A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices
Abstract
1. Introduction
2. Materials and Methods
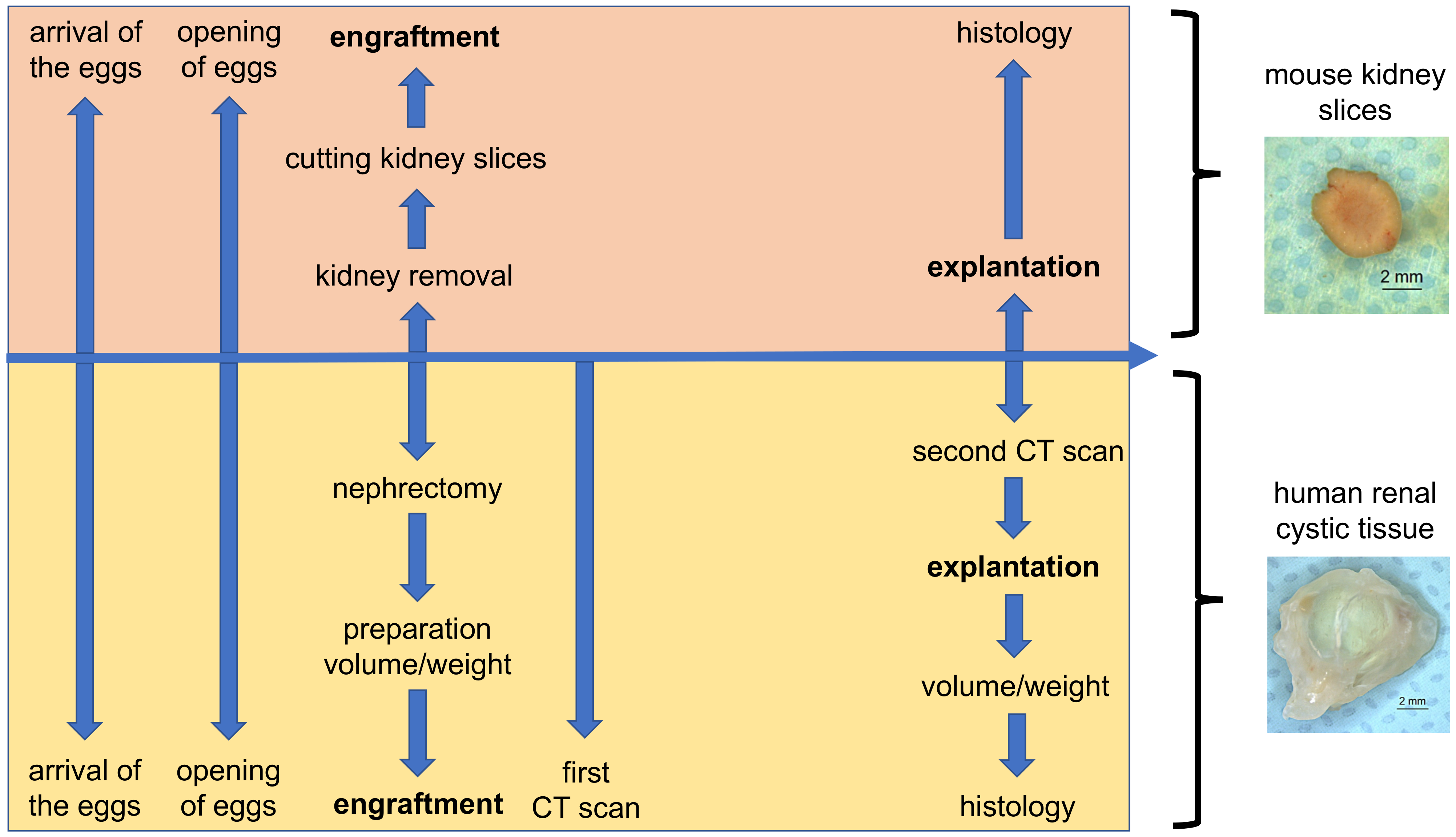
2.1. Chorioallantoic Membrane (CAM) Model
2.2. Cultivation of Mouse Kidney Slices on the CAM
2.3. Cultivation of Tissue from Human Cystic Kidneys on the CAM
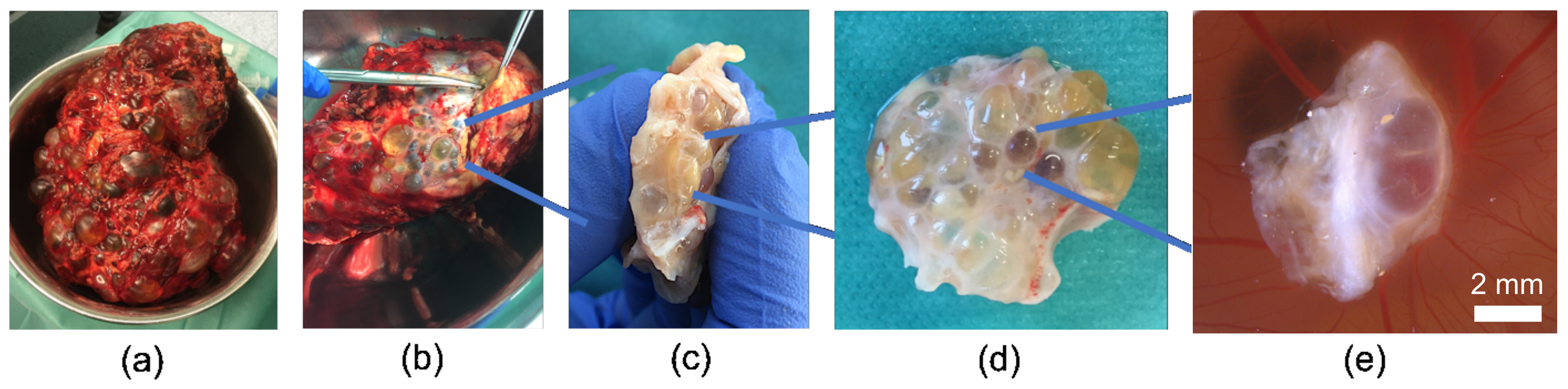
2.3.1. Preparation of Cystic Tissue
2.3.2. Ex Ovo Volume and Weight Measurement
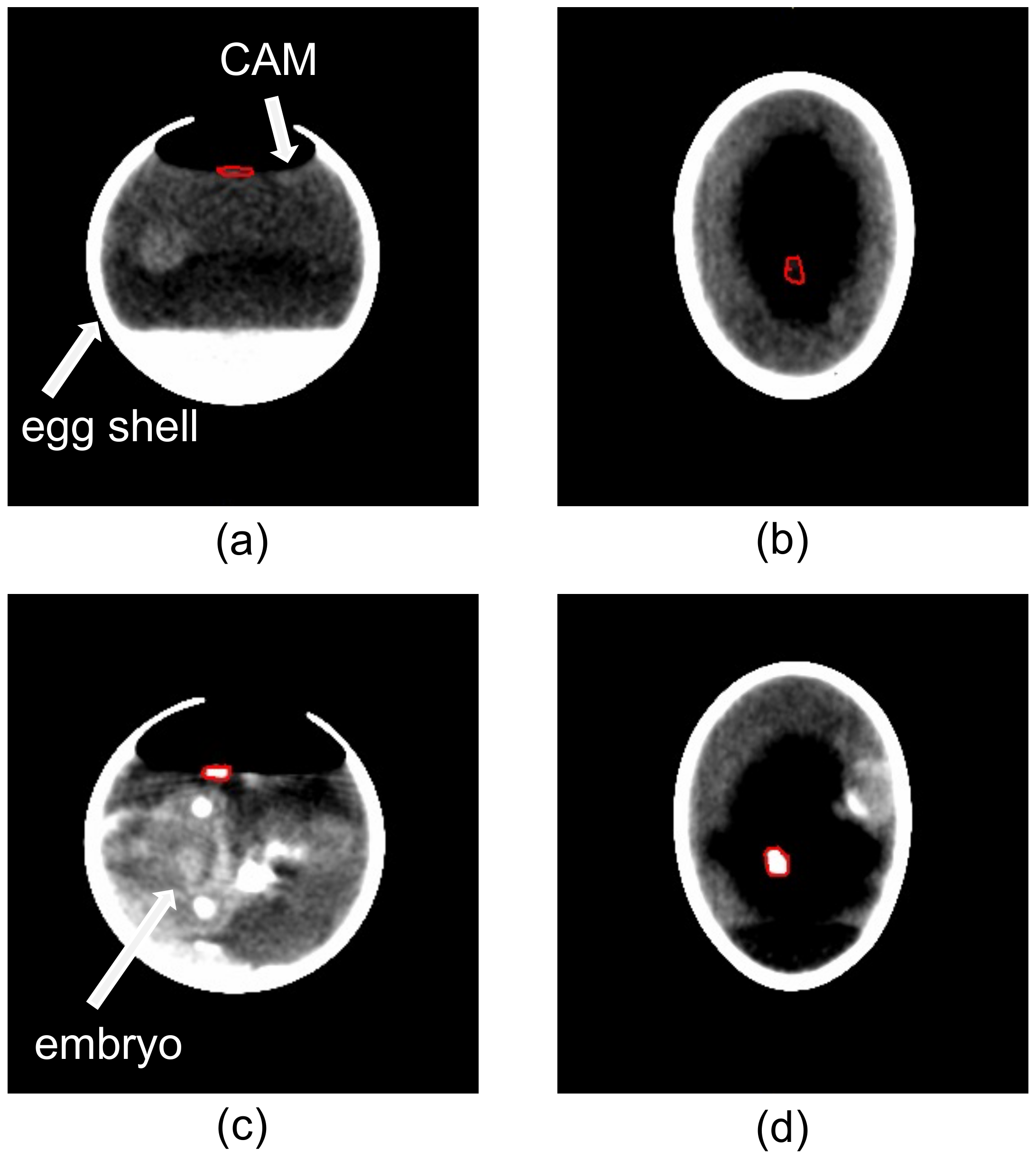
2.3.3. In Ovo Computed Tomographic (CT)-Based Volume Measurements
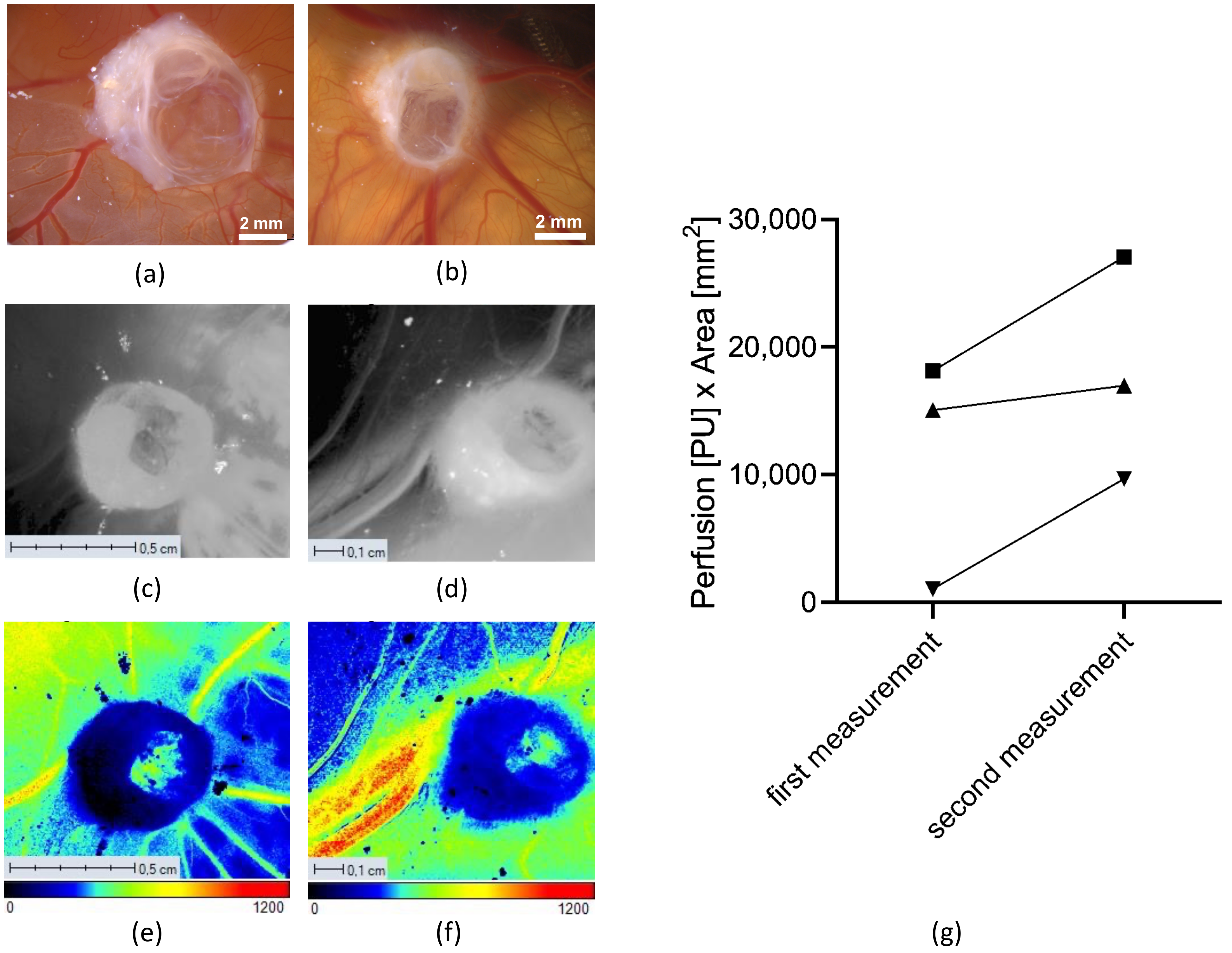
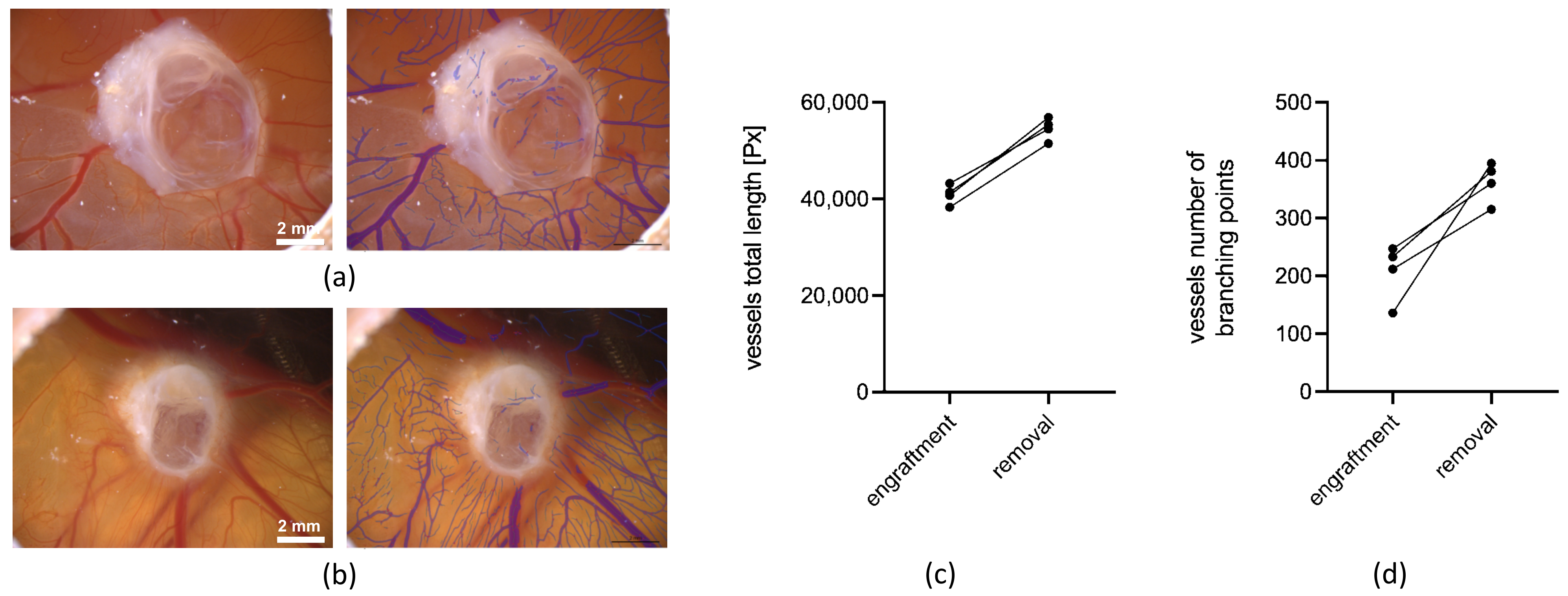
2.3.4. Measurement of Angiogenesis with LSCI and the CAM Assay Application
2.4. Immunohistochemistry and Antibodies
2.4.1. Paraffin Sections
2.4.2. Staining and Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Cultivation of Adult Mouse Kidney Slices on the CAM
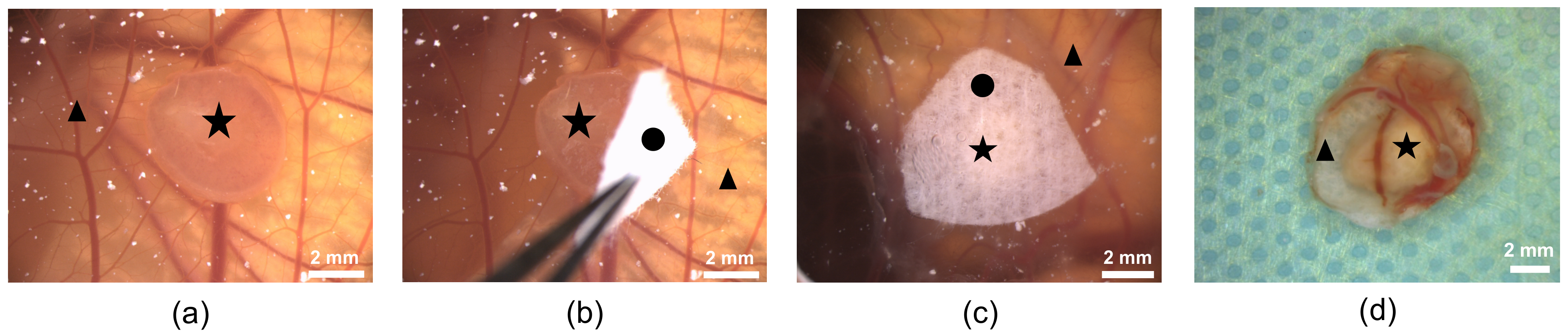
3.2. Growth of Human Cystic Kidney Tissue on the CAM
3.2.1. Angiogenesis Measurements
3.2.2. Ex Ovo 3D Volume Measurements and Weight Measurements of Human Renal Cystic Tissue
3.2.3. Comparison of In Ovo (CT) and Ex Ovo (3D) Measured Volumetric Data
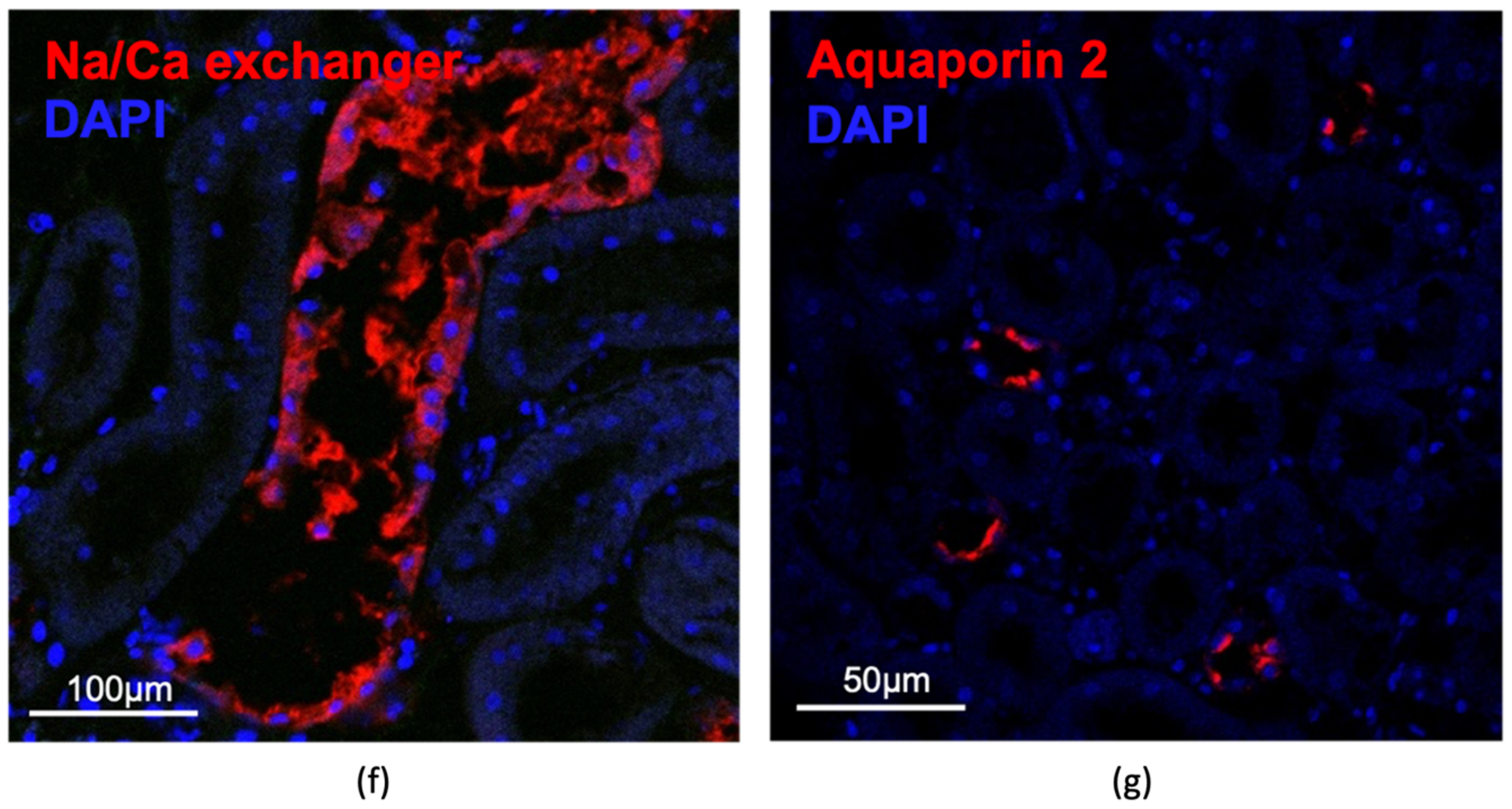
