Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Tumor-Infiltrating Lymphocytes Analysis
2.3. Immunohistochemical Analysis
2.4. Biostatistical Analysis
3. Results
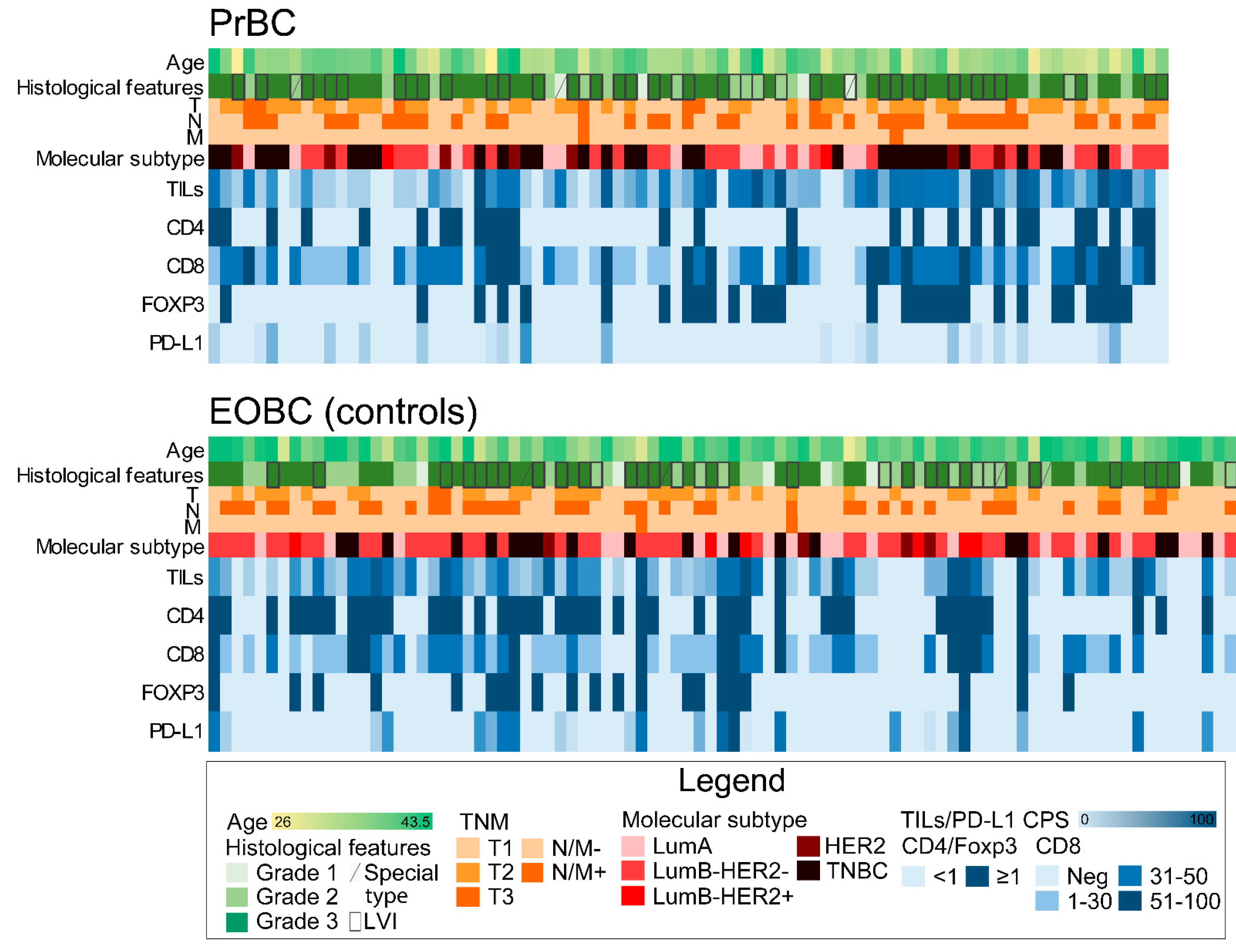
3.1. Clinicopathological Features of PrBC
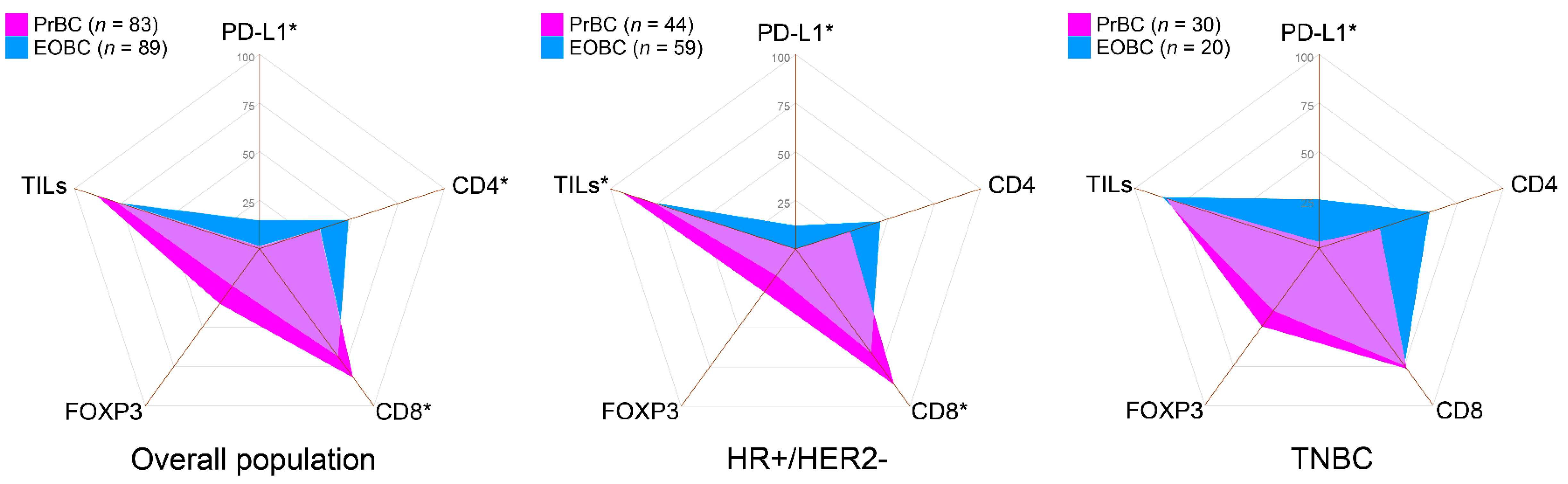
3.2. Increased CD8(+) TILs and Low/Null PD-L1 Expression in HR+/HER2– PrBC
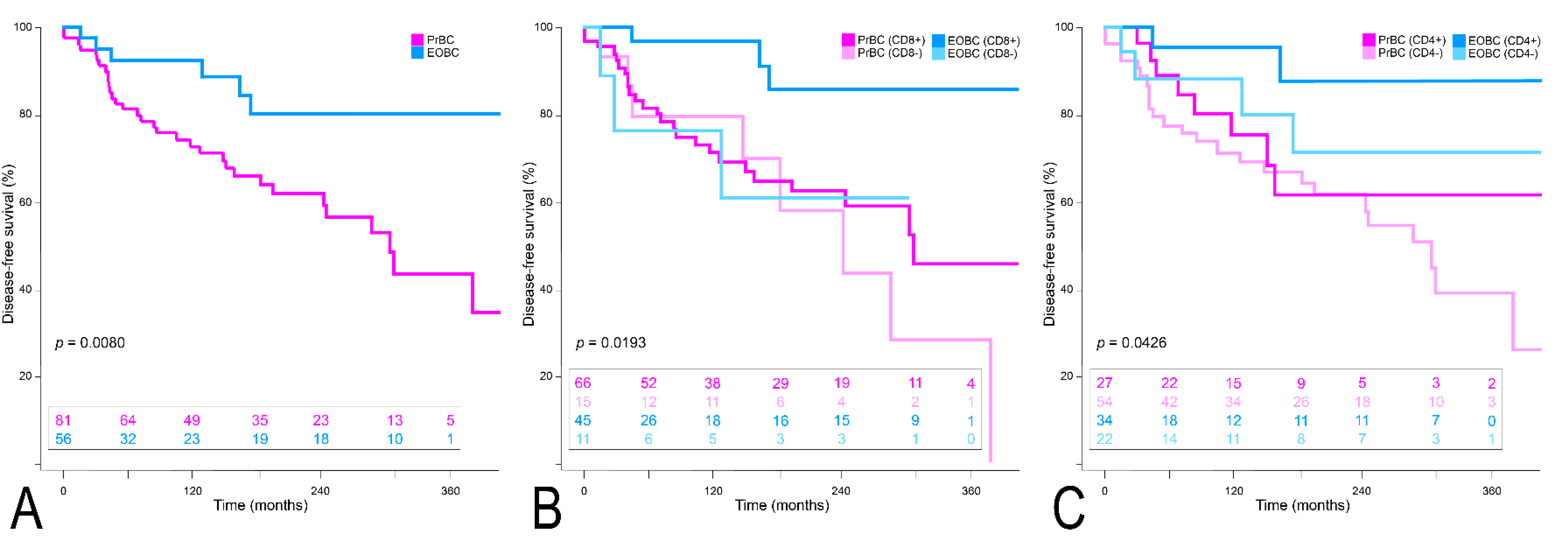
3.3. Clinical Outcome of PrBC Based on T-Cells Subpopulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amant, F.; Lefrère, H.; Borges, V.F.; Cardonick, E.; Lambertini, M.; Loibl, S.; Peccatori, F.; Partridge, A.; Schedin, P. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol. 2021, 22, 753–754. [Google Scholar] [CrossRef]
- Korenaga, T.K.; Tewari, K.S. Gynecologic cancer in pregnancy. Gynecol. Oncol. 2020, 157, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Chelmow, D.; Pearlman, M.D.; Young, A.; Bozzuto, L.; Dayaratna, S.; Jeudy, M.; Kremer, M.E.; Scott, D.M.; O’Hara, J.S. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet. Gynecol. 2020, 135, 1457–1478. [Google Scholar] [CrossRef]
- Muñoz-Montaño, W.R.; Cabrera-Galeana, P.; De la Garza-Ramos, C.; Azim, H.A., Jr.; Tabares, A.; Perez, V.; Porras Reyes, F.; Sanchez Benitez, D.; Alvarado-Miranda, A.; Lara-Medina, F.; et al. Prognosis of breast cancer diagnosed during pregnancy and early postpartum according to immunohistochemical subtype: A matched case-control study. Breast Cancer Res. Treat. 2021, 188, 489–500. [Google Scholar] [CrossRef]
- Gooch, J.C.; Chun, J.; Kaplowitz, E.; Guth, A.; Axelrod, D.; Shapiro, R.; Roses, D.; Schnabel, F. Pregnancy-associated breast cancer in a contemporary cohort of newly diagnosed women. Breast J. 2020, 26, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.T.; Bermejo, B.; Hernando, C.; Gambardella, V.; Cejalvo, J.M.; Lluch, A. Breast cancer in pregnant patients: A review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 222–227. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A., Jr. Managing pregnancy-associated breast cancer: Is more really better? Breast 2016, 30, 215–216. [Google Scholar] [CrossRef]
