Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account
Abstract
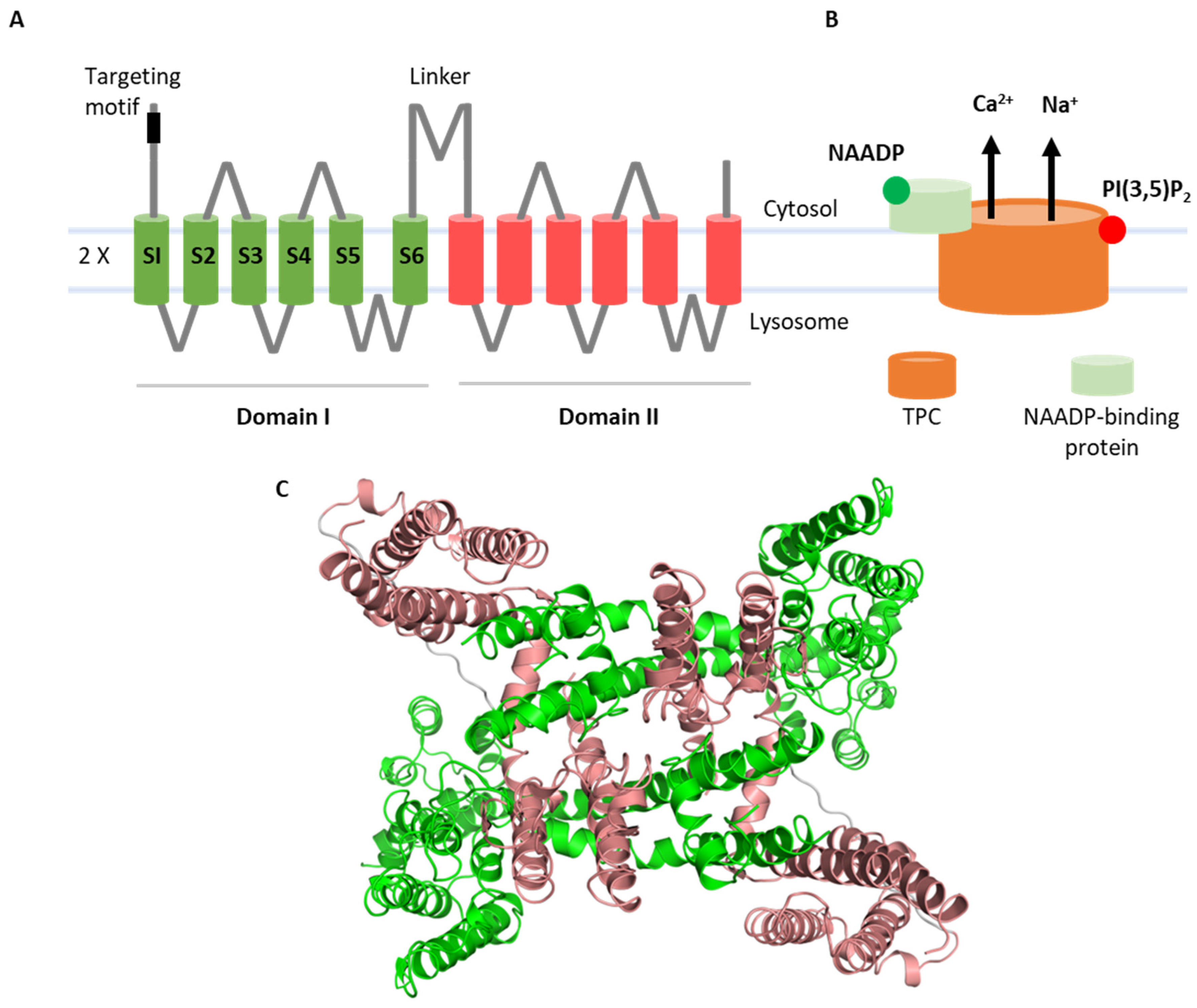
:1. Introduction
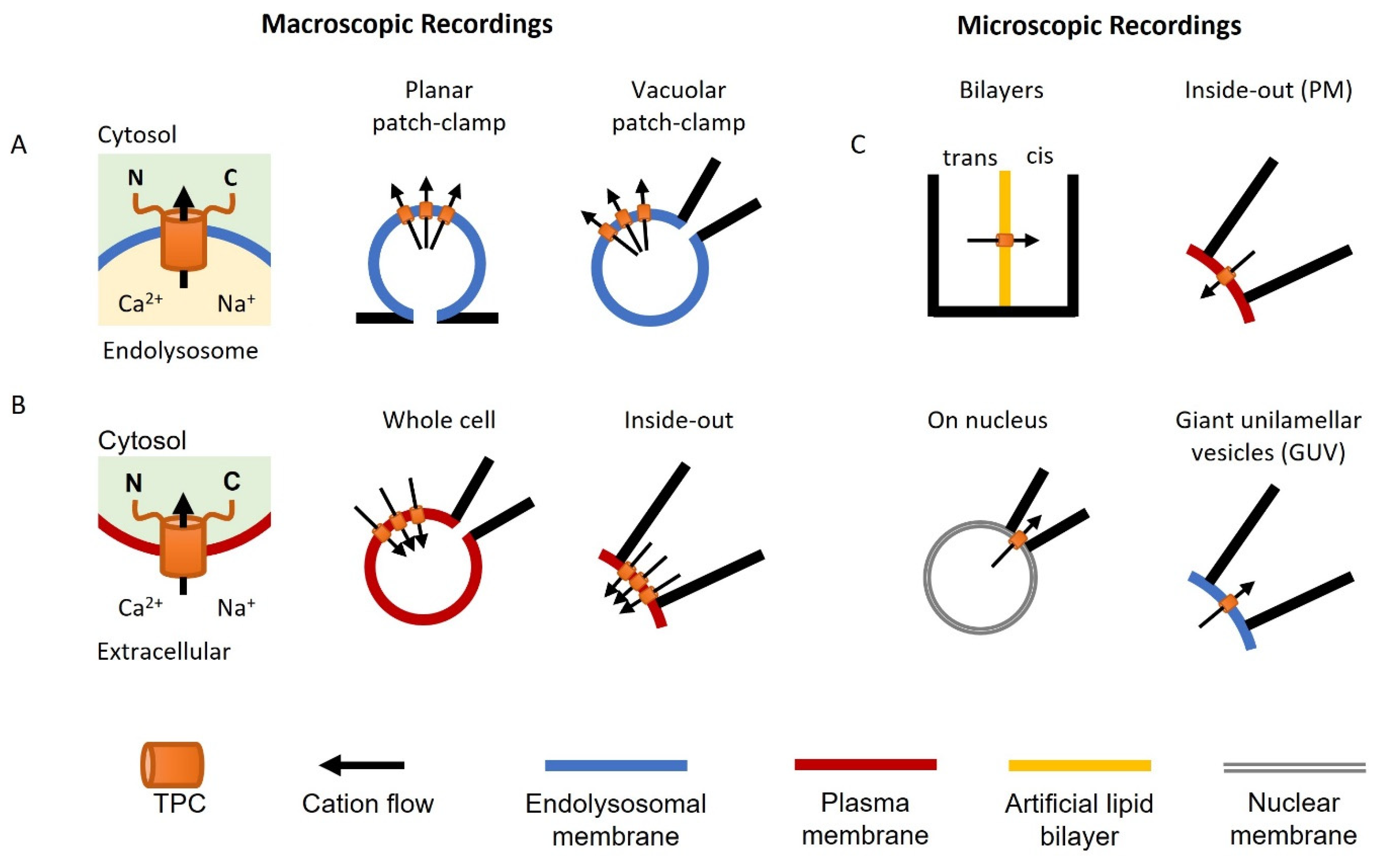
2. Methodology
2.1. Macroscopic Recordings
2.2. Microscopic (Single-Channel) Recordings
3. Lysosomal Patch-Clamp Analyses of TPCs
3.1. Regulation of TPCs
3.2. Pharmacology of TPCs
3.3. TPC Variants
3.4. Endogenous TPC Activity
4. Conventional Patch-Clamp Analyses of TPCs
5. Single-Channel Analyses of TPCs
6. Conflict Resolution: On the Identification of TPC2 Agonists and NAADP-Binding Proteins
7. Outlook
Funding
Conflicts of Interest
References
- Patel, S. Function and dysfunction of two-pore channels. Sci. Signal. 2015, 8, re7. [Google Scholar] [CrossRef]
- Rahman, T.; Cai, X.; Brailoiu, G.C.; Abood, M.E.; Brailoiu, E.; Patel, S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014, 7, ra109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, G.J.; Hirata, H.; Fimia, G.M.; do Carmo, L.G.; Bincoletto, C.; Han, S.W.; Stilhano, R.S.; Ureshino, R.P.; Bloor-Young, D.; Churchill, G.; et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011, 286, 27875–27881. [Google Scholar] [CrossRef] [Green Version]
- Grimm, C.; Holdt, L.M.; Chen, C.C.; Hassan, S.; Muller, C.; Jors, S.; Cuny, H.; Kissing, S.; Schroder, B.; Butz, E.; et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kolokolstov, A.A.; Chen, C.C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Lin-Moshier, Y.; Keebler, M.V.; Hooper, R.; Boulware, M.J.; Liu, X.; Churamani, D.; Abood, M.E.; Walseth, T.F.; Brailoiu, E.; Patel, S.; et al. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. USA 2014, 111, 13087–13092. [Google Scholar] [CrossRef] [Green Version]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del, B.D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4706–E4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, B.S.; Eden, E.R.; Hockey, L.N.; Yates, E.; Futter, C.E.; Patel, S. An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 2017, 18, 1636–1645. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.C.; Morgan, A.J.; Galione, A. NAADP-regulated two-pore channels drive phagocytosis through endo-lysosomal Ca(2+) nanodomains, calcineurin and dynamin. EMBO J. 2020, 39, e104058. [Google Scholar] [CrossRef]
- Marchant, J.S.; Patel, S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans. 2015, 43, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Vassileva, K.; Marsh, M.; Patel, S. Two-pore channels as master regulators of membrane trafficking and endocytic well-being. Curr. Opin. Physiol. 2020, 17, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Foote, K.; Kunuthur, S.; Gosain, R.; Tan, N.; Tyser, R.; Zhao, Y.J.; Graeff, R.; Ganesan, A.; Duchen, M.R.; et al. Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc. Res. 2015, 108, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, O.N.; Grimm, C.; Schneider, L.S.; Chao, Y.K.; Atzberger, C.; Bartel, K.; Watermann, A.; Ulrich, M.; Mayr, D.; Wahl-Schott, C.; et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017, 77, 1427–1438. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Kilpatrick, B.S. Two-pore channels and disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1678–1686. [Google Scholar] [CrossRef]
- Chen, C.C.; Krogsaeter, E.; Kuo, C.Y.; Huang, M.C.; Chang, S.Y.; Biel, M. Endolysosomal cation channels point the way towards precision medicine of cancer and infectious diseases. Biomed. Pharmacother. 2022, 148, 112751. [Google Scholar] [CrossRef] [PubMed]
- Penny, C.J.; Vassileva, K.; Jha, A.; Yuan, Y.; Chee, X.; Yates, E.; Mazzon, M.; Kilpatrick, B.S.; Muallem, S.; Marsh, M.; et al. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Li, P.; Calvo, R.; Southall, N.; Hu, X.; Bryant-Genevier, M.; Feng, X.; Geng, Q.; Gao, C.; et al. Agonist-specific voltage-dependent gating of lysosomal two-pore Na(+) channels. eLife 2019, 8, e51423. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Schieder, M.; Cuny, H.; Fenske, S.; Gruner, C.; Rotzer, K.; Griesbeck, O.; Harz, H.; Biel, M.; Wahl-Schott, C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009, 458, 891–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brailoiu, E.; Churamani, D.; Cai, X.; Schrlau, M.G.; Brailoiu, G.C.; Gao, X.; Hooper, R.; Boulware, M.J.; Dun, N.J.; Marchant, J.S.; et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009, 186, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Aarhus, R.; Graeff, R.M.; Dickey, D.M.; Walseth, T.F.; Lee, H.C. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP+. J. Biol. Chem. 1995, 270, 30327–30333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchill, G.C.; Okada, Y.; Thomas, J.M.; Genazzani, A.A.; Patel, S.; Galione, A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 2002, 111, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, E.; Patel, S.; Dun, N.J. Modulation of spontaneous transmitter release from the frog neuromuscular junction by interacting intracellular Ca2+ stores: Critical role for nicotinic acid-adenine dinucleotide phosphate (NAADP). Biochem. J. 2003, 373, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Hoard, J.L.; Filipeanu, C.M.; Brailoiu, G.C.; Dun, S.L.; Patel, S.; Dun, N.J. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J. Biol. Chem. 2005, 280, 5646–5650. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, E.; Churamani, D.; Pandey, V.; Brailoiu, G.C.; Tuluc, F.; Patel, S.; Dun, N.J. Messenger-specific role for NAADP in neuronal differentiation. J. Biol. Chem. 2006, 281, 15923–15928. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, G.C.; Brailoiu, E.; Parkesh, R.; Galione, A.; Churchill, G.C.; Patel, S.; Dun, N.J. NAADP-mediated channel “chatter” in neurons of the rat medulla oblongata. Biochem. J. 2009, 419, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieder, M.; Rotzer, K.; Bruggemann, A.; Biel, M.; Wahl-Schott, C.A. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 2010, 285, 21219–21222. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, E.; Rahman, T.; Churamani, D.; Prole, D.L.; Brailoiu, G.C.; Hooper, R.; Taylor, C.W.; Patel, S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010, 285, 38511–38516. [Google Scholar] [CrossRef] [Green Version]
- Pitt, S.J.; Funnell, T.; Sitsapesan, M.; Venturi, E.; Rietdorf, K.; Ruas, M.; Ganesan, A.; Gosain, R.; Churchill, G.C.; Zhu, M.X.; et al. TPC2 is a novel NAADP-sensitive Ca2+-release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem. 2010, 285, 24925–24932. [Google Scholar] [CrossRef] [Green Version]
- Lin-Moshier, Y.; Walseth, T.F.; Churamani, D.; Davidson, S.M.; Slama, J.T.; Hooper, R.; Brailoiu, E.; Patel, S.; Marchant, J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012, 287, 2296–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walseth, T.F.; Lin-Moshier, Y.; Weber, K.; Marchant, J.S.; Slama, J.T.; Guse, A.H. Nicotinic Acid Adenine Dinucleotide 2′-Phosphate (NAADP) Binding Proteins in T-Lymphocytes. Messenger 2012, 1, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, G.S.; Brailoiu, E.; He, S.; Unterwald, E.M.; Patel, S.; Slama, J.T.; Walseth, T.F.; Marchant, J.S. Essential requirement for JPT2 in NAADP-evoked Ca(2+) signaling. Sci. Signal. 2021, 14, eabd5605. [Google Scholar] [CrossRef] [PubMed]
- Roggenkamp, H.G.; Khansahib, I.; Hernandez, C.L.; Zhang, Y.; Lodygin, D.; Krüger, A.; Gu, F.; Möckl, F.; Löhndorf, A.; Wolters, V.; et al. HN1L/JPT2: A signaling protein that connects NAADP generation to Ca(2+) microdomain formation. Sci. Signal. 2021, 14, eabd5647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, X.; Shah, K.; Yan, J. Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat. Commun. 2021, 12, 4739. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gunaratne, G.S.; Marchant, J.S.; Biggin, P.C.; Rahman, T. NAADP receptors: A one-two. Cell Calcium. 2021, 100, 102478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Dong, X.P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, N.; Lang, M.J.; Weisman, L.S. Phosphatidylinositol 3,5-bisphosphate: Regulation of cellular events in space and time. Biochem. Soc. Trans. 2016, 44, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, J.; Strunk, B.S.; Weisman, L.S. PI5P and PI(3,5)P(2): Minor, but Essential Phosphoinositides. Cell Struct. Funct. 