Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Biochemical Parameters
2.3. Histological Coloration and Immunostaining
2.3.1. Histology
2.3.2. Immunohistochemistry
2.3.3. Immunofluorescence
2.4. RNA Sequencing
2.5. Total RNA Extraction and Quantitative Real-Time PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
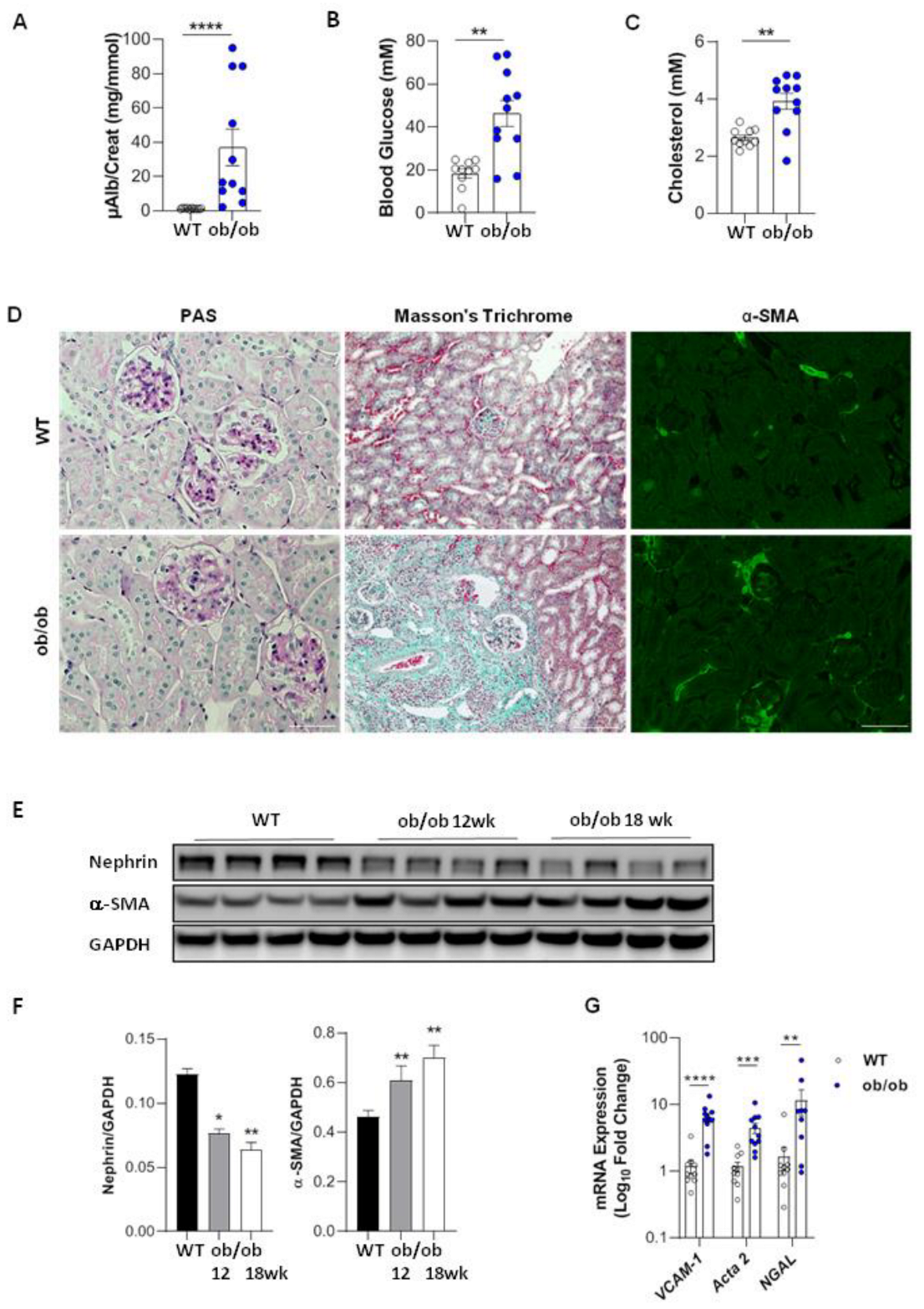
3.1. Renal Function and Structure in Diabetic Ob/Ob Mice
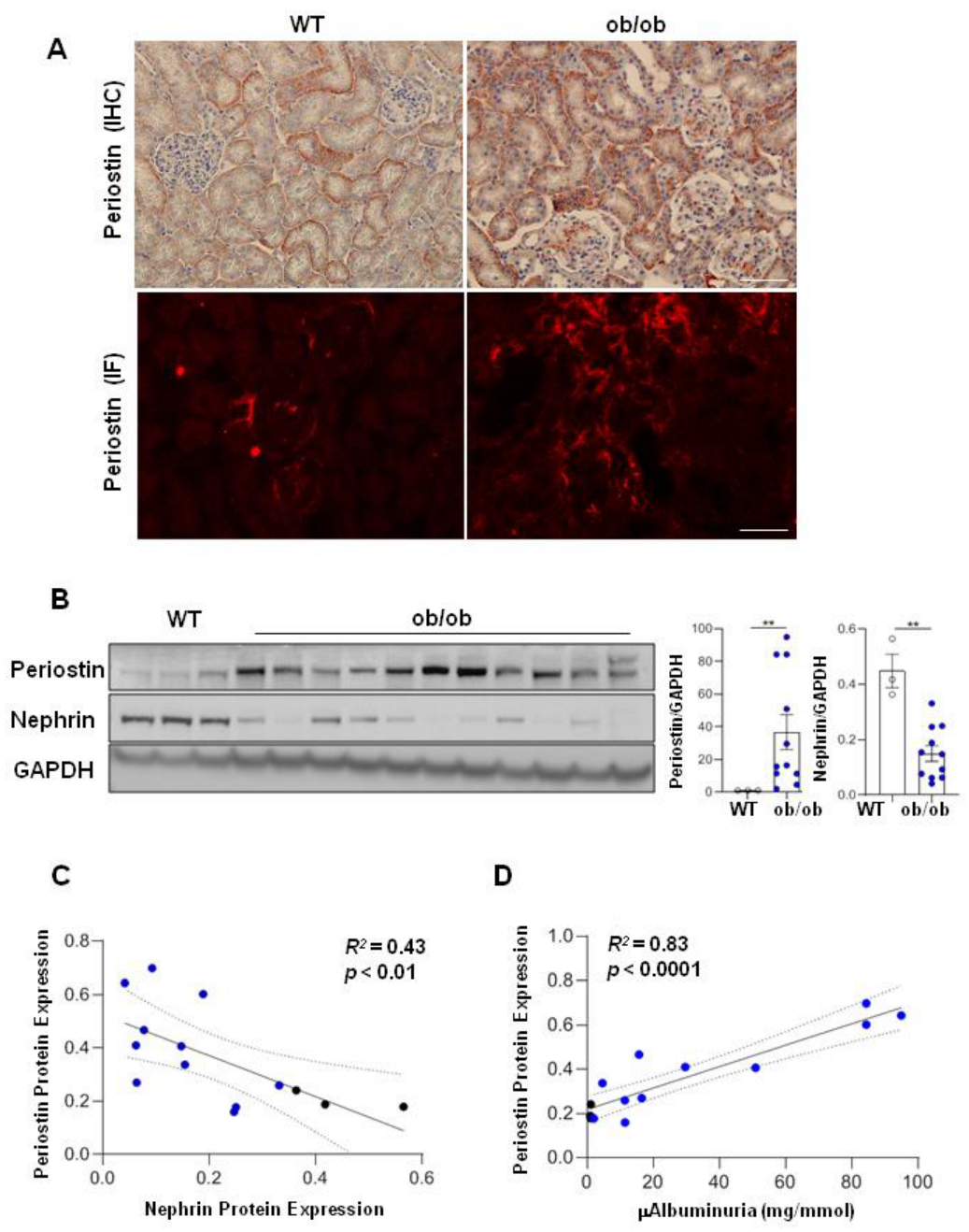
3.2. Periostin Is Associated with the Degree of Renal Injury in Diabetic Ob/Ob Mice
3.3. High Periostin Levels Are Associated with an Activation of the NF-κB Pathway
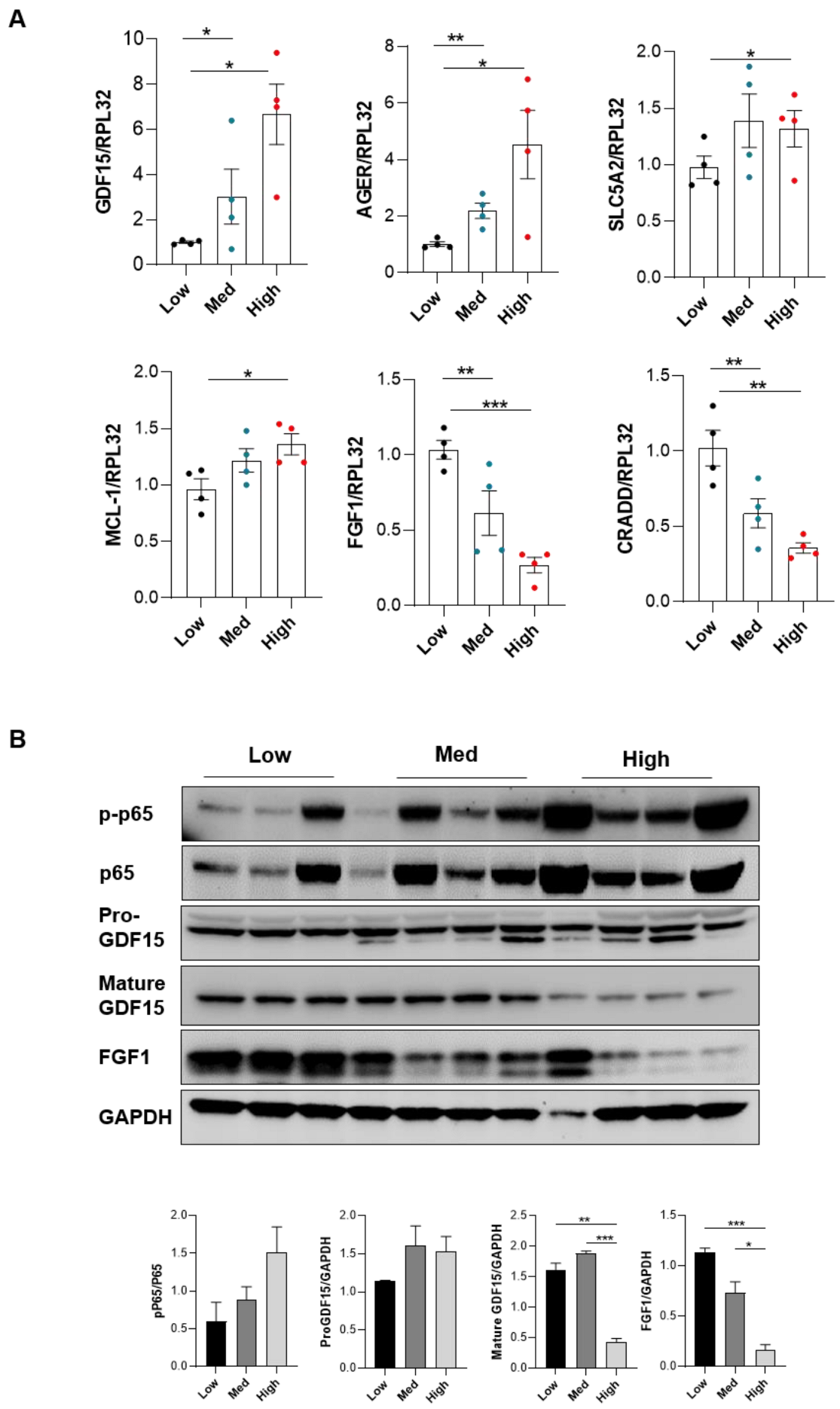
3.4. Periostin Mediates Renal Inflammation and Fibrosis through NF-κB by Repressing FGF1 and GDF15
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, M.; Rahmaninan, M.; Mirzaei, M.; Nadjarzadeh, A.; Dehghani tafti, A.A. Epidemiology of Diabetes Mellitus, Pre-Diabetes, Undiagnosed and Uncontrolled Diabetes in Central Iran: Results from Yazd Health Study. BMC Public Health 2020, 20, 166. [Google Scholar] [CrossRef]
- Mezzano, S.; Aros, C.; Droguett, A.; Burgos, M.E.; Ardiles, L.; Flores, C.; Schneider, H.; Ruiz-Ortega, M.; Egido, J. NF-κB Activation and Overexpression of Regulated Genes in Human Diabetic Nephropathy. Nephrol. Dial. Transplant. 2004, 19, 2505–2512. [Google Scholar] [CrossRef]
- Schmid, H.; Boucherot, A.; Yasuda, Y.; Henger, A.; Brunner, B.; Eichinger, F.; Nitsche, A.; Kiss, E.; Bleich, M.; Gröne, H.-J.; et al. Modular Activation of Nuclear Factor-KappaB Transcriptional Programs in Human Diabetic Nephropathy. Diabetes 2006, 55, 2993–3003. [Google Scholar] [CrossRef] [Green Version]
- Arendse, L.B.; Danser, A.H.J.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, D.; Winocour, P.; Chowdhury, T.A.; De, P.; Wahba, M.; Montero, R.; Fogarty, D.; Frankel, A.H.; Karalliedde, J.; Mark, P.B.; et al. Management of Hypertension and Renin-Angiotensin-Aldosterone System Blockade in Adults with Diabetic Kidney Disease: Association of British Clinical Diabetologists and the Renal Association UK Guideline Update 2021. BMC Nephrol. 2022, 23, 9. [Google Scholar] [CrossRef]