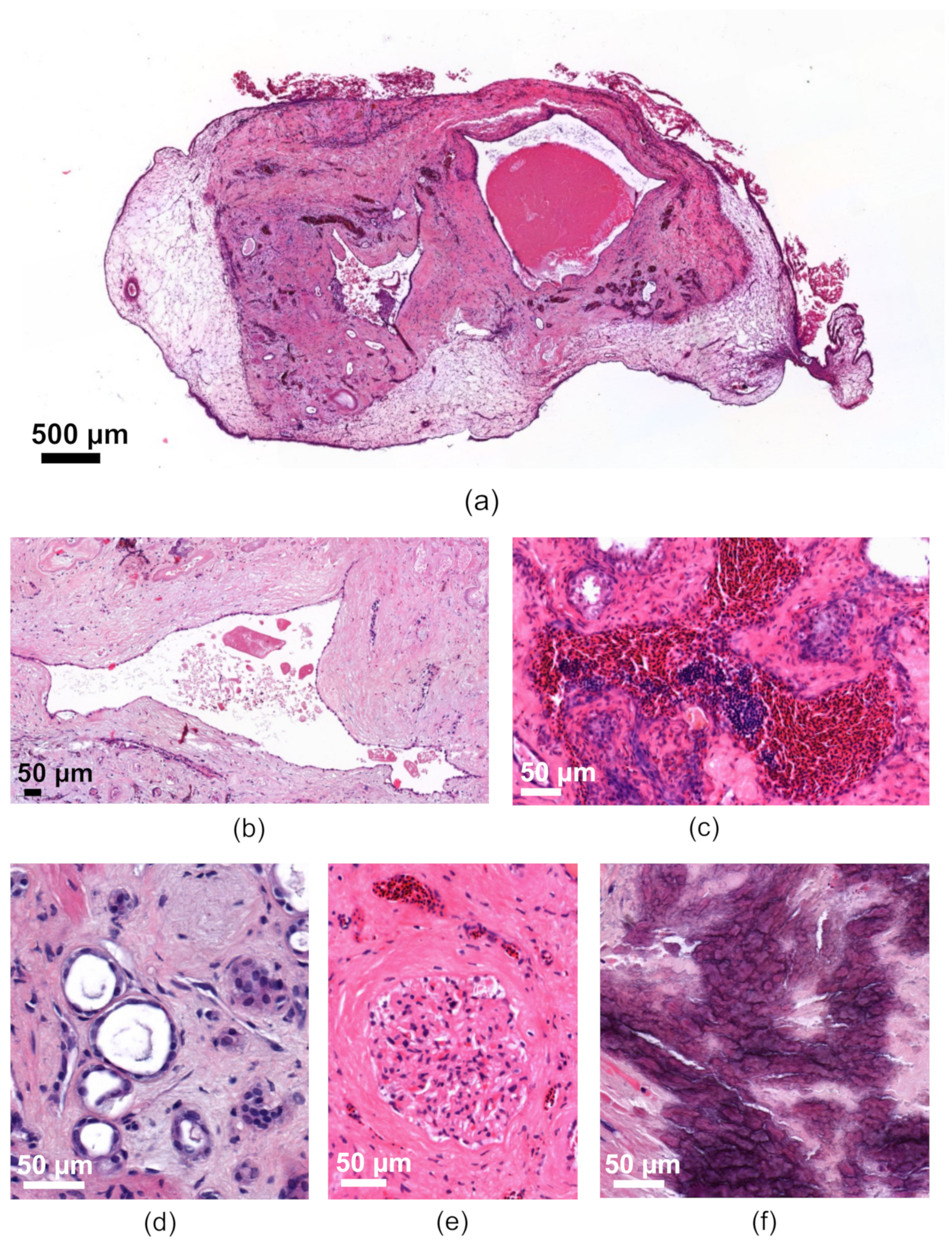
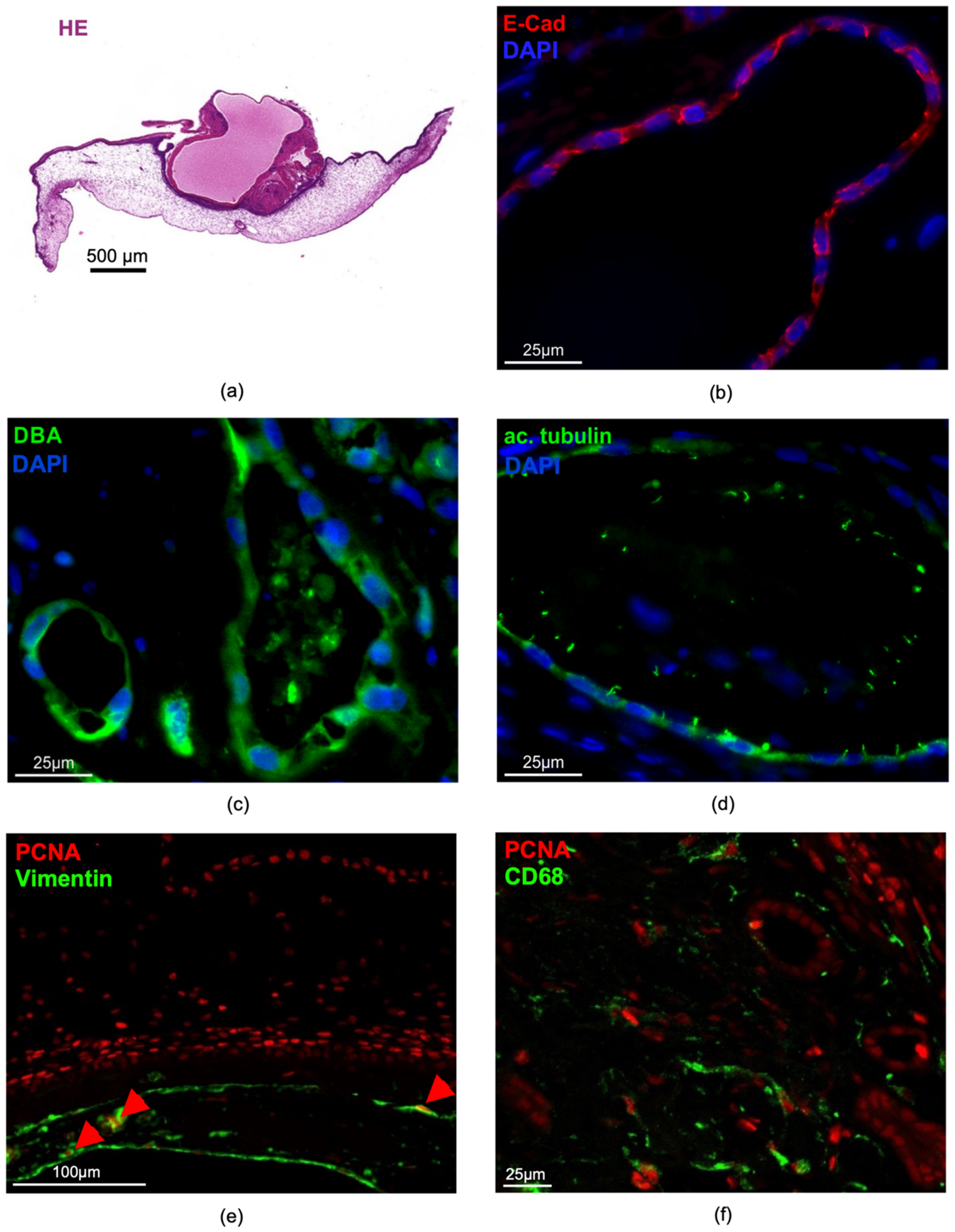
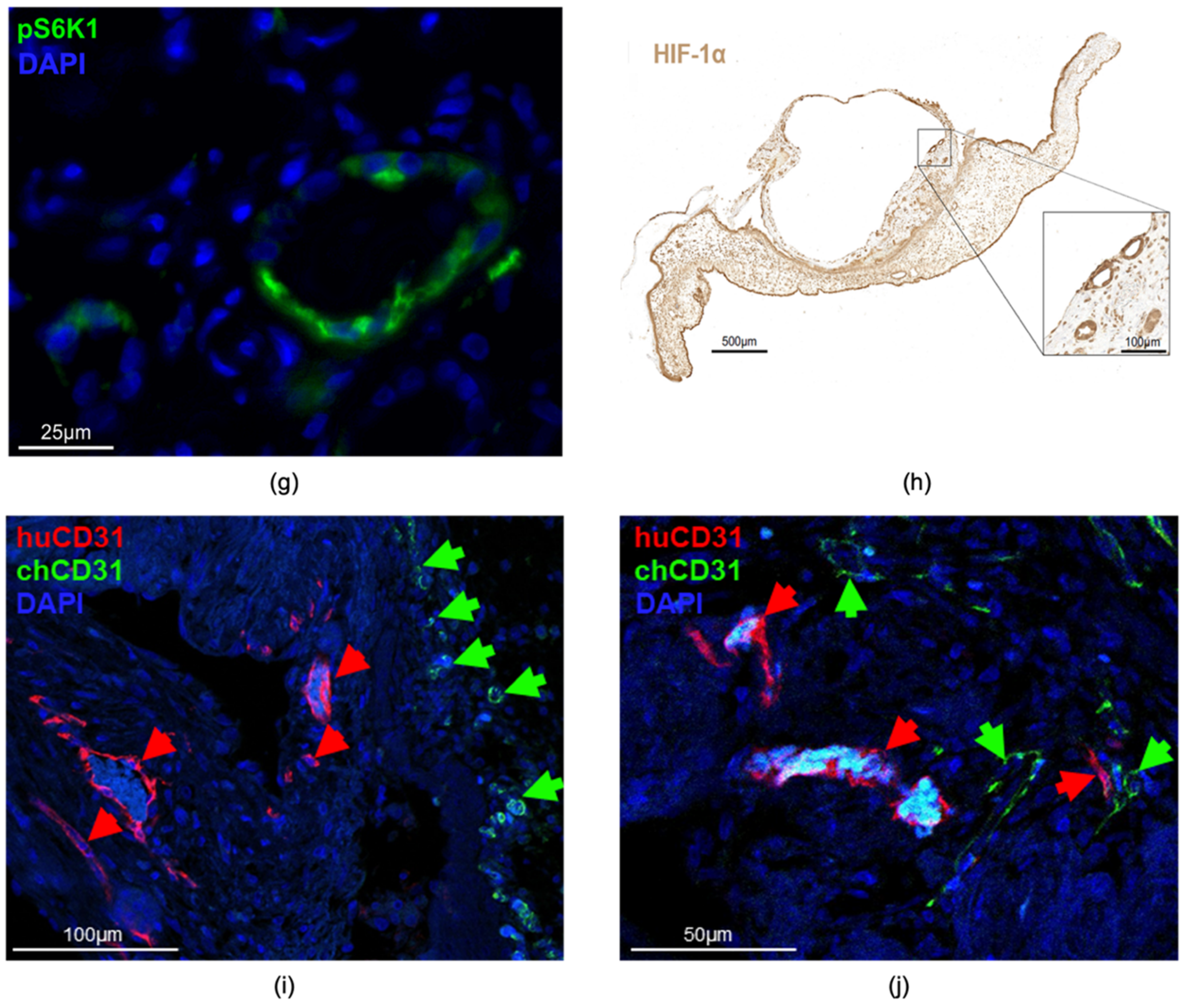
3.2.4. Characterization of the Human Renal Cystic Tissue after Growth on the CAM (Histological Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.C.; Torres, V.E. Polycystic kidney disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Mulamalla, S.; Swenson-Fields, K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2011, 7, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Tolvaptan: A Review in Autosomal Dominant Polycystic Kidney Disease. Drugs 2019, 79, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Patil, A.; Sweeney, W.E.; Pan, C.G.; Avner, E.D.; Gunay-Aygun, M. Novel Treatments for Polycystic Kidney Disease. TRD 2019, 4, 77–86. [Google Scholar] [CrossRef]
- Buchholz, B.; Eckardt, K.-U. Role of oxygen and the HIF-pathway in polycystic kidney disease. Cell Signal. 2020, 69, 109524. [Google Scholar] [CrossRef]
- Kraus, A.; Peters, D.J.M.; Klanke, B.; Weidemann, A.; Willam, C.; Schley, G.; Kunzelmann, K.; Eckardt, K.-U.; Buchholz, B. HIF-1α promotes cyst progression in a mouse model of autosomal dominant polycystic kidney disease. Kidney Int. 2018, 94, 887–899. [Google Scholar] [CrossRef]