2017, 42, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, J.; Guo, J.; Chen, Q.; Zeng, W.; Jiang, Y.; Bai, X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 2018, 556, 130–134. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Zeng, W.; Guo, J.; Chen, Q.; Bai, X.C.; Jiang, Y. Structural mechanisms of phospholipid activation of the human TPC2 channel. eLife 2019, 8, e45222. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Chen, C.C.; Chao, Y.K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. eLife 2020, 9, e54712. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Krogsaeter, E.; Patel, S.; Bracher, F.; Grimm, C. Discovery of lipophilic two-pore channel agonists. FEBS J. 2020, 287, 5284–5293. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Minicozzi, V.; Boccaccio, A.; Lagostena, L.; Gradogna, A.; Qi, T.; Costa, A.; Larisch, N.; Hamamoto, S.; Pedrazzini, E.; et al. Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters. Cells 2022, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Butz, E.S.; Chao, Y.K.; Grishchuk, Y.; Becker, L.; Heller, S.; Slaugenhaupt, S.A.; Biel, M.; Wahl-Schott, C.; Grimm, C. Small Molecules for Early Endosome-Specific Patch Clamping. Cell Chem. Biol. 2017, 24, 907–916.e904. [Google Scholar] [CrossRef] [Green Version]
- Schieder, M.; Rotzer, K.; Bruggemann, A.; Biel, M.; Wahl-Schott, C. Planar patch clamp approach to characterize ionic currents from intact lysosomes. Sci. Signal. 2010, 3, l3. [Google Scholar] [CrossRef]
- Chen, C.C.; Cang, C.; Fenske, S.; Butz, E.; Chao, Y.K.; Biel, M.; Ren, D.; Wahl-Schott, C.; Grimm, C. Patch-clamp technique to characterize ion channels in enlarged individual endolysosomes. Nat. Protoc. 2017, 12, 1639–1658. [Google Scholar] [CrossRef]
- Boccaccio, A.; Scholz-Starke, J.; Hamamoto, S.; Larisch, N.; Festa, M.; Gutla, P.V.; Costa, A.; Dietrich, P.; Uozumi, N.; Carpaneto, A. The phosphoinositide PI(3,5)P(2) mediates activation of mammalian but not plant TPC proteins: Functional expression of endolysosomal channels in yeast and plant cells. Cell Mol. Life Sci. 2014, 71, 4275–4283. [Google Scholar] [CrossRef]
- Zakharian, E. Recording of ion channel activity in planar lipid bilayer experiments. Methods Mol. Biol. 2013, 998, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybalchenko, V.; Ahuja, M.; Coblentz, J.; Churamani, D.; Patel, S.; Kiselyov, K.; Muallem, S. Membrane potential regulates NAADP dependence of the pH and Ca2+ sensitive organellar two-pore channel TPC1. J. Biol. Chem. 2012, 287, 20407–20416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Tong, B.C.; Cheng, C.W.; Hung, H.C.; Cheung, K.H. Characterization of Two-Pore Channel 2 by Nuclear Membrane Electrophysiology. Sci. Rep. 2016, 6, 20282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapovalov, G.; Ritaine, A.; Bidaux, G.; Slomianny, C.; Borowiec, A.S.; Gordienko, D.; Bultynck, G.; Skryma, R.; Prevarskaya, N. Organelle membrane derived patches: Reshaping classical methods for new targets. Sci. Rep. 2017, 7, 14082. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.P.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cang, C.; Bekele, B.; Ren, D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014, 10, 463–469. [Google Scholar] [CrossRef]
- Jha, A.; Ahuja, M.; Patel, S.; Brailoiu, E.; Muallem, S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P(2) and multiple protein kinases. EMBO J. 2014, 33, 501–511. [Google Scholar] [CrossRef]
- Kirsch, S.A.; Kugemann, A.; Carpaneto, A.; Bockmann, R.A.; Dietrich, P. Phosphatidylinositol-3,5-bisphosphate lipid-binding-induced activation of the human two-pore channel 2. Cell Mol. Life Sci. 2018, 75, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Lagostena, L.; Festa, M.; Pusch, M.; Carpaneto, A. The human two-pore channel 1 is modulated by cytosolic and luminal calcium. Sci. Rep. 2017, 7, 43900. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; Gfeller, A.; Proebsting, W.M.; Hortensteiner, S.; Chetelat, A.; Martinoia, E.; Farmer, E.E. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007, 49, 889–898. [Google Scholar] [CrossRef]
- Beyhl, D.; Hörtensteiner, S.; Martinoia, E.; Farmer, E.E.; Fromm, J.; Marten, I.; Hedrich, R. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009, 58, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Penny, C.J.; Rahman, T. Two-pore Channels Enter the Atomic Era. Structure of plant TPC revealed. Trends Biochem. Sci. 2016, 41, 475–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genazzani, A.A.; Empson, R.M.; Galione, A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem. 1996, 271, 1159911602. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Gerndt, S.; Chao, Y.K.; Zisis, T.; Nguyen, O.N.P.; Gerwien, A.; Urban, N.; Müller, C.; Gegenfurtner, F.A.; Geisslinger, F.; et al. Gene editing and synthetically accessible inhibitors reveal role for TPC2 in HCC cell proliferation and tumor growth. Cell Chem. Biol. 2021, 28, 1119–1131.e1127. [Google Scholar] [CrossRef] [PubMed]
- Pafumi, I.; Festa, M.; Papacci, F.; Lagostena, L.; Giunta, C.; Gutla, V.; Cornara, L.; Favia, A.; Palombi, F.; Gambale, F.; et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017, 7, 5121. [Google Scholar] [CrossRef] [PubMed]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.C.; Rosato, A.S.; Beyers, W.; Chao, Y.K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids increase melanin production and reduce proliferation, migration and invasion of melanoma cells by blocking endolysosomal/melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef]
