Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Cell Culture and Transfection
2.3. Recombinant-DNA Procedures
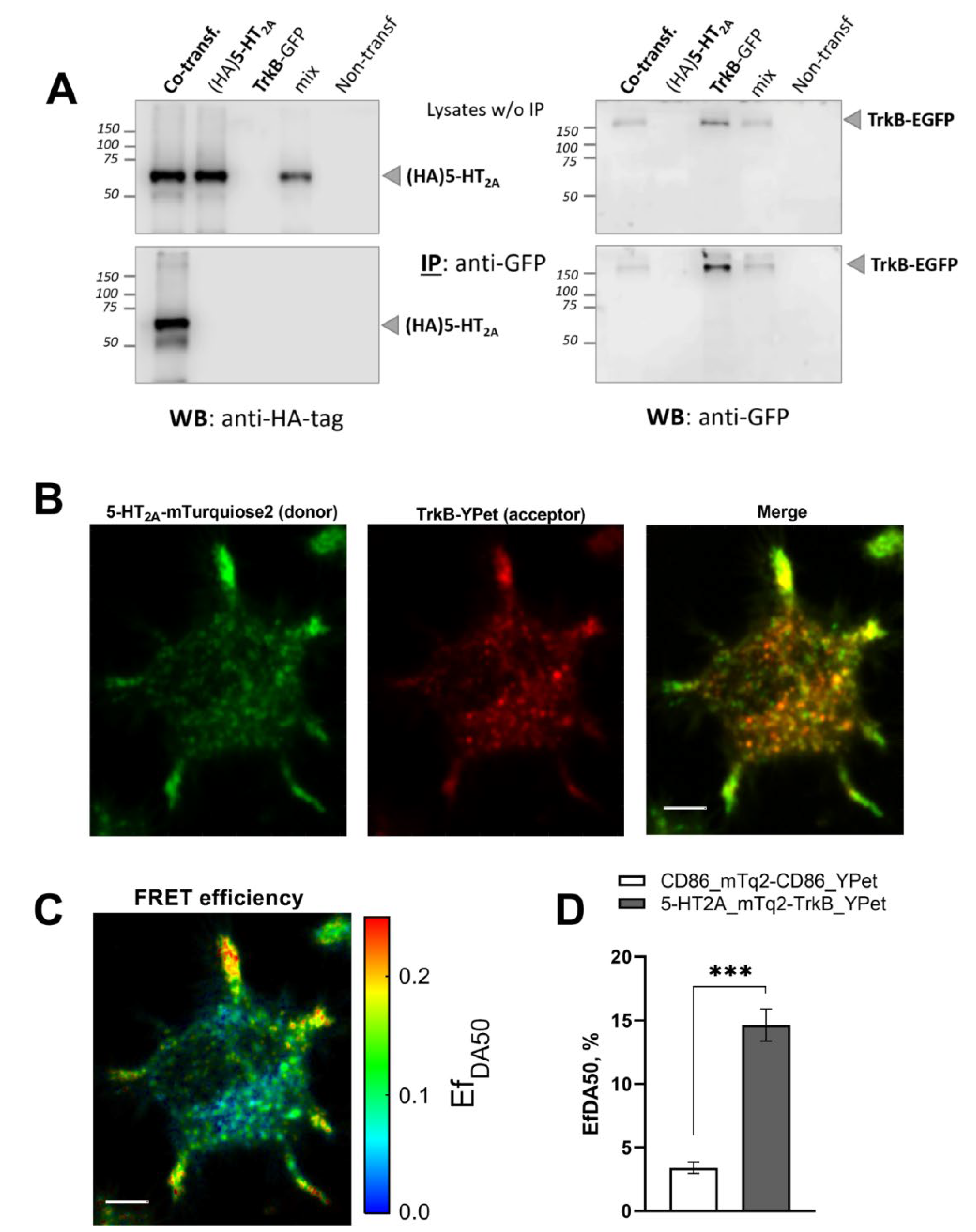
2.4. Co-Immunoprecipitation
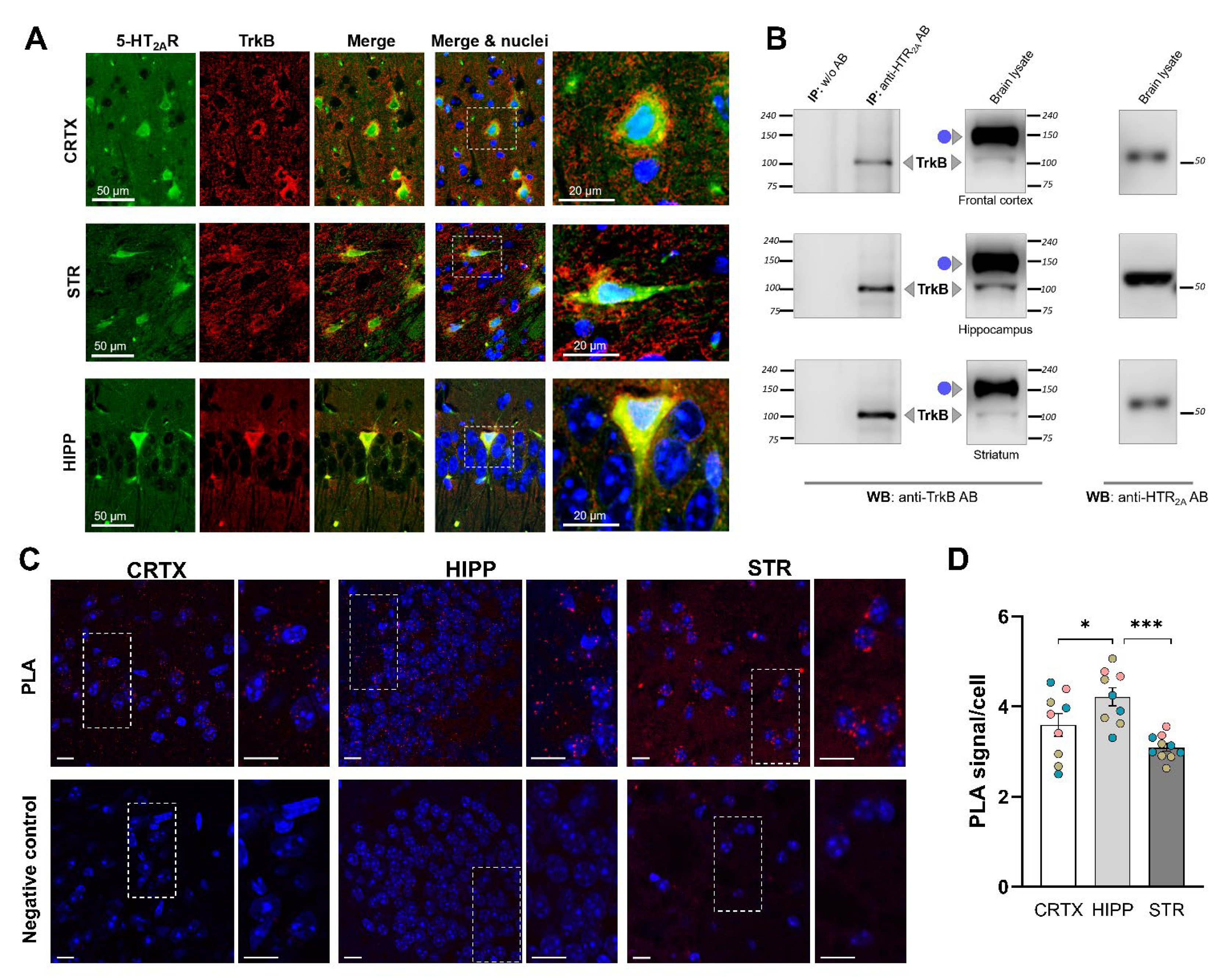
2.5. The Immunofluorescence Assay of Mouse Brain Sections
2.6. Linear Unmixing FRET Analysis
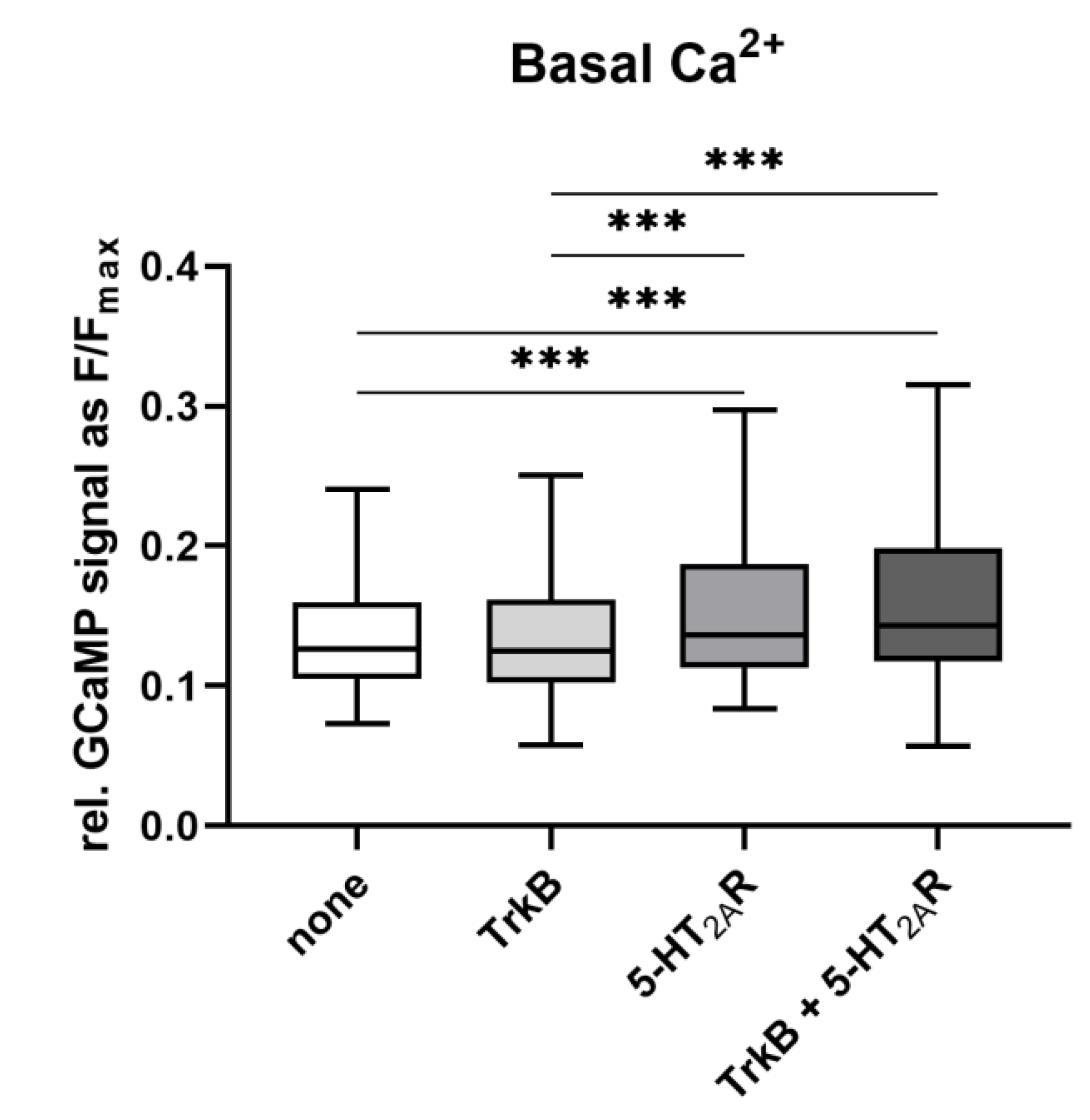
2.7. Analysis of Ca2+ Activity
2.8. In Situ Proximity Ligation Assay (PLA)
2.9. qRT-PCR
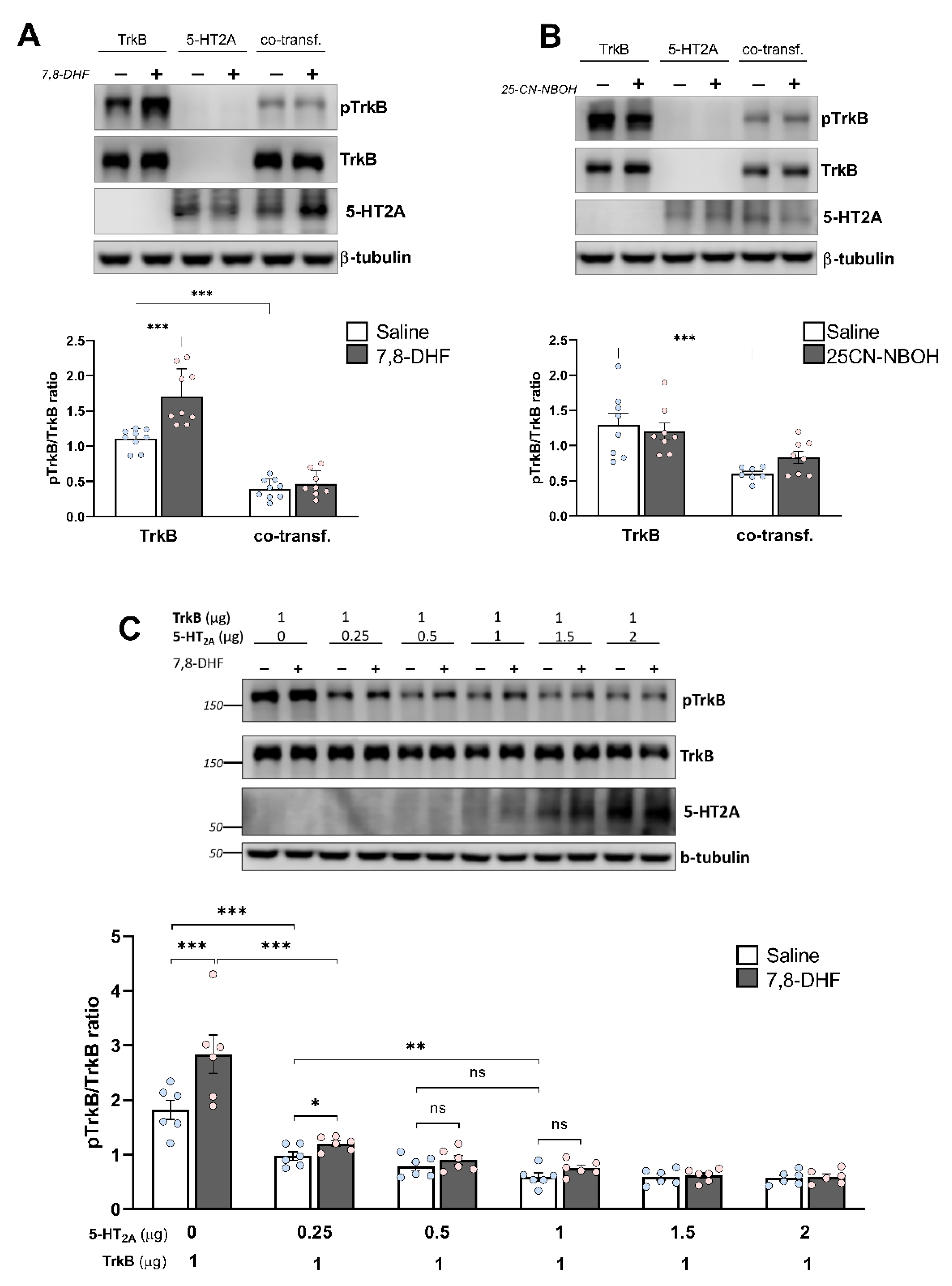
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. 5-HT2A and TrkB Receptors Form Heterodimers in Neuroblastoma N1E-115 Cells
3.2. Endogenous 5-HT2A and TrkB Receptors Form a Protein Complex in the Mouse Brain
3.3. Calcium Signaling Is Not Affected by the Heterodimerization
3.4. Heterodimerization Reduces TrkB Phosphorylation and Blunts the Response to 7,8-DHF
3.5. Expression Patterns of TrkB and 5-HT2A during Postnatal Development
3.6. Restoration of TrkB Phosphorylation by Ketanserin Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seroogy, K.B.; Lundgren, K.H.; Tran, T.M.; Guthrie, K.M.; Isackson, P.J.; Gall, C.M. Dopaminergic Neurons in Rat Ventral Midbrain Express Brain-Derived Neurotrophic Factor and Neurotrophin-3 MRNAs. J. Comp. Neurol. 1994, 342, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Hayashi, M. Expression of TrkB Subtypes in the Adult Monkey Cerebellar Cortex. J. Chem. Neuroanat. 2003, 25, 175–183. [Google Scholar] [CrossRef]
- Tang, S.; Machaalani, R.; Waters, K.A. Immunolocalization of Pro- and Mature-Brain Derived Neurotrophic Factor (BDNF) and Receptor TrkB in the Human Brainstem and Hippocampus. Brain Res. 2010, 1354, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF Signaling and Survival of Striatal Neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, G.-Y.; Kinney, C.E.; An, J.J.; Xu, B. TrkB-Expressing Neurons in the Dorsomedial Hypothalamus Are Necessary and Sufficient to Suppress Homeostatic Feeding. Proc. Natl. Acad. Sci. USA 2019, 116, 3256–3261. [Google Scholar] [CrossRef] [Green Version]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [Green Version]
- Minichiello, L. TrkB Signalling Pathways in LTP and Learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF Effects on Dendritic Spine Morphology and Hippocampal Function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Zagrebelsky, M.; Tacke, C.; Korte, M. BDNF Signaling during the Lifetime of Dendritic Spines. Cell Tissue Res. 2020, 382, 185–199. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A Key Transducer of Antidepressant Effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Rantamäki, T. TrkB Neurotrophin Receptor at the Core of Antidepressant Effects, but How? Cell Tissue Res. 2019, 377, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Pandey, S.C. Cellular Localization of Serotonin(2A) (5HT(2A)) Receptors in the Rat Brain. Brain Res. Bull. 2000, 51, 499–505. [Google Scholar] [CrossRef]