- Cabrita, I.; Kraus, A.; Scholz, J.K.; Skoczynski, K.; Schreiber, R.; Kunzelmann, K.; Buchholz, B. Cyst growth in ADPKD is prevented by pharmacological and genetic inhibition of TMEM16A in vivo. Nat. Commun. 2020, 11, 4320. [Google Scholar] [CrossRef]
- Terryn, S.; Ho, A.; Beauwens, R.; Devuyst, O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta 2011, 1812, 1314–1321. [Google Scholar] [CrossRef]
- Talbi, K.; Cabrita, I.; Kraus, A.; Hofmann, S.; Skoczynski, K.; Kunzelmann, K.; Buchholz, B.; Schreiber, R. The chloride channel CFTR is not required for cyst growth in an ADPKD mouse model. FASEB J. 2021, 35, e21897. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.; Faria, D.; Schley, G.; Schreiber, R.; Eckardt, K.-U.; Kunzelmann, K. Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int. 2014, 85, 1058–1067. [Google Scholar] [CrossRef]
- Serra, A.L.; Poster, D.; Kistler, A.D.; Krauer, F.; Raina, S.; Young, J.; Rentsch, K.M.; Spanaus, K.S.; Senn, O.; Kristanto, P.; et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2010, 363, 820–829. [Google Scholar] [CrossRef]