- Sulem, P.; Gudbjartsson, D.F.; Stacey, S.N.; Helgason, A.; Rafnar, T.; Jakobsdottir, M.; Steinberg, S.; Gudjonsson, S.A.; Palsson, A.; Thorleifsson, G.; et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat. Genet. 2008, 40, 835–837. [Google Scholar] [CrossRef]
- Chao, Y.K.; Schludi, V.; Chen, C.C.; Butz, E.; Nguyen, O.N.P.; Muller, M.; Kruger, J.; Kammerbauer, C.; Ben-Johny, M.; Vollmar, A.M.; et al. TPC2 polymorphisms associated with a hair pigmentation phenotype in humans result in gain of channel function by independent mechanisms. Proc. Natl. Acad. Sci. USA 2017, 114, E8595–E8602. [Google Scholar] [CrossRef] [Green Version]
- Böck, J.; Krogsaeter, E.; Passon, M.; Chao, Y.K.; Sharma, S.; Grallert, H.; Peters, A.; Grimm, C. Human genome diversity data reveal that L564P is the predominant TPC2 variant and a prerequisite for the blond hair associated M484L gain-of-function effect. PLoS Genet. 2021, 17, e1009236. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Davis, L.C.; Chen, C.C.; Morgan, A.J.; Chuang, K.T.; Walseth, T.F.; Grimm, C.; Garnham, C.; Powell, T.; Platt, N.; et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015, 34, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Escobar, I.E.; Oancea, E. A melanosomal two-pore sodium channel regulates pigmentation. Sci. Rep. 2016, 6, 26570. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schön, C.; Chen, C.C.; Yang, Z.; Liegl, R.; Murenu, E.; Schworm, B.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; et al. TPC2 promotes choroidal angiogenesis and inflammation in a mouse model of neovascular age-related macular degeneration. Life Sci. Alliance 2021, 4, e202101047. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Jiang, Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. USA 2017, 114, 1009–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S. Two-pore channels open up. Nature 2018, 556, 38–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S.; Jha, Y.; Li, Q.; Soyombo, A.A.; Dickinson, G.D.; Churamani, D.; Brailoiu, E.; Patel, S.; Muallem, S. Transient Receptor Potential Mucolipin 1 (TRPML1) and Two-pore Channels are Functionally Independent Organellar Ion Channels. J. Biol. Chem. 2011, 286, 22934–22942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, S.J.; Lam, A.K.; Rietdorf, K.; Galione, A.; Sitsapesan, R. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+. Sci. Signal. 2014, 7, ra46. [Google Scholar] [CrossRef]
- Milenkovic, S.; Bodrenko, I.V.; Lagostena, L.; Gradogna, A.; Serra, G.; Bosin, A.; Carpaneto, A.; Ceccarelli, M. The mechanism and energetics of a ligand-controlled hydrophobic gate in a mammalian two pore channel. Phys. Chem. Chem. Phys. 2020, 22, 15664–15674. [Google Scholar] [CrossRef]