- Varnäs, K.; Halldin, C.; Hall, H. Autoradiographic Distribution of Serotonin Transporters and Receptor Subtypes in Human Brain. Hum. Brain Mapp. 2004, 22, 246–260. [Google Scholar] [CrossRef]
- Béïque, J.-C.; Imad, M.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of the 5-Hydroxytryptamine 2A Receptor-Mediated Facilitation of Synaptic Activity in Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9870–9875. [Google Scholar] [CrossRef] [Green Version]
- García-Oscos, F.; Torres-Ramírez, O.; Dinh, L.; Galindo-Charles, L.; Pérez Padilla, E.A.; Pineda, J.C.; Atzori, M.; Salgado, H. Activation of 5-HT Receptors Inhibits GABAergic Transmission by Pre- and Post-Synaptic Mechanisms in Layer II/III of the Juvenile Rat Auditory Cortex. Synapse 2015, 69, 115–127. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and Brain Function: A Tale of Two Receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [Green Version]
- Moravčíková, L.; Csatlósová, K.; Ďurišová, B.; Ondáčová, K.; Pavlovičová, M.; Lacinová, Ľ.; Dremencov, E. Role of Serotonin-2A Receptors in Pathophysiology and Treatment of Depression. Receptors 2018, 32, 205–230. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.; Amargós-Bosch, M.; Adell, A.; Artigas, F. The Therapeutic Role of 5-HT1A and 5-HT2A Receptors in Depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar]
- McIntyre, R.S.; Soczynska, J.K.; Woldeyohannes, H.O.; Alsuwaidan, M.; Konarski, J.Z. A Preclinical and Clinical Rationale for Quetiapine in Mood Syndromes. Expert Opin. Pharmacother. 2007, 8, 1211–1219. [Google Scholar] [CrossRef]
- Sanford, M.; Keating, G.M. Quetiapine: A Review of Its Use in the Management of Bipolar Depression. CNS Drugs 2012, 26, 435–460. [Google Scholar] [CrossRef]
- Mestre, T.A.; Zurowski, M.; Fox, S.H. 5-Hydroxytryptamine 2A Receptor Antagonists as Potential Treatment for Psychiatric Disorders. Expert Opin. Investig. Drugs 2013, 22, 411–421. [Google Scholar] [CrossRef]
- Wright, B.M.; Eiland, E.H., 3rd; Lorenz, R. Augmentation with Atypical Antipsychotics for Depression: A Review of Evidence-Based Support from the Medical Literature. Pharmacotherapy 2013, 33, 344–359. [Google Scholar] [CrossRef]
- de Miranda, A.S.; Moreira, F.A.; Teixeira, A.L. The Preclinical Discovery and Development of Quetiapine for the Treatment of Mania and Depression. Expert Opin. Drug Discov. 2017, 12, 525–535. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2A Receptor-Mediated Regulation of Brain-Derived Neurotrophic Factor MRNA in the Hippocampus and the Neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef]