- Walz, G.; Budde, K.; Mannaa, M.; Nürnberger, J.; Wanner, C.; Sommerer, C.; Kunzendorf, U.; Banas, B.; Hörl, W.H.; Obermüller, N.; et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2010, 363, 830–840. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Che, P.P.; Sarogni, P.; Sminia, P.; Giovannetti, E.; Voliani, V. Tumor grafted-chick chorioallantoic membrane as an alternative model for biological cancer research and conventional/nanomaterial-based theranostics evaluation. Expert Opin. Drug Metab. Toxicol. 2021, 17, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, F.D.; Cardoso, V.M.d.O.; Ferreira, N.N.; Calixto, G.M.F.; Fontana, C.R.; Baltazar, F.; Gremião, M.P.D.; Chorilli, M. Chick embryo chorioallantoic membrane as a suitable in vivo model to evaluate drug delivery systems for cancer treatment: A review. Eur. J. Pharm. Biopharm. 2020, 153, 273–284. [Google Scholar] [CrossRef]
- Rous, P. Tumor implantation in the developing embryo. JAMA 1911, LVI, 741–742. [Google Scholar]
- Schley, G.; Scholz, H.; Kraus, A.; Hackenbeck, T.; Klanke, B.; Willam, C.; Wiesener, M.S.; Heinze, E.; Burzlaff, N.; Eckardt, K.-U.; et al. Hypoxia inhibits nephrogenesis through paracrine Vegfa despite the ability to enhance tubulogenesis. Kidney Int. 2015, 88, 1283–1292. [Google Scholar] [CrossRef]