- Blundo, C.; Giroda, M.; Fusco, N.; Sajjadi, E.; Venetis, K.; Leonardi, M.C.; Vicini, E.; Despini, L.; Rossi, C.F.; Runza, L.; et al. Early Breast Cancers During Pregnancy Treated with Breast-Conserving Surgery in the First Trimester of Gestation: A Feasibility Study. Front. Oncol. 2021, 11, 723693. [Google Scholar] [CrossRef]
- Sun, J.; Lee, M.C. Clinical Presentation, Diagnosis and Prognosis of Pregnancy-Associated Breast Cancer. In Diseases of the Breast during Pregnancy and Lactation; Alipour, S., Omranipour, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 87–93. [Google Scholar]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Ivanova, M.; Andaloro, S.; Guerini-Rocco, E.; Montagna, E.; Caldarella, P.; Veronesi, P.; Colleoni, M.; et al. Low-risk triple-negative breast cancers: Clinico-pathological and molecular features. Crit. Rev. Oncol. Hematol. 2022, 172, 103643. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi160–vi170. [Google Scholar] [CrossRef]
- Acs, B.; Madaras, L.; Tokes, A.M.; Kovacs, A.K.; Kovacs, E.; Ozsvari-Vidakovich, M.; Karaszi, A.; Birtalan, E.; Dank, M.; Szasz, A.M.; et al. PD-1, PD-L1 and CTLA-4 in pregnancy-related—and in early-onset breast cancer: A comparative study. Breast 2017, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Vingiani, A.; Peccatori, F.; Viale, G.; Loi, S.; Pruneri, G. Tumour infiltrating lymphocytes (TILs) in breast cancer during pregnancy. Breast 2015, 24, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Venet, D.; Azim, H.A.; Brown, D.; Desmedt, C.; Lambertini, M.; Majjaj, S.; Pruneri, G.; Peccatori, F.; Piccart, M.; et al. Breast cancer diagnosed during pregnancy is associated with enrichment of non-silent mutations, mismatch repair deficiency signature and mucin mutations. npj Breast Cancer 2018, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Azim, H.A., Jr.; Brohee, S.; Peccatori, F.A.; Desmedt, C.; Loi, S.; Lambrechts, D.; Dell’Orto, P.; Majjaj, S.; Jose, V.; Rotmensz, N.; et al. Biology of breast cancer during pregnancy using genomic profiling. Endocr. Relat. Cancer 2014, 21, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Korakiti, A.M.; Moutafi, M.; Zografos, E.; Dimopoulos, M.A.; Zagouri, F. The Genomic Profile of Pregnancy-Associated Breast Cancer: A Systematic Review. Front. Oncol. 