- Choi, C.-I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Wilding, J.P.H. Sodium Glucose Cotransporter 2 Inhibitors as a New Treatment for Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Currie, G.; McKay, G.; Delles, C. Biomarkers in Diabetic Nephropathy: Present and Future. World J. Diabetes 2014, 5, 763–776. [Google Scholar] [CrossRef]
- Turczyn, A.; Pańczyk-Tomaszewska, M. The Role of Periostin in Kidney Diseases. Cent. Eur. J. Immunol. 2021, 46, 494–501. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, Y.; Li, H.J. The Research Status and Prospect of Periostin in Chronic Kidney Disease. Ren. Fail. 2020, 42, 1166–1172. [Google Scholar] [CrossRef]
- Snider, P.; Standley, K.N.; Wang, J.; Azhar, M.; Doetschman, T.; Conway, S.J. Origin of Cardiac Fibroblasts and the Role of Periostin. Circ. Res. 2009, 105, 934–947. [Google Scholar] [CrossRef] [Green Version]
- Kudo, A. Periostin in Fibrillogenesis for Tissue Regeneration: Periostin Actions inside and Outside the Cell. Cell. Mol. Life Sci. 2011, 68, 3201–3207. [Google Scholar] [CrossRef] [Green Version]
- Cobo, T.; Viloria, C.G.; Solares, L.; Fontanil, T.; González-Chamorro, E.; De Carlos, F.; Cobo, J.; Cal, S.; Obaya, A.J. Role of Periostin in Adhesion and Migration of Bone Remodeling Cells. PLoS ONE 2016, 11, e0147837. [Google Scholar] [CrossRef] [Green Version]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin Promotes Chronic Allergic Inflammation in Response to Th2 Cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef] [Green Version]
- Sözmen, M.; Devrim, A.K.; Kabak, Y.B.; Devrim, T. Periostin Alters Transcriptional Profile in a Rat Model of Isoproterenol-Induced Cardiotoxicity. Hum. Exp. Toxicol. 2019, 38, 255–266. [Google Scholar] [CrossRef]
- Sen, K.; Lindenmeyer, M.T.; Gaspert, A.; Eichinger, F.; Neusser, M.A.; Kretzler, M.; Segerer, S.; Cohen, C.D. Periostin Is Induced in Glomerular Injury and Expressed de Novo in Interstitial Renal Fibrosis. Am. J. Pathol. 2011, 179, 1756–1767. [Google Scholar] [CrossRef]
- Guerrot, D.; Dussaule, J.-C.; Mael-Ainin, M.; Xu-Dubois, Y.-C.; Rondeau, E.; Chatziantoniou, C.; Placier, S. Identification of Periostin as a Critical Marker of Progression/Reversal of Hypertensive Nephropathy. PLoS ONE 2012, 7, e31974. [Google Scholar] [CrossRef] [Green Version]