- Sperling, S.; Aung, T.; Martin, S.; Rohde, V.; Ninkovic, M. Riluzole: A potential therapeutic intervention in human brain tumor stem-like cells. Oncotarget 2017, 8, 96697–96709. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, P.; Troeltzsch, M.; Cordesmeyer, R.; Heidekrueger, P.I.; Schliephake, H.; Canis, M.; Wolff, H.A.; Rave-Fraenk, M.; Stroebel, P.; Kehrer, A.; et al. Presentation of a variation of the chorioallantoic membrane set up as a potential model for individual therapy for squamous cell carcinoma of the oropharynx. Clin. Hemorheol. Microcirc. 2017, 67, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Troebs, J.; Asam, C.; Pion, E.; Prantl, L.; Aung, T.; Haerteis, S. 3D monitoring of tumor volume in an in vivo model. Clin. Hemorheol. Microcirc. 2020, 76, 123–131. [Google Scholar] [CrossRef]
- Feder, A.-L.; Pion, E.; Troebs, J.; Lenze, U.; Prantl, L.; Htwe, M.M.; Phyo, A.; Haerteis, S.; Aung, T. Extended analysis of intratumoral heterogeneity of primary osteosarcoma tissue using 3D-in-vivo-tumor-model. Clin. Hemorheol. Microcirc. 2020, 76, 133–141. [Google Scholar] [CrossRef]