- Jaggar, M.; Vaidya, V.A. 5-HT2A Receptors and BDNF Regulation: Implications for Psychopathology. In 5-HT2A Receptors in the Central Nervous System; Humana Press: Cham, Switzerland, 2018; Volume 32, ISBN 9783319704746. [Google Scholar]
- Popova, N.K.; Naumenko, V.S. Neuronal and Behavioral Plasticity: The Role of Serotonin and BDNF Systems Tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Li, X.; Tarakanov, A.O.; Savelli, D.; Narváez, M.; Shumilov, K.; Andrade-Talavera, Y.; Jimenez-Beristain, A.; Pomierny, B.; Díaz-Cabiale, Z.; et al. Existence of Brain 5-HT1A-5-HT2A Isoreceptor Complexes with Antagonistic Allosteric Receptor-Receptor Interactions Regulating 5-HT1A Receptor Recognition. ACS Omega 2017, 2, 4779–4789. [Google Scholar] [CrossRef]
- Lukasiewicz, S.; Polit, A.; Kędracka-Krok, S.; Wędzony, K.; Maćkowiak, M.; Dziedzicka-Wasylewska, M. Hetero-Dimerization of Serotonin 5-HT(2A) and Dopamine D(2) Receptors. Biochim. Biophys. Acta 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; Muguruza, C.; Umali, A.; Mortillo, S.; Holloway, T.; Pilar-Cuéllar, F.; Mocci, G.; Seto, J.; Callado, L.F.; Neve, R.L.; et al. Identification of Three Residues Essential for 5-Hydroxytryptamine 2A-Metabotropic Glutamate 2 (5-HT2A·mGlu2) Receptor Heteromerization and Its Psychoactive Behavioral Function. J. Biol. Chem. 2012, 287, 44301–44319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnenberg, S.B.; Rauer, J.; Göhr, C.; Gorinski, N.; Schade, S.K.; Abdel Galil, D.; Naumenko, V.; Zeug, A.; Bischoff, S.C.; Ponimaskin, E.; et al. The 5-HT(4) Receptor Interacts with Adhesion Molecule L1 to Modulate Morphogenic Signaling in Neurons. J. Cell Sci. 2021, 134, jcs249193. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.; Röhrs, K.-F.; Ackmann, J.; Varbanov, H.; Müller, F.E.; Jia, S.; Jahreis, K.; Vollbrecht, A.-L.; Butzlaff, M.; Schill, Y.; et al. Amelioration of Tau Pathology and Memory Deficits by Targeting 5-HT7 Receptor. Prog. Neurobiol. 2021, 197, 101900. [Google Scholar] [CrossRef] [PubMed]
- Bijata, M.; Labus, J.; Guseva, D.; Stawarski, M.; Butzlaff, M.; Dzwonek, J.; Schneeberg, J.; Böhm, K.; Michaluk, P.; Rusakov, D.A.; et al. Synaptic Remodeling Depends on Signaling between Serotonin Receptors and the Extracellular Matrix. Cell Rep. 2017, 19, 1767–1782. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A Selective TrkB Agonist with Potent Neurotrophic Activities by 7,8-Dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [Green Version]