- Cancela, J.M.; Churchill, G.C.; Galione, A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 1999, 398, 74–76. [Google Scholar] [CrossRef]
- Berg, I.; Potter, V.L.; Mayr, G.W.; Guse, A.H. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+ signaling. J. Cell Biol. 2000, 150, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunbayo, O.A.; Duan, J.; Xiong, J.; Wang, Q.; Feng, X.; Ma, J.; Zhu, M.X.; Evans, A.M. mTORC1 controls lysosomal Ca(2+) release through the two-pore channel TPC2. Sci. Signal. 2018, 11, eaao5775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walseth, T.F.; Lin-Moshier, Y.; Jain, P.; Ruas, M.; Parrington, J.; Galione, A.; Marchant, J.S.; Slama, J.T. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) proteins in sea urchin egg. J. Biol. Chem. 2012, 287, 2308–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brailoiu, E.; Hooper, R.; Cai, X.; Brailoiu, G.C.; Keebler, M.V.; Dun, N.J.; Marchant, J.S.; Patel, S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 2010, 285, 2897–2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cang, C.; Aranda, K.; Ren, D. A non-inactivating high-voltage-activated two-pore Na(+) channel that supports ultra-long action potentials and membrane bistability. Nat. Commun. 2014, 5, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimomura, T.; Kubo, Y. Phosphoinositides modulate the voltage dependence of two-pore channel 3. J. Gen. Physiol. 2019, 151, 986–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirazawa, K.; Tateyama, M.; Kubo, Y.; Shimomura, T. Phosphoinositide regulates dynamic movement of the S4 voltage sensor in the second repeat in two-pore channel 3. J. Biol. Chem. 2021, 297, 101425. [Google Scholar] [CrossRef]
- Cai, X.; Patel, S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010, 27, 2352–2359. [Google Scholar] [CrossRef] [Green Version]
| Isoform | Cell Type | Activator | Validation Method | Reference |
|---|---|---|---|---|
| HsaTPC1 | HEK cells | NAADP | siRNA, dominant negative | [51] |
| MmuTPC2 | Macrophages | PI(3,5)P2 | Double knockout mouse | [37] |
| MmuTPC1 | Macrophages | PI(3,5)P2 | Knockout mouse | [54] |
| MmuTPC2 | Macrophages | PI(3,5)P2 | Knockout mouse | [54] |
| MmuTPC1/2 | Cardiomyocytes | PI(3,5)P2 | ATP block | [54] |
| Hepatocytes | ||||
| Fibroblasts | ||||
| MmuTPC1 | Cardiomyocytes | PI(3,5)P2 | Knockout mouse | [56] |
| MmuTPC2 | MEFs | PI(3,5)P2 | Knockout mouse | [4] |
| MmuTPC2 | MEFs | NAADP | Knockout mouse | [72] |
| MmuTPC2 | Melanocytes 1 | PI(3,5)P2 | CRISPR/Cas9 knockout | [73] |
| LcaTPC2 | RPE cells | PI(3,5)P2 | [73] | |
| HsaTPC2 | HEK cells | NAADP | [53] | |