2020, 10, 1773. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune Checkpoint Molecules in Reproductive Immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Sun, H.X. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, L.; Hu, X.; Ji, J.; Mor, G.; Liao, A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum. Reprod. 2019, 34, 25–36. [Google Scholar] [CrossRef]
- Beaman, K.D.; Jaiswal, M.K.; Katara, G.K.; Kulshreshta, A.; Pamarthy, S.; Ibrahim, S.; Kwak-Kim, J.; Gilman-Sachs, A. Pregnancy is a model for tumors, not transplantation. Am. J. Reprod. Immunol. 2016, 76, 3–7. [Google Scholar] [CrossRef]
- Zagouri, F.; Psaltopoulou, T.; Dimitrakakis, C.; Bartsch, R.; Dimopoulos, M.A. Challenges in managing breast cancer during pregnancy. J. Thorac. Dis. 2013, 5, S62–S67. [Google Scholar] [PubMed]
- Hoon Tan, P.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 WHO classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Curigliano, G.; Burstein, H.J.; P Winer, E.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, G.; Lopez, G.; Ciapponi, C.; Corti, C.; Despini, L.; Gambini, D.; Runza, L.; Blundo, C.; Sciarra, A.; Fusco, N. Building Up a High-throughput Screening Platform to Assess the Heterogeneity of HER2 Gene Amplification in Breast Cancers. J. Vis. Exp. 2017, 5, e56686. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Wei, C.H.; Garcia, L.; Murata-Collins, J.; Schmolze, D.; Apple, S. Quantitative Impact of the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) Practice Guideline Update on Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: A Systematic Analysis. Arch. Pathol. Lab. Med. 2021, 145, 887–890. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [Green Version]
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 low, ultra-low, and novel complementary biomarkers: Expanding the spectrum of HER2 positivity in breast cancer. Front. Mol. Biosci. 2022, 9, 834651. [Google Scholar] [CrossRef]