- Halberstadt, A.L.; Sindhunata, I.S.; Scheffers, K.; Flynn, A.D.; Sharp, R.F.; Geyer, M.A.; Young, J.W. Effect of 5-HT2A and 5-HT2C Receptors on Temporal Discrimination by Mice. Neuropharmacology 2016, 107, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Meller, R.; Harrison, P.J.; Elliott, J.M.; Sharp, T. In Vitro Evidence That 5-Hydroxytryptamine Increases Efflux of Glial Glutamate via 5-HT(2A) Receptor Activation. J. Neurosci. Res. 2002, 67, 399–405. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of Serotonin Receptors 5-HT1A and 5-HT7 Differentially Regulates Receptor Signalling and Trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef] [Green Version]
- Wlodarczyk, J.; Woehler, A.; Kobe, F.; Ponimaskin, E.; Zeug, A.; Neher, E. Analysis of FRET Signals in the Presence of Free Donors and Acceptors. Biophys. J. 2008, 94, 986–1000. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Zeug, A.; Ponimaskin, E. Analysis of Receptor-Receptor Interaction by Combined Application of FRET and Microscopy. Methods Cell Biol. 2013, 117, 243–265. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Naumenko, V.S.; Voronova, I.P.; Tikhonova, M.A.; Popova, N.K. Quantitative RT-PCR Assay of 5-HT1A and 5-HT2A Serotonin Receptor MRNAs Using Genomic DNA as an External Standard. J. Neurosci. Methods 2005, 141, 97–101. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kulikov, A.V. Quantitative assay of 5-HT(1A) serotonin receptor gene expression in the brain. Mol. Biol. 2006, 40, 37–44. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Osipova, D.V.; Kostina, E.V.; Kulikov, A.V. Utilization of a Two-Standard System in Real-Time PCR for Quantification of Gene Expression in the Brain. J. Neurosci. Methods 2008, 170, 197–203. [Google Scholar] [CrossRef]
- Guo, H.; An, S.; Ward, R.; Yang, Y.; Liu, Y.; Guo, X.-X.; Hao, Q.; Xu, T.-R. Methods Used to Study the Oligomeric Structure of G-Protein-Coupled Receptors. Biosci. Rep. 2017, 37, BSR20160547. [Google Scholar] [CrossRef] [Green Version]
- Dorsch, S.; Klotz, K.-N.; Engelhardt, S.; Lohse, M.J.; Bünemann, M. Analysis of Receptor Oligomerization by FRAP Microscopy. Nat. Methods 2009, 6, 225–230. [Google Scholar] [CrossRef]
- Taura, J.; Fernández-Dueñas, V.; Ciruela, F. Visualizing G Protein-Coupled Receptor-Receptor Interactions in Brain Using Proximity Ligation In Situ Assay. Curr. Protoc. Cell Biol. 2015, 67, 17.17.1–17.17.16. [Google Scholar] [CrossRef]
- Shang, W.; Lu, F.; Sun, T.; Xu, J.; Li, L.-L.; Wang, Y.; Wang, G.; Chen, L.; Wang, X.; Cannell, M.B.; et al. Imaging Ca2+ Nanosparks in Heart with a New Targeted Biosensor. Circ. Res. 2014, 114, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.E.; Cherkas, V.; Stopper, G.; Caudal, L.C.; Stopper, L.; Kirchhoff, F.; Henneberger, C.; Ponimaskin, E.G.; Zeug, A. Elucidating Regulators of Astrocytic Ca2+ Signaling via Multi-Threshold Event Detection (MTED). Glia 2021, 69, 2798–2811. [Google Scholar] [CrossRef]
- Roth, B.L.; Palvimaki, E.P.; Berry, S.; Khan, N.; Sachs, N.; Uluer, A.; Choudhary, M.S. 5-Hydroxytryptamine2A (5-HT2A) Receptor Desensitization Can Occur without down-Regulation. J. Pharmacol. Exp. Ther. 1995, 275, 1638–1646. [Google Scholar]
