Organ-Specific Glucose Uptake: Does Sex Matter?
Abstract
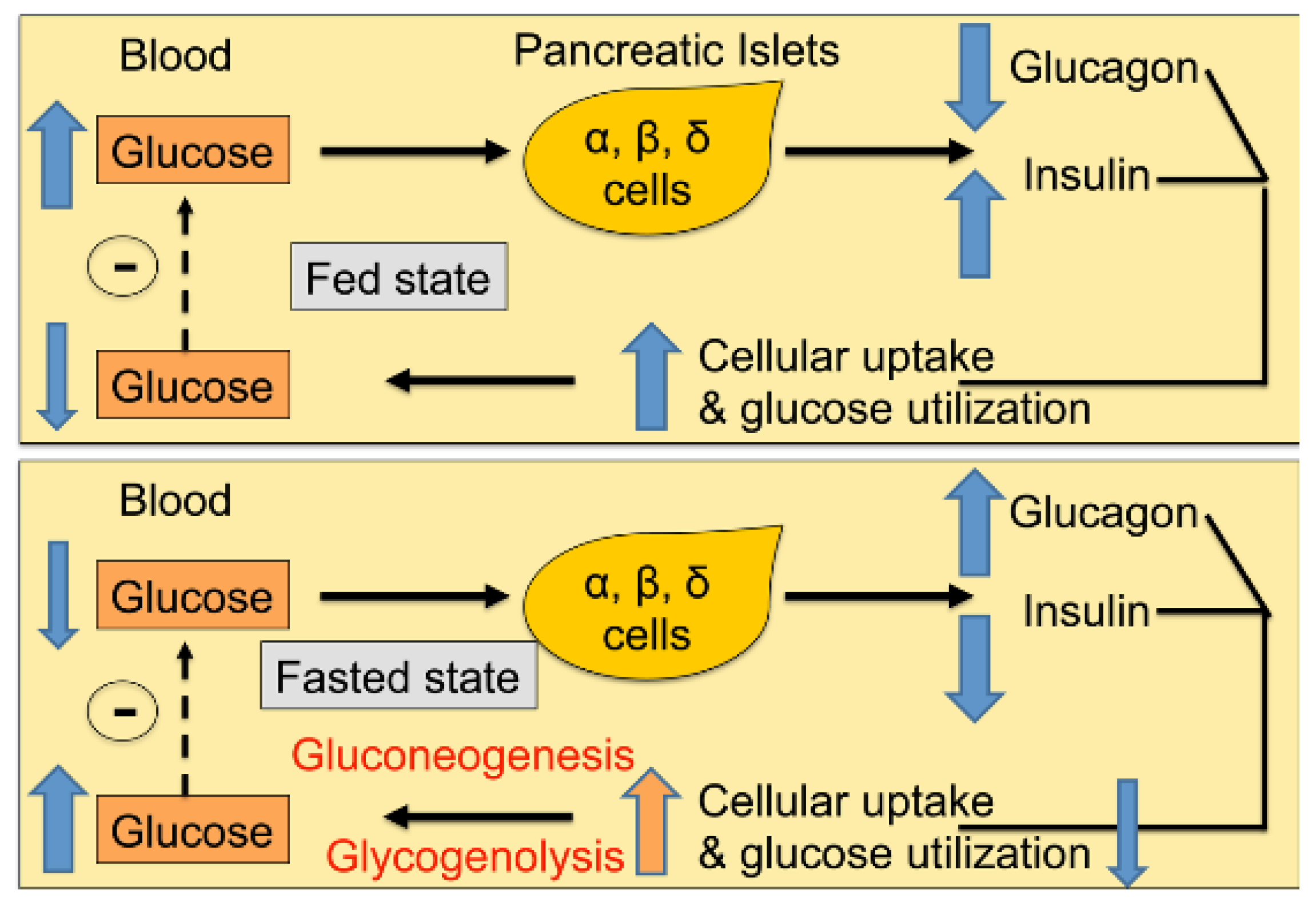
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Positron Emission Tomography (PET)
2.4. Volume of Interest (VOI) Analysis
2.5. Organ Counting
2.6. Statistical Analysis
3. Results
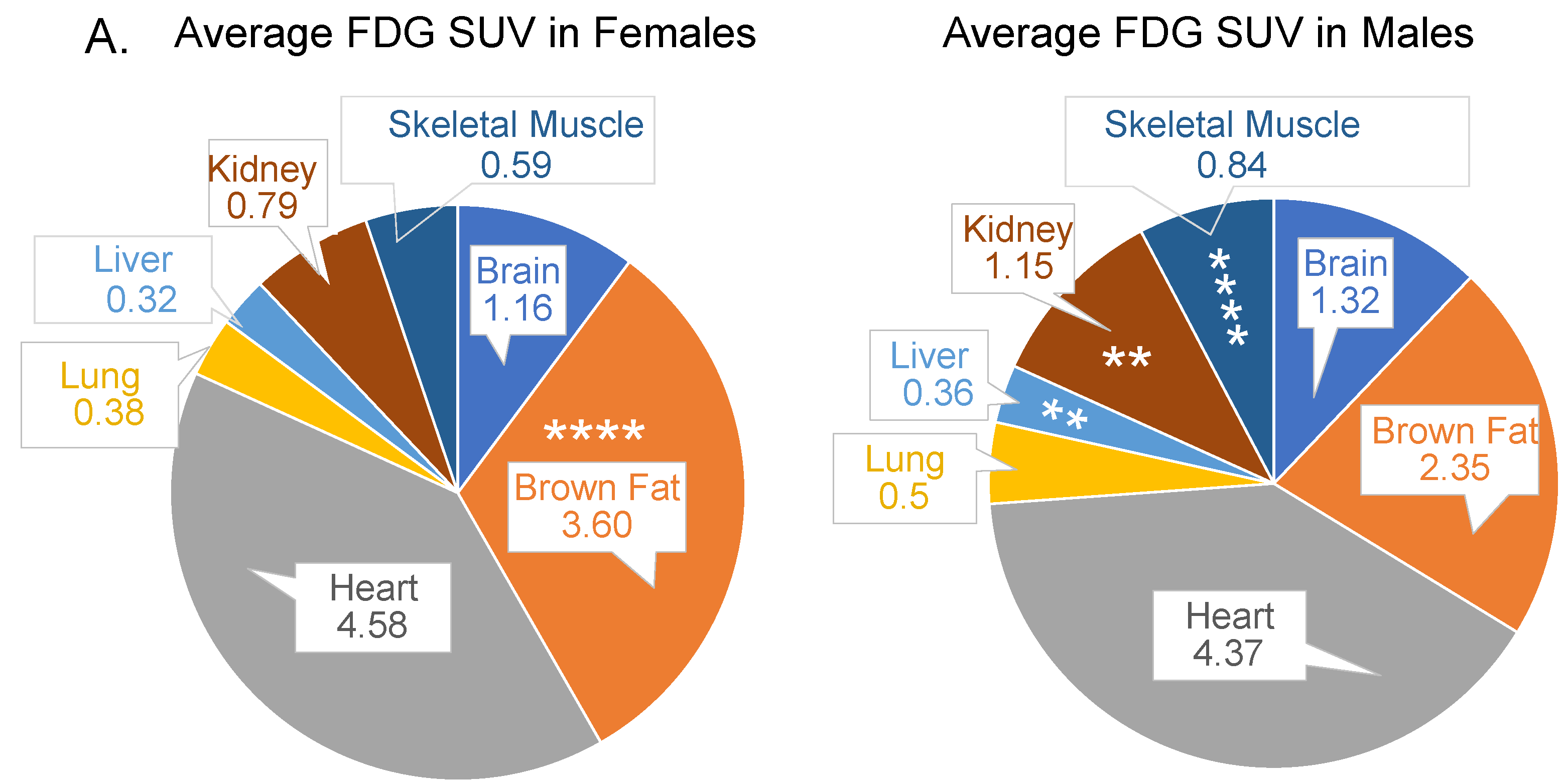
3.1. Sex Is an Important Determinant of Relative Glucose Uptake by Various Organs
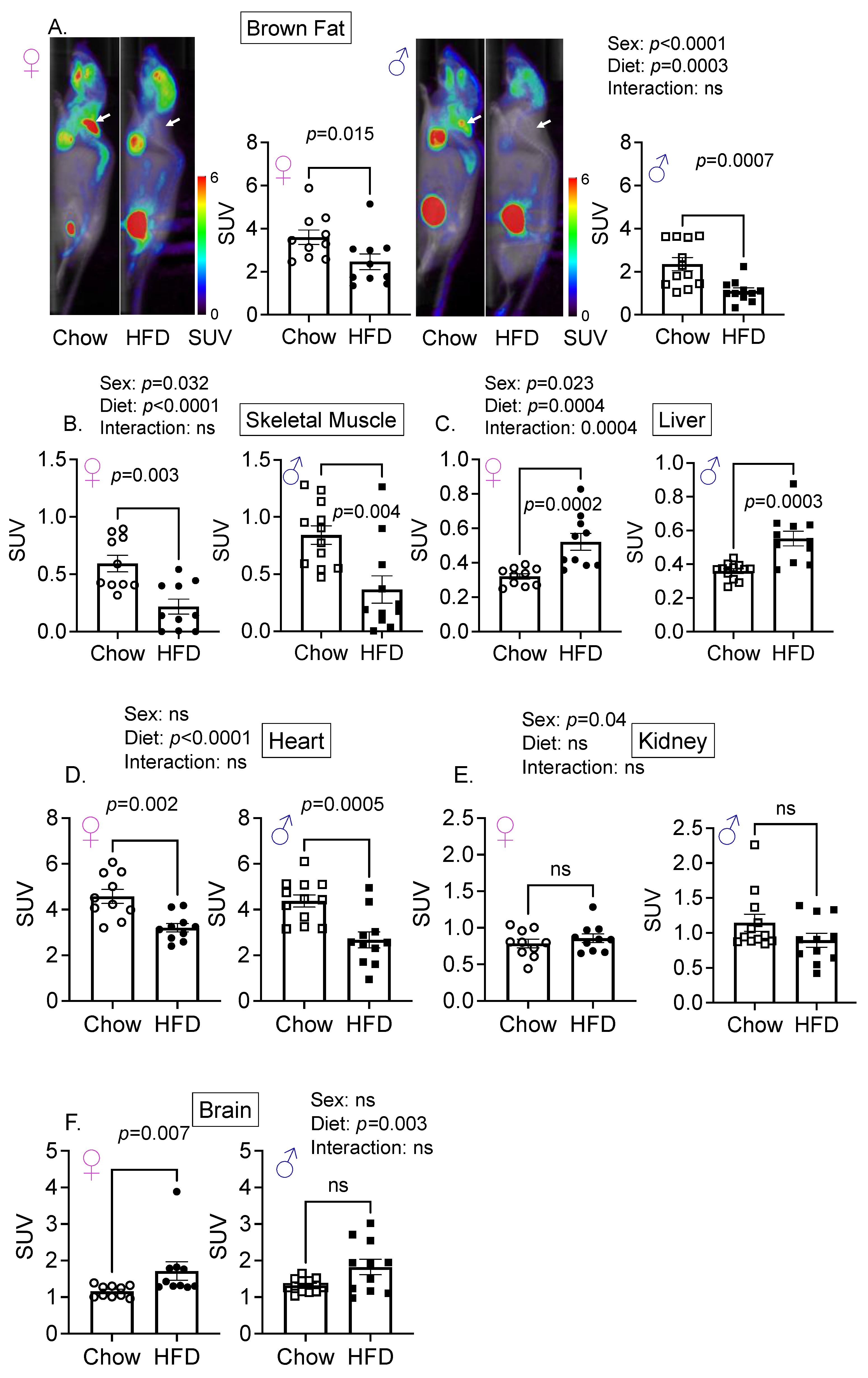
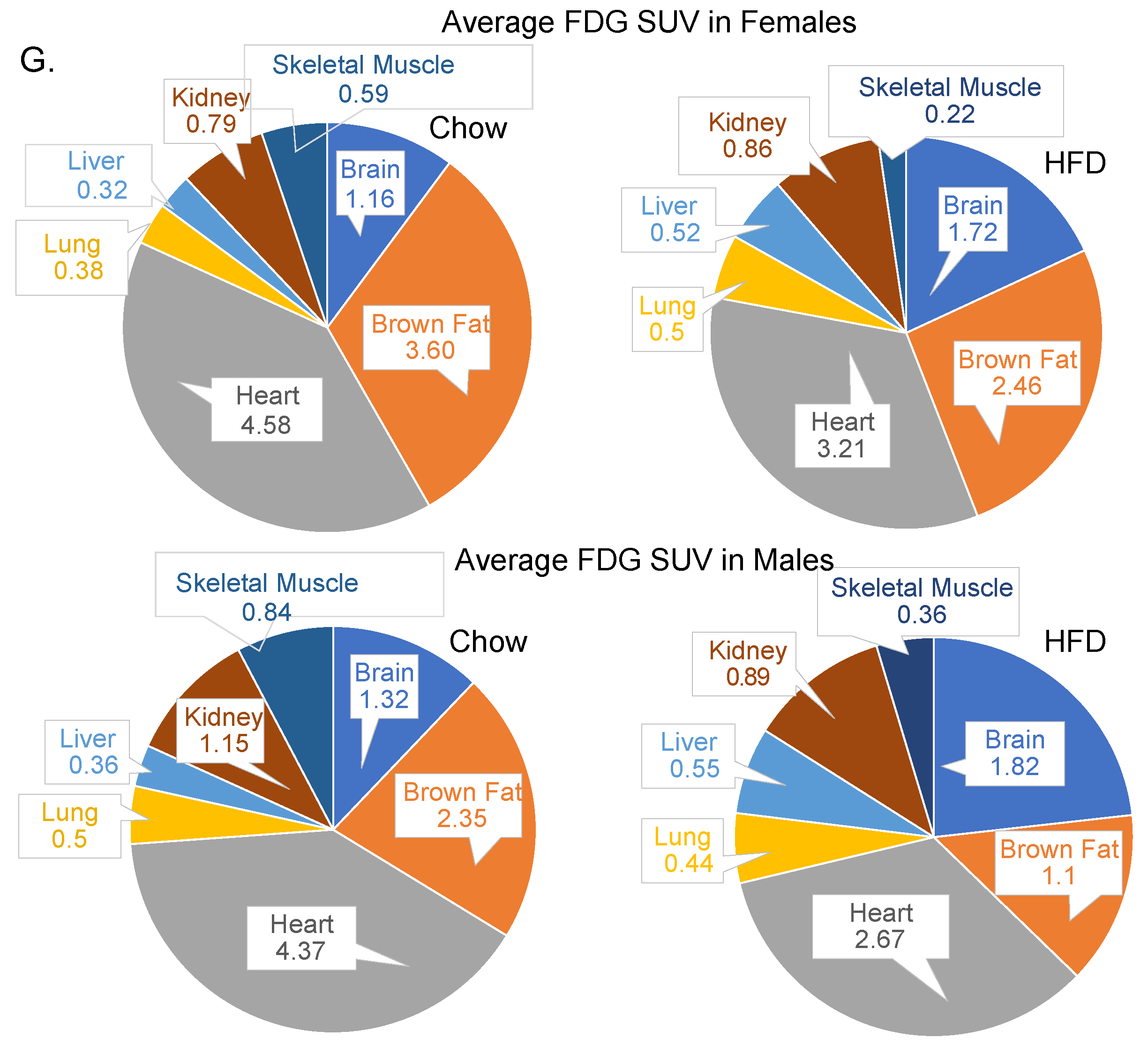
3.2. Diet and Sex Are Important Determinant of Hepatic Glucose Uptake
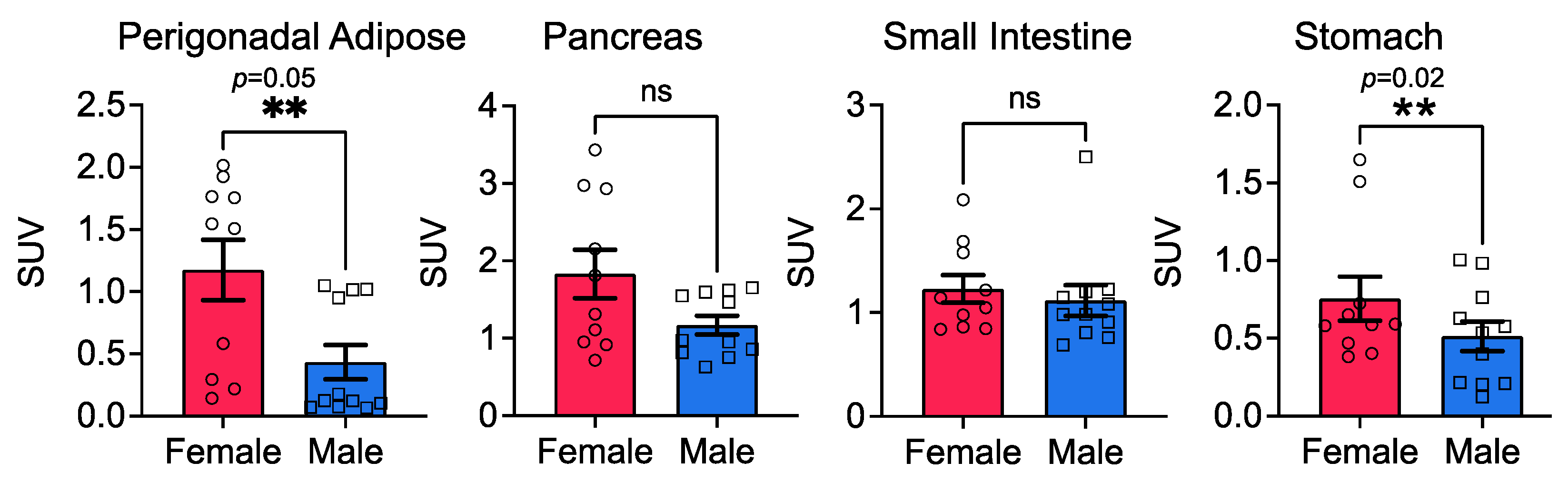
3.3. Stomach Glucose Uptake Differs between the Sexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Zeigerer, A.; Sekar, R.; Kleinert, M.; Nason, S.; Habegger, K.M.; Müller, T.D. Glucagon’s Metabolic Action in Health and Disease. Compr. Physiol. 2021, 11, 1759–1783. [Google Scholar] [CrossRef] [PubMed]
- Yankauer, A. Job Lewis Smith and the Germ Theory of Disease. Pediatrics 1994, 93, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Gao, M.H.; Giamouridis, D.; Lai, N.C.; Walenta, E.; Paschoal, V.A.; Kim, Y.C.; Miyanohara, A.; Guo, T.; Liao, M.; Liu, L.; et al. One-time