWGS 2014 | ◯ | ||||||||
| Nakamura et al., 2021 | ◯ CHS | ◯ AWGS 2014 | ◯ | ◯ | ◯ EAT-10 | |||||
| Nishida et al., 2021 | ◯ CHS | ◯ EAT-10 | ||||||||
| Serra-Prat 2012 | ◯ CHS | ◯ V-VST | ||||||||
| Suzuki et al., 2018 | ◯ AWGS 2014 | ◯ | ◯ | ◯ | ◯ | ◯ EAT-10 | ||||
| Satake et al., 2019 | ◯ FRAIL scale | ◯ | ◯ | ◯ | ◯ | ◯ | ||||
| Shimazaki et al., 2020 | ◯ KCL | ◯ | ◯ | ◯ | ◯ EAT-10 | |||||
| Takeuchi et al., 2022 | ◯ J-CHS | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ EAT-10 | |||
| Tanaka et al., 2018 | ◯ CHS | ◯ AWGS 2014 | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ Self-reported | ||
| Watanabe et al., 2017 | ◯ CHS | ◯ | ◯ | ◯ | ◯ | |||||
| Weiss 2021 | ◯ FRAIL scale | ◯ TWST | ||||||||
| Yoshida et al., 2022 | ◯ CHS and KCL | ◯ AWGS 2019 | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ EAT-10 | ||
| Yamanashi et al., 2018 | ◯ CHS | ◯ |
| Follow-Up Period | Assessed Items at Baseline | Frailty | Sarcopenia | Disability | |
|---|---|---|---|---|---|
| Horibe et al., 2018 | 2 years | Occlusal force | Adjusted OR (95% CI) of low occlusal force for frailty progression (from healthy to pre-frail, from pre-frail to frail, or from healthy to frail): 1.00 (0.99 to 1.00) | NR | NR |
| Iwasaki et al., 2018 | 5 years | Occlusal force | Adjusted HRs (95% CI) of low occlusal force: 2.78 (1.15 to 6.72) | NR | NR |
| Takeuchi et al., 2022 | 2 years | Tongue-lip motor function: ODK for /ta/ | Adjusted OR (95% CI) of ODK for /ta/: 1.85 (1.02 to 3.35). ODK for /ka/ and /pa/: Not significant. | NR | NR |
| Tanaka et al., 2018 | Frailty and sarcopenia: 2 years Disability: 45 months | Occlusal force Tongue-lip motor function; /pa/, /ta/, and /ka/ Tongue pressure Dysphagia | Low maximum occlusal force: 29% (p = 0.017) Low /pa/: 26% (p = 0.321) Low /ta/: 29% (p = 0.021) Low /ka/: 20% (p = 0.598) Low tongue pressure: 26% (p = 0.037) Presence of dysphagia: 25% (p = 0.094) | Low maximum occlusal force: 26% (p = 0.221) Low /pa/: 21% (p = 0.791) Low /ta/: 27% (p = 0.083) Low /ka/: 14% (p = 0.554) Low tongue pressure: 30% (p = 0.039) Presence of dysphagia: 26% (p = 0.078) | Low maximum occlusal force: 32% (p = 0.066) Low /pa/: 23% (p = 0.354) Low /ta/: 22% (p = 0.011) Low /ka/: 19% (p = 0.637) Low tongue pressure: 28% (p = 0.051) Presence of dysphagia: 23% (p = 0.036) |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | D | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cha et al., 2019 | * | * | * | * | ? | NA | NA | NA | * | NA | * | ? | NA | NA | ✢ |
| Chang et al., 2011 | * | * | * | * | ? | NA | NA | * | * | NA | CD | ? | NA | NA | ✢ |
| Chen et al., 2020 | * | * | * | * | * | NA | NA | NA | * | NA | * | ? | NA | NA | ✢ |
| Horibe et al., 2018 a | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Horibe et al., 2018 b | * | * | * | * | ? | * | * | NA | * | − | * | ? | − | CD | → |
| Iwasaki et al., 2017 | * | * | * | * | ? | * | * | * | * | − | * | ? | * | CD | → |
| Kera 2016 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Kugimiya et al., 2021 | * | * | * | * | ? | NA | NA | NA | * | NA | * | ? | NA | NA | ✢ |
| Machida et al., 2017 | * | * | * | * | ? | NA | NA | NA | * | NA | NA | ? | NA | NA | ✢ |
| Molfenter et al., 2018 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Motokawa 2018 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Murakami et al., 2014 | * | * | * | * | ? | NA | NA | NA | * | NA | * | ? | NA | NA | ✢ |
| Nishida et al., 2021 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Nakamura et al., 2021 | * | * | ? | * | ? | NA | NA | − | * | NA | * | * | NA | NA | ✢ |
| Serra-Prat 2012 | * | * | * | * | * | NA | NA | − | − | NA | * | ? | NA | NA | ✢ |
| Suzuki et al., 2018 | * | * | ? | * | ? | NA | NA | NA | * | NA | * | ? | NA | NA | ✢ |
| Satake et al., 2019 | * | * | ? | * | ? | NA | NA | − | * | NA | * | ? | NA | NA | ✢ |
| Shimazaki et al., 2020 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Takeuchi et al., 2022 | * | * | * | * | − | * | * | NA | * | * | * | ? | * | CD | → |
| Tanaka et al., 2018 | * | * | * | * | ? | * | * | NA | * | * | * | ? | * | CD | → |
| Watanabe et al., 2017 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Weiss 2021 | * | − | ? | ? | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Yamanashi et al., 2018 | * | * | * | * | ? | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
| Yoshida et al., 2022 | * | * | ? | * | * | NA | NA | * | * | NA | * | ? | NA | NA | ✢ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, K.; Nakayama, E.; Yoneoka, D.; Sakata, N.; Iijima, K.; Tanaka, T.; Hayashi, K.; Sakuma, K.; Hoshino, E. Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Cells 2022, 11, 2199. https://doi.org/10.3390/cells11142199
Sakai K, Nakayama E, Yoneoka D, Sakata N, Iijima K, Tanaka T, Hayashi K, Sakuma K, Hoshino E. Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Cells. 2022; 11(14):2199. https://doi.org/10.3390/cells11142199
Chicago/Turabian StyleSakai, Kotomi, Enri Nakayama, Daisuke Yoneoka, Nobuo Sakata, Katsuya Iijima, Tomoki Tanaka, Kuniyoshi Hayashi, Kunihiro Sakuma, and Eri Hoshino. 2022. "Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis" Cells 11, no. 14: 2199. https://doi.org/10.3390/cells11142199
APA StyleSakai, K., Nakayama, E., Yoneoka, D., Sakata, N., Iijima, K., Tanaka, T., Hayashi, K., Sakuma, K., & Hoshino, E. (2022). Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Cells, 11(14), 2199. https://doi.org/10.3390/cells11142199