- Fusco, N.; Lopez, G.; Corti, C.; Pesenti, C.; Colapietro, P.; Ercoli, G.; Gaudioso, G.; Faversani, A.; Gambini, D.; Michelotti, A.; et al. Mismatch Repair Protein Loss as a Prognostic and Predictive Biomarker in Breast Cancers Regardless of Microsatellite Instability. JNCI Cancer Spectr. 2018, 2, pky056. [Google Scholar] [CrossRef] [Green Version]
- Fusco, N.; Guerini-Rocco, E.; Augello, C.; Terrasi, A.; Ercoli, G.; Fumagalli, C.; Vacirca, D.; Braidotti, P.; Parafioriti, A.; Jaconi, M.; et al. Recurrent NAB2-STAT6 gene fusions and oestrogen receptor-α expression in pulmonary adenofibromas. Histopathology 2017, 70, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Salcedo, A.; Lin, X.; Walkiewicz, M.; Murone, C.; Ameratunga, M.; Asadi, K.; Deb, S.; Barnett, S.A.; Knight, S.; et al. The Immune Microenvironment, Genome-wide Copy Number Aberrations, and Survival in Mesothelioma. J. Thorac. Oncol. 2017, 12, 850–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018, 52, 16–25. [Google Scholar] [PubMed]
- Sajjadi, E.; Venetis, K.; Scatena, C.; Fusco, N. Biomarkers for precision immunotherapy in the metastatic setting: Hope or reality? Ecancermedicalscience 2020, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Guerini-Rocco, E.; Viale, G.; Fumagalli, C.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Malapelle, U.; Fusco, N. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anticancer Agents Med. Chem. 2021, 22, 787–800. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [Green Version]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.L.V.; Andersson, T.M.; Hsieh, C.C.; Jirstrom, K.; Cnattingius, S.; Fredriksson, I.; Dickman, P.W.; Lambe, M. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int. J. Cancer. 2018, 142, 1343–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser-Weippl, K.; Ramchandani, R.; Fan, L.; Li, J.; Hurlbert, M.; Finkelstein, D.; Shao, Z.M.; Goss, P.E. Pregnancy-associated breast cancer in women from Shanghai: Risk and prognosis. Breast Cancer Res. Treat. 2015, 149, 255–261. [Google Scholar] [CrossRef]