- Mael-Ainin, M.; Abed, A.; Conway, S.J.; Dussaule, J.-C.; Chatziantoniou, C. Inhibition of Periostin Expression Protects against the Development of Renal Inflammation and Fibrosis. J. Am. Soc. Neprol. 2014, 25, 1724–1736. [Google Scholar] [CrossRef] [Green Version]
- Prakoura, N.; Kavvadas, P.; Kormann, R.; Dussaule, J.-C.; Chadjichristos, C.E.; Chatziantoniou, C. NF-κB-Induced Periostin Activates Integrin-Β3 Signaling to Promote Renal Injury in GN. J. Am. Soc. Nephrol. 2017, 28, 1475–1490. [Google Scholar] [CrossRef] [Green Version]
- Satirapoj, B.; Tassanasorn, S.; Charoenpitakchai, M.; Supasyndh, O. Periostin as a Tissue and Urinary Biomarker of Renal Injury in Type 2 Diabetes Mellitus. PLoS ONE 2015, 10, e0124055. [Google Scholar] [CrossRef] [PubMed]
- Hudkins, K.L.; Pichaiwong, W.; Wietecha, T.; Kowalewska, J.; Banas, M.C.; Spencer, M.W.; Mühlfeld, A.; Koelling, M.; Pippin, J.W.; Shankland, S.J.; et al. BTBR Ob/ob Mutant Mice Model Progressive Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 1533–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Ma, Q. In Vivo Activation and Pro-Fibrotic Function of NF-κB in Fibroblastic Cells During Pulmonary Inflammation and Fibrosis Induced by Carbon Nanotubes. Front. Pharmacol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Sodium-Glucose Cotransporter 2-Mediated Oxidative Stress Augments Advanced Glycation End Products-Induced Tubular Cell Apoptosis. Diabetes Metab. Res. Rev. 2013, 29, 406–412. [Google Scholar] [CrossRef]
- Ahmad, M.; Srinivasula, S.M.; Wang, L.; Talanian, R.V.; Litwack, G.; Fernandes-Alnemri, T.; Alnemri, E.S. CRADD, a Novel Human Apoptotic Adaptor Molecule for Caspase-2, and FasL/Tumor Necrosis Factor Receptor-Interacting Protein RIP. Cancer Res. 1997, 57, 615–619. [Google Scholar]
- Jiang, C.; Lin, X. Regulation of NF-κB by the CARD Proteins. Immunol. Rev. 2012, 246, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, J.; Yuan, Y.; Xia, Z.; Chen, M.; Xie, L.; Ma, X.; Wang, J.; Ouyang, S.; Wu, Q.; et al. Regulation of Mcl-1 by Constitutive Activation of NF-κB Contributes to Cell Viability in Human Esophageal Squamous Cell Carcinoma Cells. BMC Cancer 2014, 14, 98. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, W.; Tanaka, N. The Anti-Apoptotic Protein MCL1, a Novel Target of Lung Cancer Therapy. J. Cancer Treat. Diagn. 2018, 2, 54–58. [Google Scholar]
