Pseudoislet Aggregation of Pancreatic β-Cells Improves Glucose Stimulated Insulin Secretion by Altering Glucose Metabolism and Increasing ATP Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. shRNA Knockdown of Connexin36
2.3. Human Islet Isolation and Culture
2.4. Viability Staining
2.5. Glucose Stimulated Insulin Secretion Assay
2.6. Enzyme Activity Assays
- (i)
- Low Km hexokinase (HK) and glucokinase (GK): main reagent (MR) = 50 mM Hepes (pH 7.8), 100 mM KCl, 2 mM MgCl2, 6 mM ATP/Mg2+, 1 mM NAD, 2 mM DTT, and 1.5 U/mL glucose 6-P dehydrogenase. A glucose concentration of 0.5 mM was used to measure low Km HK activity and 50 mM glucose to measure total HK activity. GK activity was calculated by the difference in activity between these two concentrations.
- (ii)
- Phosphoglucoisomerase (PGI): MR = 50 mM Hepes, 1 mM MgCl2, 0.5 mM NAD, 2 mM fructose 6-phosphate, 1 mM DTT, and 2.5 U/mL glucose 6-P dehydrogenase.
- (iii)
- Phosphofructokinase 1 (PFK1): MR = 20 mM Tris, 100 mM KCl, 2 mM NH4Cl2, 3 mM MgCl2, 1 mM ATP/MgCl2, 0.16 mM NADH, 2 mM AMP, 10 mM fructose 6-phosphate, 1 mU/mL α-glycerophosphate dehydrogenase, 10 mU/mL triosephosphate isomerase, and 1 mU/mL aldolase.
- (iv)
- Glyceraldehyde 3-phosphate dehydrogenase (GAPDH): MR = 0.1 M triethanolamine (pH 8), 6 mM glycerate 3-phosphate, 0.18 mM EDTA, 1.12 mM ATP, 0.4 mM NADH, 5 mM MgSO4, and 14.8 U/mL Phosphoglycerokinase.
- (v)
- Aldolase (ALD): MR = 20 mM Tris, 100 mM KCl, 0.32 mM NADH, 10 mM fructose 1,6-bisphosphate, 1 mU/mL α-glycerophosphate dehydrogenase and 10 mU/mL triosephosphate isomerase.
- (vi)
- Pyruvate kinase (PK): MR = 0.1 M triethanolamine, 4 mM phospho(enol)pyruvic acid (PEP), 4 mM ADP, 0.4 mM NADH, 5 mM MgSO4, and 34 U/mL LDH.
- (vii)
- Lactate Dehydrogenase (LDH) activity was measured in main reagent containing 0.2 M KPi, 0.2 mM NADH, and 2.8 mM pyruvate.
- (viii)
- Pyruvate Carboxylase (PC): MR = 80 mM Tris, 2mM ATP, 16 mM Sodium pyruvate, 22 mM KHCO3, 9 mM MgSO4, 0.32 mM acetyl CoA, 0.16 mM NADH, and 10 U/mL malate dehydrogenase.
- (ix)
- Citrate Synthase (CS): MR = 50 mM Tris (pH 8), 0.3 mM Acetyl CoA, 0.24 mM oxaloacetate, and 0.2 mM DTNB. Absorbance was read at 412 nm.
- (x)
- Isocitrate Dehydrogenase (ICD): MR = 50 mM KPi (pH 7.4), 10 mM MgCl2, 2.5 mM isocitrate, and 0.25 mM NADP.
- (xi)
- α-ketoglutarate Dehydrogenase (αKG): MR = 100 mM Tris (pH 8), 0.5 mM NAD, 3 mM MgCl2, 0.2 mM Thiamine Pyrophosphate, 0.04 mM Coenzyme A, 2.5 µM rotenone, and 5 mM α-ketoglutarate.
- (xii)
- Malate dehydrogenase (MDH): MR = 0.2 M KPi, 0.2 mM NADH, and 2.78 mM oxaloacetate.