injection of AAV8 encoding urocortin 2 provides long-term resolution of insulin resistance. JCI Insight 2016, 1, e88322. [Google Scholar] [CrossRef] [Green Version]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Godsland, I.F.; Gangar, K.; Walton, C.; Cust, M.P.; Whitehead, M.I.; Wynn, V.; Stevenson, J.C. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism 1993, 42, 846–853. [Google Scholar] [CrossRef]
- Díaz, A.; Lopez-Grueso, R.; Gambini, J.; Monleón, D.; Mas-Bargues, C.; Abdelaziz, K.M.; Viña, J.; Borrás, C. Sex Differences in Age-Associated Type 2 Diabetes in Rats-Role of Estrogens and Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 6734836. [Google Scholar] [CrossRef]
- Nuutila, P.; Knuuti, M.J.; Mäki, M.; Laine, H.; Ruotsalainen, U.; Teräs, M.; Haaparanta, M.; Solin, O.; Yki-Järvinen, H. Gender and Insulin Sensitivity in the Heart and in Skeletal Muscles: Studies Using Positron Emission Tomography. Diabetes 1995, 44, 31–36. [Google Scholar] [CrossRef]
- Paruthiyil, S.; Hagiwara, S.-I.; Kundassery, K.; Bhargava, A. Sexually dimorphic metabolic responses mediated by CRF2 receptor during nutritional stress in mice. Biol. Sex Differ. 