- Wienke, J.; Brouwers, L.; van der Burg, L.M.; Mokry, M.; Scholman, R.C.; Nikkels, P.G.; van Rijn, B.B.; van Wijk, F. Human Tregs at the materno-fetal interface show site-specific adaptation reminiscent of tumor Tregs. JCI Insight 2020, 5, e137926. [Google Scholar] [CrossRef]
- Kinder, J.M.; Turner, L.H.; Stelzer, I.A.; Miller-Handley, H.; Burg, A.; Shao, T.Y.; Pham, G.; Way, S.S. CD8(+) T Cell Functional Exhaustion Overrides Pregnancy-Induced Fetal Antigen Alloimmunization. Cell Rep. 2020, 31, 107784. [Google Scholar] [CrossRef]
- Lissauer, D.; Piper, K.; Goodyear, O.; Kilby, M.D.; Moss, P.A. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 2012, 189, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Lyons, T.R.; Schedin, P.J.; Borges, V.F. Pregnancy and breast cancer: When they collide. J. Mammary Gland Biol. Neoplasia 2009, 14, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, A.L.; Andersson, T.M.; Hsieh, C.C.; Jirstrom, K.; Dickman, P.; Cnattingius, S.; Lambe, M. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res. Treat. 2013, 139, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.E.; Mayer, E.L.; Partridge, A. Prognosis of pregnancy-associated breast cancer. Breast Cancer Res. Treat. 2017, 163, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.K.; Eslick, G.D. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: A meta-analysis. Breast Cancer Res. Treat. 2016, 160, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; von Minckwitz, G.; Han, S.N.; Bontenbal, M.; Ring, A.E.; Giermek, J.; Wildiers, H.; Fehm, T.; Linn, S.C.; Schlehe, B.; et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: Results from an international collaborative study. J. Clin. Oncol. 2013, 31, 2532–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, N.; Kwon, H.J.; Park, M.H.; Kang, S.H.; Bae, Y.K. Prognostic Value of Tumor-Infiltrating Lymphocyte Density Assessed Using a Standardized Method Based on Molecular Subtypes and Adjuvant Chemotherapy in Invasive Breast Cancer. Ann. Surg. Oncol. 2018, 25, 937–946. [Google Scholar] [CrossRef] [PubMed]


| PrBC | EOBC | p-Value | |
|---|---|---|---|