2.7. Seahorse Analysis
2.8. Western Blotting
2.9. RT-PCR
2.10. Statistical Analysis
3. Results
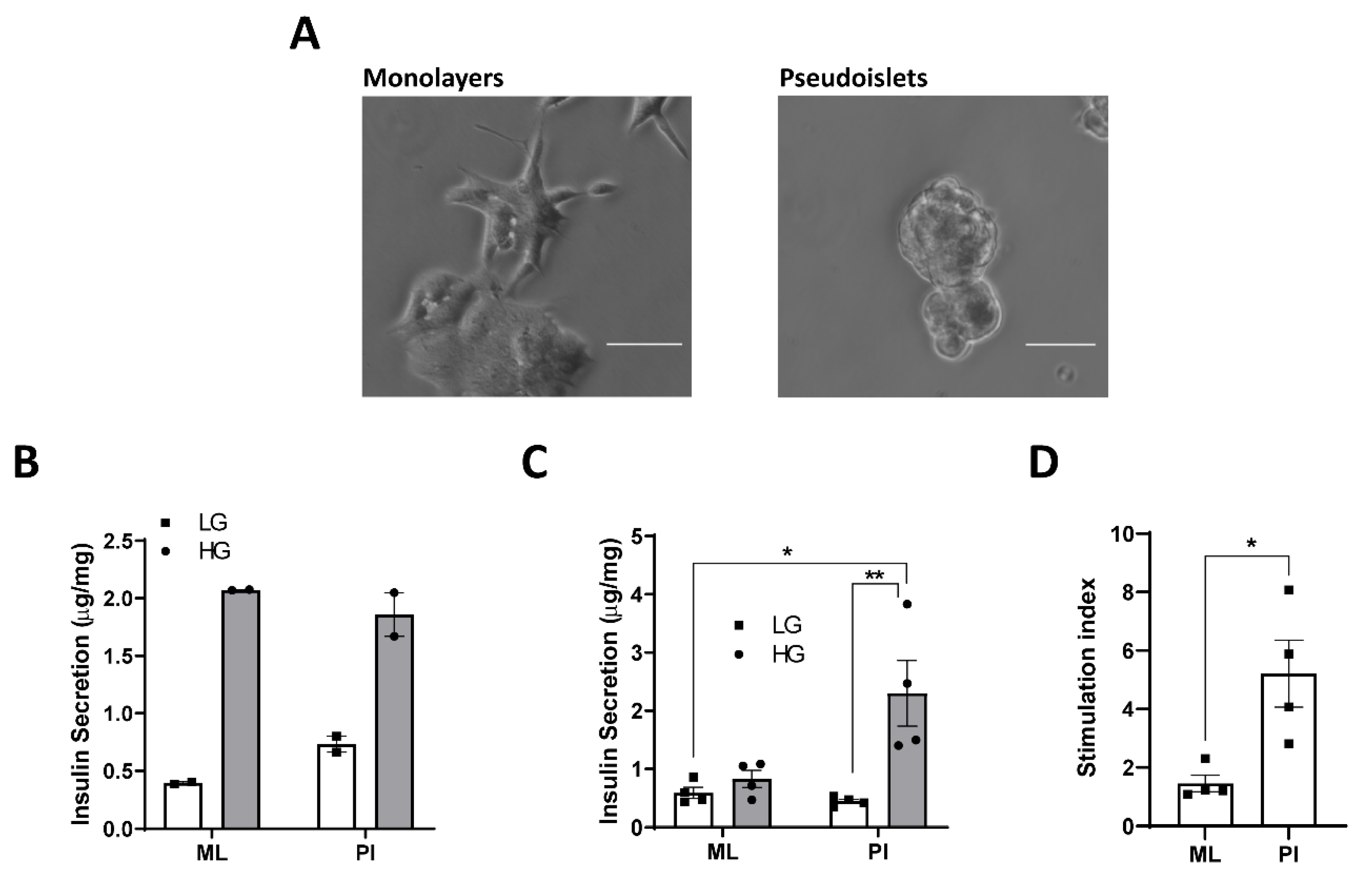
3.1. Configuration of MIN6 as Pseudoislets Improves Glucose-Responsiveness
3.2. Pseudoislet Formation Increases ATP Production in Response to Glucose
3.3. Interpreting the ECAR Rate in MIN6 ML and PI
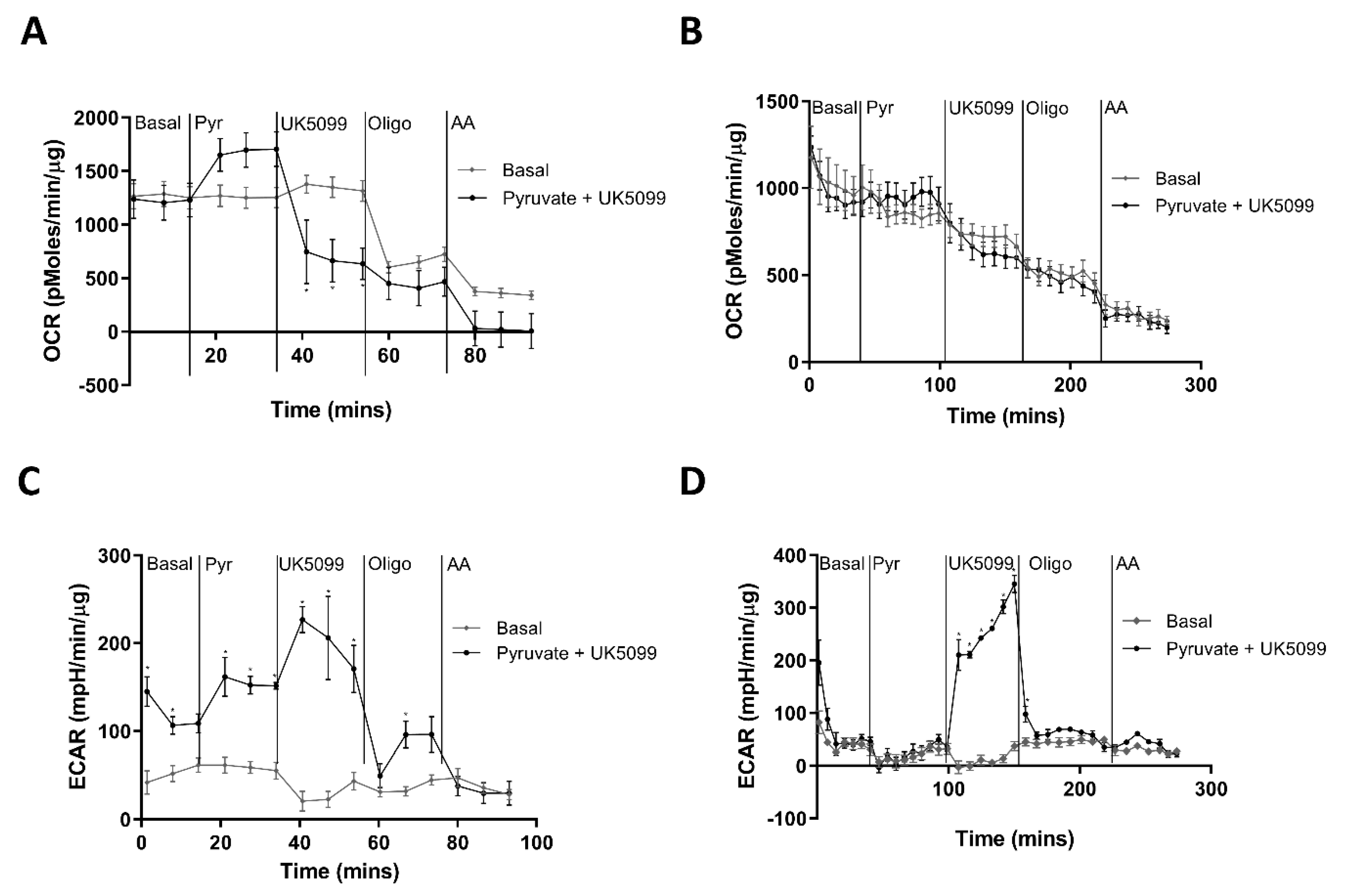
3.4. Human Islets Show Increased OCR and ECAR in Response to Glucose Stimulation
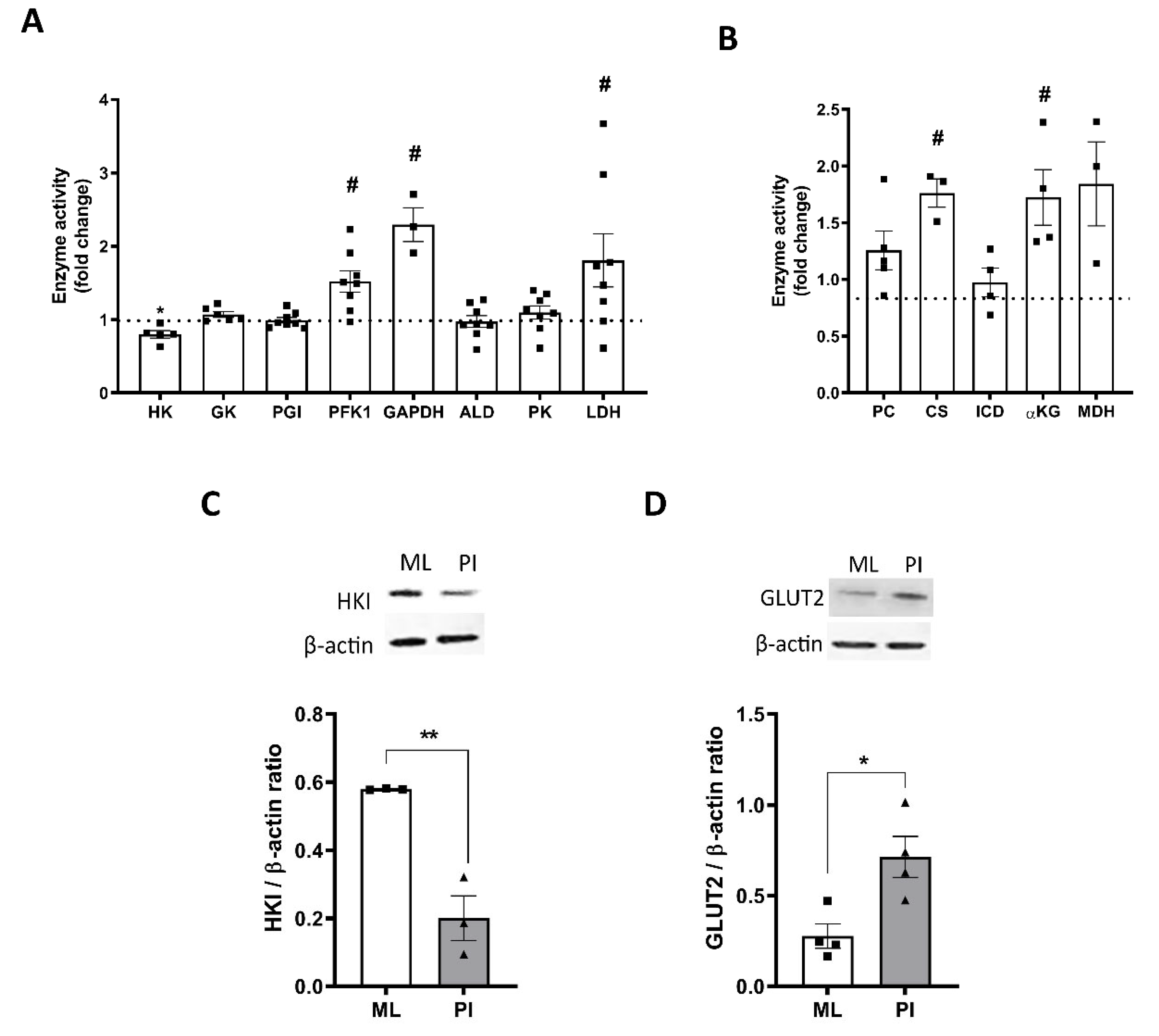
3.5. Pseudoislet Formation Alters Expression and Activity of Glycolytic and TCA Cycle Enzymes
3.6. Role for Hypoxia in Mediating Changes in Glucose Responsiveness
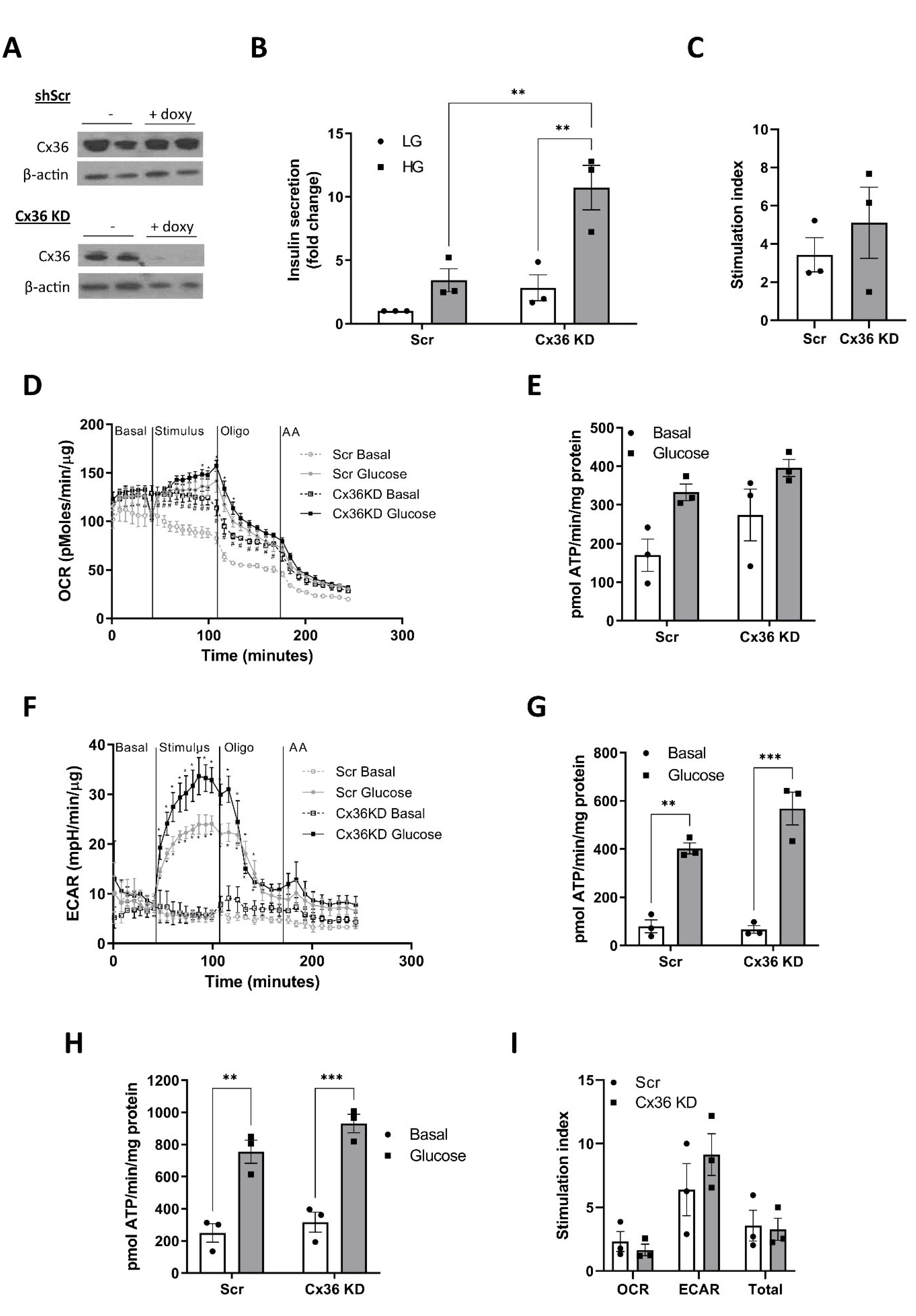
3.7. Connexin 36 Does Not Significantly Impact on GSIS or Metabolic Flux in Pseudoislets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell. Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Migliorini, A.; Gegg, M.; Lickert, H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 2016, 12, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Pipeleers, D.; In’t Veld, P.; Maes, E.; Van de Winkel, M. Glucose-induced insulin release dep5ends on functional cooperation between islet cells. Proc. Natl. Acad. Sci. USA 1982, 79, 7322–7325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halban, P.A.; Blondel, B.; Meda, P.; Niesor, E.N.; Mintz, D.H. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinol 1982, 111, 86–94. [Google Scholar] [CrossRef]
- Halban, P.A.; Powers, S.L.; George, K.L. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregated with three-dimensional architecture typical of native islets. Diabetes 1987, 38, 783–792. [Google Scholar] [CrossRef]