- Pion, E.; Asam, C.; Feder, A.-L.; Felthaus, O.; Heidekrueger, P.I.; Prantl, L.; Haerteis, S.; Aung, T. Laser speckle contrast analysis (LASCA) technology for the semiquantitative measurement of angiogenesis in in-ovo-tumor-model. Microvasc. Res. 2021, 133, 104072. [Google Scholar] [CrossRef]
- Kohl, C.; Aung, T.; Haerteis, S.; Papathemelis, T. Assessment of breast cancer primary tumor material in a 3D in vivo model. Clin. Hemorheol. Microcirc. 2021, 79, 157–166. [Google Scholar] [CrossRef]
- Kauffmann, P.; Troeltzsch, M.; Brockmeyer, P.; Bohnenberger, H.; Heidekrüger, P.I.; Manzke, M.; Canis, M.; Gaayathiri, S.; Schliephake, H.; Prantl, L.; et al. First experience of chick chorioallantoic membrane (CAM) assay in the clinical work flow with oral squamous cell carcinoma patients. Clin. Hemorheol. Microcirc. 2018, 70, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gobé, G.; Willgoss, D.; Hogg, N.; Schoch, E.; Endre, Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999, 56, 1299–1304. [Google Scholar] [CrossRef]
- Power, E.A.; Fernandez-Torres, J.; Zhang, L.; Yaun, R.; Lucien, F.; Daniels, D.J. Chorioallantoic membrane (CAM) assay to study treatment effects in diffuse intrinsic pontine glioma. PLoS ONE 2022, 17, e0263822. [Google Scholar] [CrossRef]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the