- Alves, M.; Calegari, V.C.; Cunha, D.A.; Saad, M.J.A.; Velloso, L.A.; Rocha, E.M. Increased Expression of Advanced Glycation End-Products and Their Receptor, and Activation of Nuclear Factor Kappa-B in Lacrimal Glands of Diabetic Rats. Diabetologia 2005, 48, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Breyer, M.D.; Böttinger, E.; Brosius, F.C.; Coffman, T.M.; Harris, R.C.; Heilig, C.W.; Sharma, K.; AMDCC. Mouse Models of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2005, 16, 27–45. [Google Scholar] [CrossRef]
- Tesch, G.H.; Nikolic-Paterson, D.J. Recent Insights into Experimental Mouse Models of Diabetic Nephropathy. Nephron. Exp. Nephrol. 2006, 104, e57–e62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wantanasiri, P.; Satirapoj, B.; Charoenpitakchai, M.; Aramwit, P. Periostin: A Novel Tissue Biomarker Correlates with Chronicity Index and Renal Function in Lupus Nephritis Patients. Lupus 2015, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, J.P.; Kim, C.T.; Yang, S.H.; Kim, J.H.; An, J.N.; Moon, K.C.; Lee, H.; Oh, Y.K.; Joo, K.W.; et al. Urinary Periostin Excretion Predicts Renal Outcome in IgA Nephropathy. Am. J. Nephrol. 2016, 44, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.P.; Quante, M.T.; Reif, G.A.; Nivens, E.; Ahmed, F.; Hempson, S.J.; Blanco, G.; Yamaguchi, T. Periostin Induces Proliferation of Human Autosomal Dominant Polycystic Kidney Cells through AV-Integrin Receptor. Am. J. Physiol. Ren. Physiol. 2008, 295, F1463–F1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satirapoj, B. Tubulointerstitial Biomarkers for Diabetic Nephropathy. J. Diabetes Res. 2018, 2018, 2852398. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Attenuation of Diabetic Nephropathy by Tocotrienol: Involvement of NF-κB Signaling Pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Albino, A.H.; Arias, S.C.A.; Faustino, V.D.; Zambom, F.F.F.; Cenedeze, M.A.; Elias, R.M.; Malheiros, D.M.A.C.; Camara, N.O.S.; Fujihara, C.K.; et al. NF-κB System Is Chronically Activated and Promotes Glomerular Injury in Experimental Type 1 Diabetic Kidney Disease. Front. Physiol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking Inflammation and Immunity to Cancer Development and Progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- He, X.; Young, S.-H.; Schwegler-Berry, D.; Chisholm, W.P.; Fernback, J.E.; Ma, Q. Multiwalled Carbon Nanotubes Induce a Fibrogenic Response by Stimulating Reactive Oxygen Species Production, Activating NF-κB Signaling, and Promoting Fibroblast-to-Myofibroblast Transformation. Chem. Res. Toxicol. 2011, 24, 2237–2248. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, B.A.; Sadasivan, S.M.; Chen, Y.; Kravtsov, O.; Palangmonthip, W.; Arora, K.; Gupta, N.S.; Williamson, S.; Bobbitt, K.; Chitale, D.A.; et al. Growth and Differentiation Factor 15 and NF-κB Expression in Benign Prostatic Biopsies and Risk of Subsequent Prostate Cancer Detection. Cancer Med. 2021, 10, 3013–3025. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Song, L.; Chen, Z.; Qian, Y.; Xie, J.; Zhao, L.; Lin, Q.; Zhu, G.; Tan, Y.; Li, X.; et al. Fibroblast Growth Factor 1 Ameliorates Diabetic Nephropathy by an Anti-Inflammatory Mechanism. Kidney Int. 2018, 93, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Ratnam, N.M.; Peterson, J.M.; Talbert, E.E.; Ladner, K.J.; Rajasekera, P.V.; Schmidt, C.R.; Dillhoff, M.E.; Swanson, B.J.; Haverick, E.; Kladney, R.D.; et al. NF-κB Regulates GDF-15 to Suppress Macrophage Surveillance during Early Tumor Development. J. Clin. Investig. 2017, 127, 3796–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Jin, M.; Zhao, X.; Zhao, T.; Lin, W.; He, Z.; Fan, M.; Jin, W.; Zhou, J.; Jin, L.; et al. FGF1ΔHBS Ameliorates Chronic Kidney Disease via PI3K/AKT Mediated Suppression of Oxidative Stress and Inflammation. Cell Death Dis. 2019, 10, 464. [Google Scholar] [CrossRef]
- Baelde, H.J.; Eikmans, M.; Doran, P.P.; Lappin, D.W.P.; de Heer, E.; Bruijn, J.A. Gene Expression Profiling in Glomeruli from Human Kidneys with Diabetic Nephropathy. Am. J. Kidney Dis. 2004, 43, 636–650. [Google Scholar] [CrossRef]