- Hauge-Evans, A.C.; Squires, P.E.; Persaud, S.J.; Jones, P.M. Pancreatic β-cell-to-β-cell interactions are required for integrated responses to nutrient stimuli: Enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999, 48, 1402–1408. [Google Scholar] [CrossRef]
- Luther, M.J.; Hauge-Evans, A.; Souza, K.L.; Jörns, A.; Lenzen, S.; Persaud, S.J.; Jones, P.M. MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem. Biophys. Res. Commun. 2006, 343, 99–104. [Google Scholar] [CrossRef]
- Lecomte, M.J.; Pechberty, S.; Machado, C.; Da Barroca, S.; Ravassard, P.; Scharfmann, R.; Czernichow, P.; Duvillié, B. Aggregation of Engineered Human β-Cells Into Pseudoislets: Insulin Secretion and Gene Expression Profile in Normoxic and Hypoxic Milieu. Cell. Med. 2016, 8, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Guo-Parke, H.; McCluskey, J.T.; Kelly, C.; Hamid, M.; McClenaghan, N.H.; Flatt, P.R. Configuration of electrofusion-derived human insulin-secreting cell line as pseudoislets enhances functionality and therapeutic utility. J. Endocrinol. 2012, 214, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.J.; Hodgkin, M.N.; Squires, P.E. E-cadherin and cell adhesion: A role in architecture and function in the pancreatic islet. Cell. Physiol. Biochem. 2007, 20, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Ravier, M.A.; Güldenagel, M.; Charollais, A.; Gjinovci, A.; Caille, D.; Söhl, G.; Wollheim, C.B.; Willecke, K.; Henquin, J.C.; Meda, P. Loss of connexin36 channels alters β-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005, 54, 1798–1807. [Google Scholar] [CrossRef] [Green Version]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015, 466, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.R. Metabolic signaling in fuel-induced insulin secretion. Cell. Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef] [Green Version]