2018, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Halseth, A.E.; Bracy, D.P.; Wasserman, D.H. Limitations to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am. J. Physiol. Metab. 2000, 279, E1064–E1071. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, W.; Kong, X.; Xu, A.; Wang, Z.; Sweeney, G.; Wu, D. Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake. Diabetologia 2009, 52, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coate, K.C.; Scott, M.; Farmer, B.; Moore, M.C.; Smith, M.; Roop, J.; Neal, D.W.; Williams, P.; Cherrington, A.D. Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. Am. J. Physiol. Metab. 2010, 299, E887–E898. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Cypess, A.M.; Haft, C.R.; Laughlin, M.R.; Hu, H.H. Brown Fat in Humans: Consensus Points and Experimental Guidelines. Cell Metab. 2014, 20, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Loening, A.M.; Gambhir, S.S. Amide: A Free Software Tool for Multimodality Medical Image Analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]
- Huang, S.C. Anatomy of SUV. Standardized uptake value. Nucl. Med. Biol. 2000, 27, 643–646. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R.; Bjorkman, O.; Olsson, M.; Wahren, J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef]
- Mitrou, P.; Boutati, E.; Lambadiari, V.; Maratou, E.; Papakonstantinou, A.; Komesidou, V.; Sidossis, L.; Tountas, N.; Katsilambros, N.; Economopoulos, T.; et al. Rates of glucose uptake in adipose tissue and muscle in vivo after a mixed meal in women with morbid obesity. J. Clin. Endocrinol. Metab. 2009, 94, 2958–2961. [Google Scholar] [CrossRef] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, M.; Kulkarni, R.N.; Tse, H.M.; Mauvais-Jarvis, F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 2018, 15, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F. Liver and kidney metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Rajas, F.; Bruni, N.; Montano, S.; Zitoun, C.; Mithieux, G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: Regulation of expression in fasted and diabetic rats. Gastroenterology 1999, 117, 132–139. [Google Scholar] [CrossRef]
- Féry, F.; Plat, L.; Melot, C.; Balasse, E.O. Role of fat-derived substrates in the regulation of gluconeogenesis during fasting. Am. J. Physiol. Metab. 1996, 270, E822–E830. [Google Scholar] [CrossRef]