- Saucier, C.; Albert, P.R. Identification of an Endogenous 5-Hydroxytryptamine2A Receptor in NIH-3T3 Cells: Agonist-Induced down-Regulation Involves Decreases in Receptor RNA and Number. J. Neurochem. 1997, 68, 1998–2011. [Google Scholar] [CrossRef]
- Aloyo, V.J.; Berg, K.A.; Spampinato, U.; Clarke, W.P.; Harvey, J.A. Current Status of Inverse Agonism at Serotonin2A (5-HT2A) and 5-HT2C Receptors. Pharmacol. Ther. 2009, 121, 160–173. [Google Scholar] [CrossRef]
- Yoshii, A.; Murata, Y.; Kim, J.; Zhang, C.; Shokat, K.M.; Constantine-Paton, M. TrkB and Protein Kinase Mζ Regulate Synaptic Localization of PSD-95 in Developing Cortex. J. Neurosci. 2011, 31, 11894–11904. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chan, C.B.; Ye, K. 7,8-Dihydroxyflavone, a Small Molecular TrkB Agonist, Is Useful for Treating Various BDNF-Implicated Human Disorders. Transl. Neurodegener. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Tsybko, A.S.; Ilchibaeva, T.V.; Filimonova, E.A.; Eremin, D.V.; Popova, N.K.; Naumenko, V.S. The Chronic Treatment With 5-HT2A Receptor Agonists Affects the Behavior and the BDNF System in Mice. Neurochem. Res. 2020, 45, 3059–3075. [Google Scholar] [CrossRef]
- Flajolet, M.; Wang, Z.; Futter, M.; Shen, W.; Nuangchamnong, N.; Bendor, J.; Wallach, I.; Nairn, A.C.; Surmeier, D.J.; Greengard, P. FGF Acts as a Co-Transmitter through Adenosine A(2A) Receptor to Regulate Synaptic Plasticity. Nat. Neurosci. 2008, 11, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Di Palma, M.; Sartini, S.; Lattanzi, D.; Cuppini, R.; Pita-Rodriguez, M.; Diaz-Carmenate, Y.; Narvaez, M.; Fuxe, K.; Borroto-Escuela, D.O.; Ambrogini, P. Evidence for the Existence of A2AR-TrkB Heteroreceptor Complexes in the Dorsal Hippocampus of the Rat Brain: Potential Implications of A2AR and TrkB Interplay upon Ageing. Mech. Ageing Dev. 2020, 190, 111289. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Mudó, G.; Pérez-Alea, M.; Ciruela, F.; Tarakanov, A.O.; Narvaez, M.; Di Liberto, V.; Agnati, L.F.; Belluardo, N.; et al. Fibroblast Growth Factor Receptor 1-5-Hydroxytryptamine 1A Heteroreceptor Complexes and Their Enhancement of Hippocampal Plasticity. Biol. Psychiatry 2012, 71, 84–91. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered Gene Expression of Brain-Derived Neurotrophic Factor and Receptor Tyrosine Kinase B in Postmortem Brain of Suicide Subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Pandey, G.N.; Ren, X.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Dwivedi, Y. Brain-Derived Neurotrophic Factor and Tyrosine Kinase B Receptor Signalling in Post-Mortem Brain of Teenage Suicide Victims. Int. J. Neuropsychopharmacol. 