- Maechler, P.; Wollheim, C.B. Mitochondrial function in normal and diabetic β-cells. Nature 2001, 414, 807–812. [Google Scholar] [CrossRef]
- Minami, K.; Yano, H.; Miki, T.; Nagashima, K.; Wang, C.Z.; Tanaka, H.; Miyazaki, J.I.; Seino, S. Insulin secretion and differential gene expression in glucose-responsive and -unresponsive MIN6 sublines. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E773–E781. [Google Scholar] [CrossRef]
- Malmgren, S.; Nicholls, D.G.; Taneera, J.; Bacos, K.; Koeck, T.; Tamaddon, A.; Wibom, R.; Groop, L.; Ling, C.; Mulder, H.; et al. Tight coupling between glucose and mitochondrial metabolism in clonal β-cells is required for robust insulin secretion. J. Biol. Chem. 2009, 284, 32395–32404. [Google Scholar] [CrossRef] [Green Version]
- Patanè, G.; Anello, M.; Piro, S.; Vigneri, R.; Purrello, F.; Rabuazzo, A.M. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes 2002, 51, 2749–2756. [Google Scholar] [CrossRef] [Green Version]
- Piro, S.; Rabuazzo, A.M.; Renis, M.; Purrello, F. Effects of metformin on oxidative stress, adenine nucleotides balance, and glucose-induced insulin release impaired by chronic free fatty acids exposure in rat pancreatic islets. J. Endocrinol. Investig. 2012, 35, 504–510. [Google Scholar]
- Chowdhury, A.; Satagopam, V.P.; Manukyan, L.; Artemenko, K.A.; Fung, Y.M.; Schneider, R.; Bergquist, J.; Bergsten, P. Signaling in insulin-secreting MIN6 pseudoislets and monolayer cells. J. Proteome Res. 2013, 12, 5954–5962. [Google Scholar] [CrossRef]