chick embryo chorioallantoic membrane (CAM) as a platform for anti-metastatic drug testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Sudha, T.; Mousa, D.S.; El-Far, A.H.; Mousa, S.A. Pomegranate (Punica granatum) Fruit Extract Suppresses Cancer Progression and Tumor Angiogenesis of Pancreatic and Colon Cancer in Chick Chorioallantoic Membrane Model. Nutr. Cancer 2021, 73, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kaisto, S.; Saarela, U.; Dönges, L.; Raykhel, I.; Skovorodkin, I.; Vainio, S.J. Optimization of Renal Organoid and Organotypic Culture for Vascularization, Extended Development, and Improved Microscopy Imaging. J. Vis. Exp. 2020, 157, e60995. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.; Grantham, J.J. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: Clinical and CT study in 84 patients. Am. J. Roentgenol. 1992, 159, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.; Seith, A.; Sud, K.; Kohli, H.S.; Singh, S.K.; Sakhuja, V.; Suri, S. CT in the evaluation of complicated autosomal dominant polycystic kidney disease. Acta Radiol. 2000, 41, 280–284. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bichlmayer, E.-M.; Mahl, L.; Hesse, L.; Pion, E.; Haller, V.; Moehwald, A.; Hackl, C.; Werner, J.M.; Schlitt, H.J.; Schwarz, S.; et al. A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices. Cells 2022, 11, 2269. https://doi.org/10.3390/cells11152269
Bichlmayer E-M, Mahl L, Hesse L, Pion E, Haller V, Moehwald A, Hackl C, Werner JM, Schlitt HJ, Schwarz S, et al. A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices. Cells. 2022; 11(15):2269. https://doi.org/10.3390/cells11152269
Chicago/Turabian StyleBichlmayer, Eva-Marie, Lina Mahl, Leo Hesse, Eric Pion, Victoria Haller, Andreas Moehwald, Christina Hackl, Jens M. Werner, Hans J. Schlitt, Siegfried Schwarz, and et al. 2022. "A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices" Cells 11, no. 15: 2269. https://doi.org/10.3390/cells11152269
APA StyleBichlmayer, E.-M., Mahl, L., Hesse, L., Pion, E., Haller, V., Moehwald, A., Hackl, C., Werner, J. M., Schlitt, H. J., Schwarz, S., Kainz, P., Brochhausen, C., Groeger, C., Steger, F., Kölbl, O., Daniel, C., Amann, K., Kraus, A., Buchholz, B., ... Haerteis, S. (2022). A 3D In Vivo Model for Studying Human Renal Cystic Tissue and Mouse Kidney Slices. Cells, 11(15), 2269. https://doi.org/10.3390/cells11152269