2008, 11, 1047–1061. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased MRNA and Protein Expression of BDNF, NGF, and Their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin. Med. Insights Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rizavi, H.S.; Zhang, H.; Mondal, A.C.; Roberts, R.C.; Conley, R.R.; Pandey, G.N. Neurotrophin Receptor Activation and Expression in Human Postmortem Brain: Effect of Suicide. Biol. Psychiatry 2009, 65, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockmeier, C.A. Involvement of Serotonin in Depression: Evidence from Postmortem and Imaging Studies of Serotonin Receptors and the Serotonin Transporter. J. Psychiatr. Res. 2003, 37, 357–373. [Google Scholar] [CrossRef]
- Bach, H.; Arango, V. Neuroanatomy of Serotonergic Abnormalities in Suicide. In the Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-3881-5. [Google Scholar]
- Pandey, G.N.; Dwivedi, Y.; Rizavi, H.S.; Ren, X.; Pandey, S.C.; Pesold, C.; Roberts, R.C.; Conley, R.R.; Tamminga, C.A. Higher Expression of Serotonin 5-HT(2A) Receptors in the Postmortem Brains of Teenage Suicide Victims. Am. J. Psychiatry 2002, 159, 419–429. [Google Scholar] [CrossRef]
- Cahir, M.; Ardis, T.; Reynolds, G.P.; Cooper, S.J. Acute and Chronic Tryptophan Depletion Differentially Regulate Central 5-HT1A and 5-HT2A Receptor Binding in the Rat. Psychopharmacology 2007, 190, 497–506. [Google Scholar] [CrossRef]
- Jennings, K.A.; Sheward, W.J.; Harmar, A.J.; Sharp, T. Evidence That Genetic Variation in 5-HT Transporter Expression Is Linked to Changes in 5-HT2A Receptor Function. Neuropharmacology 2008, 54, 776–783. [Google Scholar] [CrossRef]
- Jørgensen, L.M.; Weikop, P.; Villadsen, J.; Visnapuu, T.; Ettrup, A.; Hansen, H.D.; Baandrup, A.O.; Andersen, F.L.; Bjarkam, C.R.; Thomsen, C.; et al. Cerebral 5-HT Release Correlates with [(11)C]Cimbi36 PET Measures of 5-HT2A Receptor Occupancy in the Pig Brain. J. Cereb. Blood Flow Metab. 2017, 37, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Di Lieto, A.; Rantamäki, T.; Vesa, L.; Yanpallewar, S.; Antila, H.; Lindholm, J.; Rios, M.; Tessarollo, L.; Castrén, E. The Responsiveness of TrkB to BDNF and Antidepressant Drugs Is Differentially Regulated during Mouse Development. PLoS ONE 2012, 7, e32869. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [Green Version]
- Castrén, E.; Antila, H. Neuronal Plasticity and Neurotrophic Factors in Drug Responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Casarotto, P.C.; Resstel, L.; Joca, S.R.L. Beyond Good and Evil: A Putative Continuum-Sorting Hypothesis for the Functional Role of ProBDNF/BDNF-Propeptide/MBDNF in Antidepressant Treatment. Neurosci. Biobehav. Rev. 2018, 90, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Carr, G.V.; Lucki, I. The Role of Serotonin Receptor Subtypes in Treating Depression: A Review of Animal Studies. Psychopharmacology 2011, 213, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.K.; Bhatt, S.; Jindal, A.; Gautam, B. Effect of Combination of Ketanserin and Escitalopram on Behavioral Anomalies after Olfactory Bulbectomy: Prediction of Quick Onset of Antidepressant Action. Indian J. Pharmacol. 