- Labrèche, C.; Cook, D.P.; Abou-Hamad, J.; Pascoal, J.; Pryce, B.R.; Al-Zahrani, K.N.; Sabourin, L.A. Periostin Gene Expression in Neu-Positive Breast Cancer Cells Is Regulated by a FGFR Signaling Cross Talk with TGFβ/PI3K/AKT Pathways. Breast Cancer Res. 2021, 23, 107. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin Secreted by Epithelial Ovarian Carcinoma Is a Ligand for αVβ3 and αVβ5 Integrins and Promotes Cell Motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar]
- Mori, S.; Wu, C.-Y.; Yamaji, S.; Saegusa, J.; Shi, B.; Ma, Z.; Kuwabara, Y.; Lam, K.S.; Isseroff, R.R.; Takada, Y.K.; et al. Direct Binding of Integrin αVβ3 to FGF1 Plays a Role in FGF1 Signaling. J. Biol. Chem. 2008, 283, 18066–18075. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Jin, R.; Norris, R.A.; Zhang, L.; Yu, S.; Wu, F.; Markwald, R.R.; Nanda, A.; Conway, S.J.; Smyth, S.S.; et al. Periostin Mediates Vascular Smooth Muscle Cell Migration through the Integrins αVβ3 and αVβ5 and Focal Adhesion Kinase (FAK) Pathway. Atherosclerosis 2010, 208, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; ten Dijke, P. Immunoregulation by Members of the TGFβ Superfamily. Nat. Rev. Immunol. 2016, 16, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, S.; Yu, H.; Zhang, Y.; Gao, W.; Cui, M.; Li, L. The Predictive Value of Growth Differentiation Factor-15 in Recurrence of Atrial Fibrillation after Catheter Ablation. Mediat. Inflamm. 2020, 2020, 8360936. [Google Scholar] [CrossRef] [PubMed]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a Novel Macrophage Inhibitory Cytokine, Is a Divergent Member of the TGF-β Superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Perez-Gomez, M.V.; Pizarro-Sanchez, S.; Gracia-Iguacel, C.; Cano, S.; Cannata-Ortiz, P.; Sanchez-Rodriguez, J.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. Urinary Growth Differentiation Factor-15 (GDF15) Levels as a Biomarker of Adverse Outcomes and Biopsy Findings in Chronic Kidney Disease. J. Nephrol. 2021, 34, 1819–1832. [Google Scholar] [CrossRef]