| (n = 83) | (n = 89) | ||
| Age at diagnosis, years | <0.0001 * | ||
| Mean ± SD | 35.1 ± 4.3 | 38.9 ± 3.7 | |
| min, max | 26, 43 | 28, 43 | |
| Histological type, n (%) | 0.137 | ||
| NST | 80 (96.4) | 85 (95.5) | |
| Other | 3 (3.6) | 4 (4.5) | |
| LVI, n (%) | 39 (47.0) | 34 (38.2) | 0.2441 |
| T, n (%) | 0.0898 | ||
| T1 | 37 (44.6) | 52 (58.4) | |
| T2 | 38 (45.8) | 34 (38.2) | |
| T3/4 | 8 (9.6) | 3 (3.4) | |
| N, n (%) | 0.6582 | ||
| N0 | 43 (51.8) | 43 (52.4) | |
| N1 | 23 (27.7) | 17 (20.7) | |
| N2 | 10 (12.1) | 12 (14.6) | |
| N3 | 7 (8.4) | 10 (12.2) | |
| M1, n (%) | 2 (2.5) | 0 (0.0) | 0.2709 |
| ER positive, n (%) | 46 (55.4) | 65 (73.0) | 0.0158* |
| PgR positive, n (%) | 43 (51.8) | 63 (70.8) | 0.0105* |
| Ki67 high, n (%) | 65 (78.3) | 66 (74.2) | 0.5227 |
| HER2 positive, n (%) | 9 (10.8) | 10 (11.2) | 0.9346 |
| Molecular subtype, n (%) | 0.1445 | ||
| Luminal-A | 15 (18.1) | 19 (21.4) | |
| Luminal-B (HER2–) | 29 (34.9) | 40 (44.9) | |
| Luminal-B (HER2+) | 2 (2.4) | 6 (6.7) | |
| HER2-type | 7 (8.4) | 4 (4.5) | |
| TNBC | 30 (36.1) | 20 (22.5) | |
| Subtypes, n (%) | 0.1331 | ||
| HR+/HER2– | 44 (53.0) | 59 (66.3) | 0.0758 |
| HER2+ | 9 (10.8) | 10 (11.2) | 0.9346 |
| HR-/HER2– | 30 (36.1) | 20 (22.5) | 0.0485 * |
| Total Population | HR+/HER2– | HR-/HER2– | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PrBC | EOBC | p-Value | PrBC | EOBC | p-Value | PrBC | EOBC | p-Value | |
| (n = 83) | (n = 89) | (n = 44) | (n = 59) | (n = 30) | (n = 20) | ||||
| TILs, n (%) | |||||||||
| Absence | 10 (12.0) | 20 (22.5) | 0.0718 | 3 (6.8) | 14 (23.7) | 0.0222 * | 5 (16.7) | 3 (15.0) | 0.8749 |
| Presence | 73 (88.0) | 69 (77.5) | 41 (93.2) | 45 (76.3) | 25 (83.3) | 17 (85.0) | |||
| Low | 52 (71.2) | 45 (65.2) | 30 (73.2) | 33 (73.3) | 16 (64.0) | 9 (52.9) | |||
| Intermediate | 12 (16.4) | 13 (18.8) | 6 (14.6) | 6 (13.3) | 5 (20.0) | 6 (35.3) | |||
| High | 9 (12.3) | 11 (15.9) | 5 (12.2) | 6 (13.3) | 4 (16.0) | 2 (11.8) | |||
| CD8, n (%) | |||||||||
| Absence | 15 (18.1) | 28 (31.5) | 0.0427 * | 6 (13.6) | 20 (33.9) | 0.0192 * | 7 (23.0) | 5 (25.0) | 0.8925 |