- Oh, K.-J.; Han, H.-S.; Kim, M.-J.; Koo, S.-H. Transcriptional regulators of hepatic gluconeogenesis. Arch. Pharmacal Res. 2013, 36, 189–200. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.G.; Perkovic, V.; Chalmers, J.; Woodward, M.; Li, Q.; Cooper, M.E.; Hamet, P.; Harrap, S.; Heller, S.; MacMahon, S.; et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: Advance-on. Diabetes Care 2016, 39, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Johnsson, K.M.; Ptaszynska, A.; Schmitz, B.; Sugg, J.; Parikh, S.J.; List, J.F. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J. Diabetes Its Complicat. 2013, 27, 479–484. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Kosi, L.; Lin, J.; Mihaljevic, R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: Results from patient-level pooled data of six randomized controlled trials. Diabetes Obes. Metab. 2015, 17, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautzky-Willer, A.; Harreiter, J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017, 131, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Leitner, B.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, J.M.; Valencak, T.G. Sex differences and aging: Is there a role of brown adipose tissue? Mol. Cell. Endocrinol. 2021, 531, 111310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [Green Version]
- Basu, R.; Dalla Man, C.; Campioni, M.; Basu, A.; Klee, G.; Toffolo, G.; Cobelli, C.; Rizza, R.A. Effects of Age and Sex on Postprandial Glucose Metabolism. Diabetes 2006, 55, 2001–2014. [Google Scholar] [CrossRef] [Green Version]
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 2424–2447. [Google Scholar] [CrossRef]
- Shah, A.; Laferrère, B. Diabetes after Bariatric Surgery. Can. J. Diabetes 2017, 41, 401–406. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, A.; Tang, R.; Seo, Y.; Bhargava, A. Organ-Specific Glucose Uptake: Does Sex Matter? Cells 2022, 11, 2217. https://doi.org/10.3390/cells11142217
Gandhi A, Tang R, Seo Y, Bhargava A. Organ-Specific Glucose Uptake: Does Sex Matter? Cells. 2022; 11(14):2217. https://doi.org/10.3390/cells11142217
Chicago/Turabian StyleGandhi, Adithi, Ryan Tang, Youngho Seo, and Aditi Bhargava. 2022. "Organ-Specific Glucose Uptake: Does Sex Matter?" Cells 11, no. 14: 2217. https://doi.org/10.3390/cells11142217