- Carlsson, A.C.; Nowak, C.; Lind, L.; Östgren, C.J.; Nyström, F.H.; Sundström, J.; Carrero, J.J.; Riserus, U.; Ingelsson, E.; Fall, T.; et al. Growth Differentiation Factor 15 (GDF-15) Is a Potential Biomarker of Both Diabetic Kidney Disease and Future Cardiovascular Events in Cohorts of Individuals with Type 2 Diabetes: A Proteomics Approach. Ups. J. Med. Sci. 2020, 125, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-I.; Shin, H.-W.; Chun, Y.-S.; Park, J.-W. CST3 and GDF15 Ameliorate Renal Fibrosis by Inhibiting Fibroblast Growth and Activation. Biochem. Biophys. Res. Commun. 2018, 500, 288–295. [Google Scholar] [CrossRef]


| Forward | Reverse | |
|---|---|---|
| ACTA2 | GACACCACCCACCCAGAGT | ACATAGCTGGAGCAGCGTCT |
| AGER | GCCACTGGAATTGTCGATGAGG | GCTGTGAGTTCAGAGGCAGGAT |
| CRADD | AAGGCGAGAGAGGAAGTCACAG | AGCTGGTTAATCTGCTGGTCTGA |
| FGF1 | CTGTGACAGCGCAAAGGCTG | TGGTAGGCTCAGCACTGAAGAA |
| GDFF15 | CATAGGGGTGACTTGCCTGAA | CCCCACAGAACATGTGATGGA |
| HPRT | GGAGCGGTAGCACCTCCT | CTGGTTCATCATCGCTAATCAC |
| KIM | CCAACATCAATCAGAGTCTCTACC | TGTCTCATGGGGACAAAATG |
| MCL1 | AGCTTCATCGAACCATTAGCAGAA | CCTTCTAGGTCCTGTACGTGGA |
| RPL32 | GCTGCCATCTGTTTTACGG | TGACTGGTGCCTGATGAACT |
| SLC2A5 | ATCGCTGCCTTTGGCTCATCCT | AGCAGCGTCAAGGTGAAGGACT |
| VCAM1 | TGGTGAAATGGAATCTGAACC | CCCAGATGGTGGTTTCCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbad, L.; Prakoura, N.; Michon, A.; Chalghoumi, R.; Reichelt-Wurm, S.; Banas, M.C.; Chatziantoniou, C. Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy. Cells 2022, 11, 2212. https://doi.org/10.3390/cells11142212
Abbad L, Prakoura N, Michon A, Chalghoumi R, Reichelt-Wurm S, Banas MC, Chatziantoniou C. Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy. Cells. 2022; 11(14):2212. https://doi.org/10.3390/cells11142212
Chicago/Turabian StyleAbbad, Lilia, Niki Prakoura, Arthur Michon, Rym Chalghoumi, Simone Reichelt-Wurm, Miriam C. Banas, and Christos Chatziantoniou. 2022. "Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy" Cells 11, no. 14: 2212. https://doi.org/10.3390/cells11142212
APA StyleAbbad, L., Prakoura, N., Michon, A., Chalghoumi, R., Reichelt-Wurm, S., Banas, M. C., & Chatziantoniou, C. (2022). Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy. Cells, 11(14), 2212. https://doi.org/10.3390/cells11142212