- Arden, C.; Trainer, A.; de la Iglesia, N.; Scougall, K.T.; Gloyn, A.L.; Lange, A.J.; Shaw, J.A.; Matschinsky, F.M.; Agius, L. Cell biology assessment of glucokinase mutations V62M and G72R in pancreatic β-cells: Evidence for cellular instability of catalytic activity. Diabetes 2007, 56, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.C.; Zhao, M.; Jones, P.; Persaud, S.; Ramracheya, R.; Löbner, K.; Christie, M.R.; Banga, J.P.; Peakman, M.; Sirinivsan, P.; et al. The development of new density gradient media for purifying human islets and islet-quality assessments. Transplant 2004, 77, 143–145. [Google Scholar] [CrossRef]
- Ishihara, H.; Asano, T.; Tsukuda, K.; Katagiri, H.; Inukai, K.; Anai, M.; Kikuchi, M.; Yazaki, Y.; Miyazaki, J.I.; Oka, Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993, 36, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, Vol. 4; Verlag Chemie: Weinheim, Germany, 1974; pp. 1–1088. [Google Scholar]
- Plitzko, B.; Loesgen, S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio-Protocol 2018, 8, e2850. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Brand, M.D. Measurement and Analysis of Extracellular Acid Production to Determine Glycolytic Rate. J. Vis. Exp. 2015, 106, e53464. [Google Scholar] [CrossRef] [Green Version]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Arden, C.; Petrie, J.L.; Tudhope, S.J.; Al-Oanzi, Z.; Claydon, A.J.; Beynon, R.J.; Towle, H.C.; Agius, L. Elevated glucose represses liver glucokinase and induces its regulatory protein to safeguard hepatic phosphate homeostasis. Diabetes 2011, 60, 3110–3120. [Google Scholar] [CrossRef] [Green Version]