2014, 46, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and MGlu2/3 Receptors in Postmortem Prefrontal Cortex of Subjects with Major Depressive Disorder: Effect of Antidepressant Treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef]
- Guiard, B.P.; Di Giovanni, G. Central Serotonin-2A (5-HT2A) Receptor Dysfunction in Depression and Epilepsy: The Missing Link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Jha, S.; Rajendran, R.; Fernandes, K.A.; Vaidya, V.A. 5-HT2A/2C Receptor Blockade Regulates Progenitor Cell Proliferation in the Adult Rat Hippocampus. Neurosci. Lett. 2008, 441, 210–214. [Google Scholar] [CrossRef]
- Pilar-Cuéllar, F.; Vidal, R.; Pazos, A. Subchronic Treatment with Fluoxetine and Ketanserin Increases Hippocampal Brain-Derived Neurotrophic Factor, β-Catenin and Antidepressant-like Effects. Br. J. Pharmacol. 2012, 165, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Chlan-Fourney, J.; Ashe, P.; Nylen, K.; Juorio, A.V.; Li, X.M. Differential Regulation of Hippocampal BDNF MRNA by Typical and Atypical Antipsychotic Administration. Brain Res. 2002, 954, 11–20. [Google Scholar] [CrossRef]
- Bai, O.; Chlan-Fourney, J.; Bowen, R.; Keegan, D.; Li, X.-M. Expression of Brain-Derived Neurotrophic Factor MRNA in Rat Hippocampus after Treatment with Antipsychotic Drugs. J. Neurosci. Res. 2003, 71, 127–131. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, C.H.; Cho, H.Y.; You, Y.S.; Lee, B.J.; Lee, J.G.; Park, S.W.; Kim, Y.H. Effects of Antipsychotic Drugs on the Expression of Synapse-Associated Proteins in the Frontal Cortex of Rats Subjected to Immobilization Stress. Psychiatry Res. 2015, 229, 968–974. [Google Scholar] [CrossRef]
- Fumagalli, F.; Molteni, R.; Bedogni, F.; Gennarelli, M.; Perez, J.; Racagni, G.; Riva, M.A. Quetiapine Regulates FGF-2 and BDNF Expression in the Hippocampus of Animals Treated with MK-801. Neuroreport 2004, 15, 2109–2112. [Google Scholar] [CrossRef]
- Angelucci, F.; Aloe, L.; Iannitelli, A.; Gruber, S.H.M.; Mathé, A.A. Effect of Chronic Olanzapine Treatment on Nerve Growth Factor and Brain-Derived Neurotrophic Factor in the Rat Brain. Eur. Neuropsychopharmacol. 2005, 15, 311–317. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; He, J.; Haimanot, S.; Li, X.; Dyck, L.; Li, X.-M. Synergetic Effects of Quetiapine and Venlafaxine in Preventing the Chronic Restraint Stress-Induced Decrease in Cell Proliferation and BDNF Expression in Rat Hippocampus. Hippocampus 2006, 16, 551–559. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, S.-K.; Kim, J.-M.; Yoon, J.-S.; Kim, Y.-H. Effects of Quetiapine on the Brain-Derived Neurotrophic Factor Expression in the Hippocampus and Neocortex of Rats. Neurosci. Lett. 2006, 402, 25–29. [Google Scholar] [CrossRef]