| Presence | 68 (81.9) | 61 (68.5) | 38 (86.4) | 39 (66.1) | 23 (77.0) | 15 (75.0) | |||
| Low | 23 (27.7) | 30 (33.7) | 16 (42.1) | 20 (51.3) | 5 (21.7) | 7 (46.7) | |||
| Intermediate | 29 (34.9) | 19 (21.3) | 14 (36.8) | 14 (35.9) | 11 (47.8) | 3 (20.0) | |||
| High | 16 (19.3) | 12 (13.5) | 8 (21.1) | 5 (12.8) | 7 (30.4) | 5 (33.3) | |||
| CD4, n (%) | |||||||||
| Absence | 56 (67.5) | 46 (51.7) | 0.0352 * | 31 (70.5) | 32 (54.2) | 0.0948 | 20 (66.7) | 8 (40.0) | 0.0627 |
| Presence | 27 (32.5) | 43 (48.3) | 13 (29.5) | 27 (45.8) | 10 (33.3) | 12 (60.0) | |||
| FOXP3, n (%) | |||||||||
| Absence | 54 (65.1) | 68 (76.4) | 0.1016 | 32 (72.7) | 49 (83.1) | 0.206 | 15 (50.0) | 12 (60.0) | 0.487 |
| Presence | 29 (34.9) | 21 (23.6) | 12 (27.3) | 10 (16.9) | 15 (50.0) | 8 (40.0) | |||
| PD-L1 CPS, n (%) | |||||||||
| <10 | 82 (98.8) | 76 (85.4) | 0.0013 * | 44 (100) | 52 (88.1) | 0.0179 * | 29 (96.7) | 15 (75.0) | 0.0209 * |
| ≥10 | 1 (1.2) | 13 (14.6) | 0 | 7 (11.9) | 1 (3.3) | 5 (25.0) | |||
| Disease Recurrence | Died of Disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PrBC | EOBC | p-Value | PrBC | EOBC | p-Value | |||||
| Yes | No | Yes | No | Yes | No | Yes | No | |||
| Cases with FU, n (%) | 36 (44.4) | 45 (55.6) | 6 (10.7) | 50 (89.3) | 0.0080 * | 16 (19.8) | 65 (80.2) | 2 (3.6) | 54 (96.4) | 0.0059 * |
| HR+HER2– | 17 (38.6) | 27 (61.4) | 5 (13.5) | 32 (86.5) | 0.0113 * | 6 (13.6) | 38 (86.4) | 1 (2.7) | 36 (97.3) | 0.0811 |
| HER2+ | 5 (55.6) | 4 (44.4) | 0 | 6 (100) | 0.0253 * | 2 (20.0) | 8 (80.0) | 0 | 6 (100) | 0.2416 |
| HR-/HER2– | 14 (50.0) | 14 (50.0) | 1 (7.7) | 12 (92.3) | 0.0089 * | 8 (28.6) | 20 (71.4) | 1 (7.7) | 12 (92.3) | 0.1328 |
| Presence of TILs, n (%) | 31 (43.7) | 40 (56.3) | 3 (6.1) | 46 (93.9) | <0.0001 * | 13 (18.3) | 58 (81.7) | 0 | 49 (100) | 0.0015 * |
| Low | 23 (46.0) | 27 (44.0) | 3 (9.4) | 29 (90.6) | 0.0005 * | 10 (20.0) | 40 (80.0) | 0 | 32 (100) | 0.0069 * |
| Intermediate | 6 (50.0) | 6 (50.0) | 0 | 9 (100) | 0.0121 * | 2 (16.6) | 10 (83.3) | 0 | 9 (100) | 0.1978 |
| High | 2 (22.2) | 7 (77.8) | 0 | 8 (100) | 0.1557 | 1 (11.1) | 8 (88.9) | 0 | 8 (100) | 0.3312 |