- O’Driscoll, L.; Gammell, P.; McKiernan, E.; Ryan, E.; Jeppesen, P.B.; Rani, S.; Clynes, M. Phenotypic and global gene expression profile changes between low passage and high passage MIN-6 cells. J. Endocrinol. 2006, 191, 665–676. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 2018, 293, 12649–12652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Windt, G.J.W.; Chang, C.H.; Pearce, E.L. Measuring bioenergetics in T cells using a Seahorse Extracellular Flux Analyser. Curr. Protoc. Immunol. 2016, 113, 3.16B.1–3.16B.14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildyard, J.C.; Ammala, C.; Dukes, I.D.; Thomson, S.A.; Halestrap, A.P. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim. Biophys. Acta. 2005, 1707, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilderink, J.; Spijker, S.; Carlotti, F.; Lange, L.; Engelse, M.; van Blitterswijk, C.; de Koning, E.; Karperien, M.; van Apeldoorn, A. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J. Cell. Mol. Med. 2015, 19, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Lock, L.T.; Laychock, S.G.; Tzanakakis, E.S. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J. Biotechnol. 2011, 151, 278–286. [Google Scholar] [CrossRef]
- Head, W.S.; Orseth, M.L.; Nunemaker, C.S.; Satin, L.S.; Piston, D.W.; Benninger, R.K. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 2012, 61, 1700–1707. [Google Scholar] [CrossRef] [Green Version]
- Pullen, T.J.; Khan, A.M.; Barton, G.; Butcher, S.A.; Sun, G.; Rutter, G.A. Identification of genes selectively disallowed in the pancreatic islet. Islets 2010, 2, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Cantley, J.; Walters, S.N.; Jung, M.H.; Weinberg, A.; Cowley, M.J.; Whitworth, T.P.; Kaplan, W.; Hawthorne, W.J.; O’Connell, P.J.; Weir, G.; et al. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant. 2013, 22, 2147–2159. [Google Scholar] [CrossRef]