| HsaTPC2 | Adult fibroblasts | PI(3,5)P2 | WT vs. M484L | [70] |
| HsaTPC2 | T24 cells | PI(3,5)P2 | Tetrandrine block | [14] |
| HsaTPC1/2 | HAP1 cells | Clomipramine | CRISPR/Cas9 knockout | [18] |
| MmuTPC2 | Macrophages | TPC2-A1-N/P | Knockout mouse | [43] |
| MmuTPC2 | RIL175 cells | PI(3,5)P2 | CRISPR/CAS9 knockout | [66] |
| MmuTPC2 | Macrophages | PI(3,5)P2 | Knockout mouse | [74] |
| Microglia | ||||
| HsaTPC2 | Adult fibroblasts | PI(3,5)P2 | WT vs. K376R/G387D | [71] |
| Isoform | Ca2+ | Ba2+ | Na+ | K+ | Cs+ | Reference |
|---|---|---|---|---|---|---|
| HsaTPC1 | 50/200 pS | [51] | ||||
| HsaTPC1 | 19 pS | 68 pS | 87 pS | [78] | ||
| HsaTPC1 1 | 5.5 pS | [79] | ||||
| HsaTPC2 | 15 pS | 300 pS | [30] | |||
| HsaTPC2 | 40 pS | 128 pS | [29] | |||
| HsaTPC2 | 100 pS | [77] | ||||
| HsaTPC2 | 208 pS | 78 pS | [52] | |||
| HsaTPC2 2 | 207 pS | [53] | ||||
| HsaTPC2 | 85 pS | [17] |
| Isoform | Sensitive? | NAADP PCa/PNa | PCa/PK | Sensitive? | PI(3,5)P2 PNa/PCa | PNa/PK | Reference |
|---|---|---|---|---|---|---|---|
| HsaTPC1 | √ | 2.2 1 | [51] | ||||
| HsaTPC1 | √ | 0.98 | 0.11 | [78] | |||
| MmuTPC1 | × | √ | 212.3 | 78.1 | [56] | ||
| HsaTPC1 | × | √ | 10–20 | 35.7 | [59] | ||
| MmuTPC1 | √ | 11.4 | 65.8 | [40] | |||
| MmuTPC2 | √ | >1000 | [28] | ||||
| HsaTPC2 | √ | 2.6 | [30] | ||||
| MmuTPC2 2 | √ | 0.7 | 340 | √ | [4] | ||
| MmuTPC2 2 | √ | 0.57, 0.86 | 286 | [72] | |||
| HsaTPC2 | × | √ | 10 | 33.3 | [37] | ||
| HsaTPC2 3 | × | √ | ~12 | ~11 | [49] | ||
| MmuTPC2 4 | √ | ≥100 | ≥50 | [73] | |||
| HsaTPC2 | × | √ | 16.8 | 23.8 | [75] | ||
| HsaTPC2 | √ | ~10 | [70] | ||||
| PCa/PNa = 0.76 ± 0.1 | PCa/PNa = 0.06 ± 0.02 | ||||||
| III | III | ||||||
| HsaTPC2 | √ | 0.73 | √ | 0.08 | [43] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Yuan, Y.; Chen, C.-C.; Jaślan, D.; Gunaratne, G.; Grimm, C.; Rahman, T.; Marchant, J.S. Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account. Cells 2022, 11, 2368. https://doi.org/10.3390/cells11152368
Patel S, Yuan Y, Chen C-C, Jaślan D, Gunaratne G, Grimm C, Rahman T, Marchant JS. Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account. Cells. 2022; 11(15):2368. https://doi.org/10.3390/cells11152368
Chicago/Turabian StylePatel, Sandip, Yu Yuan, Cheng-Chang Chen, Dawid Jaślan, Gihan Gunaratne, Christian Grimm, Taufiq Rahman, and Jonathan S. Marchant. 2022. "Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account" Cells 11, no. 15: 2368. https://doi.org/10.3390/cells11152368
APA StylePatel, S., Yuan, Y., Chen, C.-C., Jaślan, D., Gunaratne, G., Grimm, C., Rahman, T., & Marchant, J. S. (2022). Electrophysiology of Endolysosomal Two-Pore Channels: A Current Account. Cells, 11(15), 2368. https://doi.org/10.3390/cells11152368