| CD8 TILs, n (%) | 28 (42.4) | 38 (57.6) | 3 (6.7) | 42 (93.3) | <0.0001 * | 13 (19.7) | 53 (80.3) | 0 | 45 (100) | 0.0015 * |
| Low | 14 (63.6) | 8 (36.4) | 2 (8.7) | 21(91.3) | 0.0001 * | 8 (36.4) | 14 (63.6) | 0 | 23 (100) | 0.0014 * |
| Intermediate | 7 (25.0) | 21 (75.0) | 1 (8.3) | 11(91.7) | 0.2272 | 3 (10.7) | 25 (89.3) | 0 | 12 (100) | 0.2384 |
| High | 7 (43.7) | 9 (56.3) | 0 | 10 (100) | 0.0144 * | 2 (12.5) | 14 (87.5) | 0 | 10 (100) | 0.2445 |
| CD4 TILs, n (%) | 9 (33.3) | 18 (66.7) | 2 (5.6) | 32 (94.4) | 0.0056 * | 2 (7.4) | 25 (92.6) | 0 | 34 (100) | 0.1066 |
| FOXP3, n (%) | 9 (33.3) | 18 (66.7) | 0 | 21 (100) | 0.0033 * | 3 (11.1) | 24 (88.9) | 0 | 21 (100) | 0.1146 |
| PD-L1 CPS ≥ 10, n (%) | - | - | 0 | 9 (100) | - | - | - | 0 | 9 (100) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjadi, E.; Venetis, K.; Noale, M.; Azim, H.A., Jr.; Blundo, C.; Bonizzi, G.; Di Loreto, E.; Scarfone, G.; Ferrero, S.; Maggi, S.; et al. Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles. Cells 2022, 11, 2286. https://doi.org/10.3390/cells11152286
Sajjadi E, Venetis K, Noale M, Azim HA Jr., Blundo C, Bonizzi G, Di Loreto E, Scarfone G, Ferrero S, Maggi S, et al. Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles. Cells. 2022; 11(15):2286. https://doi.org/10.3390/cells11152286
Chicago/Turabian StyleSajjadi, Elham, Konstantinos Venetis, Marianna Noale, Hatem A. Azim, Jr., Concetta Blundo, Giuseppina Bonizzi, Eugenia Di Loreto, Giovanna Scarfone, Stefano Ferrero, Stefania Maggi, and et al. 2022. "Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles" Cells 11, no. 15: 2286. https://doi.org/10.3390/cells11152286
APA StyleSajjadi, E., Venetis, K., Noale, M., Azim, H. A., Jr., Blundo, C., Bonizzi, G., Di Loreto, E., Scarfone, G., Ferrero, S., Maggi, S., Barberis, M., Veronesi, P., Galimberti, V. E., Viale, G., Fusco, N., Peccatori, F. A., & Guerini-Rocco, E. (2022). Breast Cancer during Pregnancy as a Special Type of Early-Onset Breast Cancer: Analysis of the Tumor Immune Microenvironment and Risk Profiles. Cells, 11(15), 2286. https://doi.org/10.3390/cells11152286