- Wang, N.; Khan, S.A.; Prabhakar, N.R.; Nanduri, J. Impairment of pancreatic β-cell function by chronic intermittent hypoxia. Exp. Physiol. 2013, 98, 1376–1385. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornell, D.; Miwa, S.; Georgiou, M.; Anderson, S.J.; Honkanen-Scott, M.; Shaw, J.A.M.; Arden, C. Pseudoislet Aggregation of Pancreatic β-Cells Improves Glucose Stimulated Insulin Secretion by Altering Glucose Metabolism and Increasing ATP Production. Cells 2022, 11, 2330. https://doi.org/10.3390/cells11152330
Cornell D, Miwa S, Georgiou M, Anderson SJ, Honkanen-Scott M, Shaw JAM, Arden C. Pseudoislet Aggregation of Pancreatic β-Cells Improves Glucose Stimulated Insulin Secretion by Altering Glucose Metabolism and Increasing ATP Production. Cells. 2022; 11(15):2330. https://doi.org/10.3390/cells11152330
Chicago/Turabian StyleCornell, Deborah, Satomi Miwa, Merilin Georgiou, Scott James Anderson, Minna Honkanen-Scott, James A. M. Shaw, and Catherine Arden. 2022. "Pseudoislet Aggregation of Pancreatic β-Cells Improves Glucose Stimulated Insulin Secretion by Altering Glucose Metabolism and Increasing ATP Production" Cells 11, no. 15: 2330. https://doi.org/10.3390/cells11152330
APA StyleCornell, D., Miwa, S., Georgiou, M., Anderson, S. J., Honkanen-Scott, M., Shaw, J. A. M., & Arden, C. (2022). Pseudoislet Aggregation of Pancreatic β-Cells Improves Glucose Stimulated Insulin Secretion by Altering Glucose Metabolism and Increasing ATP Production. Cells, 11(15), 2330. https://doi.org/10.3390/cells11152330