- Pillai, A.; Terry, A.V.J.; Mahadik, S.P. Differential Effects of Long-Term Treatment with Typical and Atypical Antipsychotics on NGF and BDNF Levels in Rat Striatum and Hippocampus. Schizophr. Res. 2006, 82, 95–106. [Google Scholar] [CrossRef]
- Balu, D.T.; Hoshaw, B.A.; Malberg, J.E.; Rosenzweig-Lipson, S.; Schechter, L.E.; Lucki, I. Differential Regulation of Central BDNF Protein Levels by Antidepressant and Non-Antidepressant Drug Treatments. Brain Res. 2008, 1211, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Cheon, Y.; Modi, H.R.; Rapoport, S.I.; Rao, J.S. Effects of Chronic Clozapine Administration on Markers of Arachidonic Acid Cascade and Synaptic Integrity in Rat Brain. Psychopharmacology 2012, 222, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Lee, C.H.; Cho, H.Y.; Seo, M.K.; Lee, J.G.; Lee, B.J.; Seol, W.; Kee, B.S.; Kim, Y.H. Effects of Antipsychotic Drugs on the Expression of Synaptic Proteins and Dendritic Outgrowth in Hippocampal Neuronal Cultures. Synapse 2013, 67, 224–234. [Google Scholar] [CrossRef]


| Target Gene | Primer Sequences | Annealing Temperature, °C | Amplicon Length, bp |
|---|---|---|---|
| Ntrk2 | F 5′-cattcactgtgagaggcaacc-3′ R 5′-atcagggtgtagtctccgttatt-3′ | 63 | 175 |
| Htr2a | F 5′-agaagccaccttgtgtgtga-3′ R 5′-ttgctcattgctgatggact-3′ | 61 | 169 |
| Polr2a | F 5′-gttgtcgggcagcagaatgtag-3′ R 5′-tcaatgagaccttctcgtcctcc-3′ | 63 | 188 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilchibaeva, T.; Tsybko, A.; Zeug, A.; Müller, F.E.; Guseva, D.; Bischoff, S.; Ponimaskin, E.; Naumenko, V. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells 2022, 11, 2384. https://doi.org/10.3390/cells11152384
Ilchibaeva T, Tsybko A, Zeug A, Müller FE, Guseva D, Bischoff S, Ponimaskin E, Naumenko V. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells. 2022; 11(15):2384. https://doi.org/10.3390/cells11152384
Chicago/Turabian StyleIlchibaeva, Tatiana, Anton Tsybko, Andre Zeug, Franziska E. Müller, Daria Guseva, Stephan Bischoff, Evgeni Ponimaskin, and Vladimir Naumenko. 2022. "Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes" Cells 11, no. 15: 2384. https://doi.org/10.3390/cells11152384
APA StyleIlchibaeva, T., Tsybko, A., Zeug, A., Müller, F. E., Guseva, D., Bischoff, S., Ponimaskin, E., & Naumenko, V. (2022). Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells, 11(15), 2384. https://doi.org/10.3390/cells11152384






