Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Self-Administration
2.2. Dataset
2.3. Protein Extraction
2.4. Quantification of Brain Innate Immune Proteins by BioPlex
2.5. Statistical Analyses
3. Results
3.1. Cocaine Self-Administration in WT and HIV-1Tg Rats
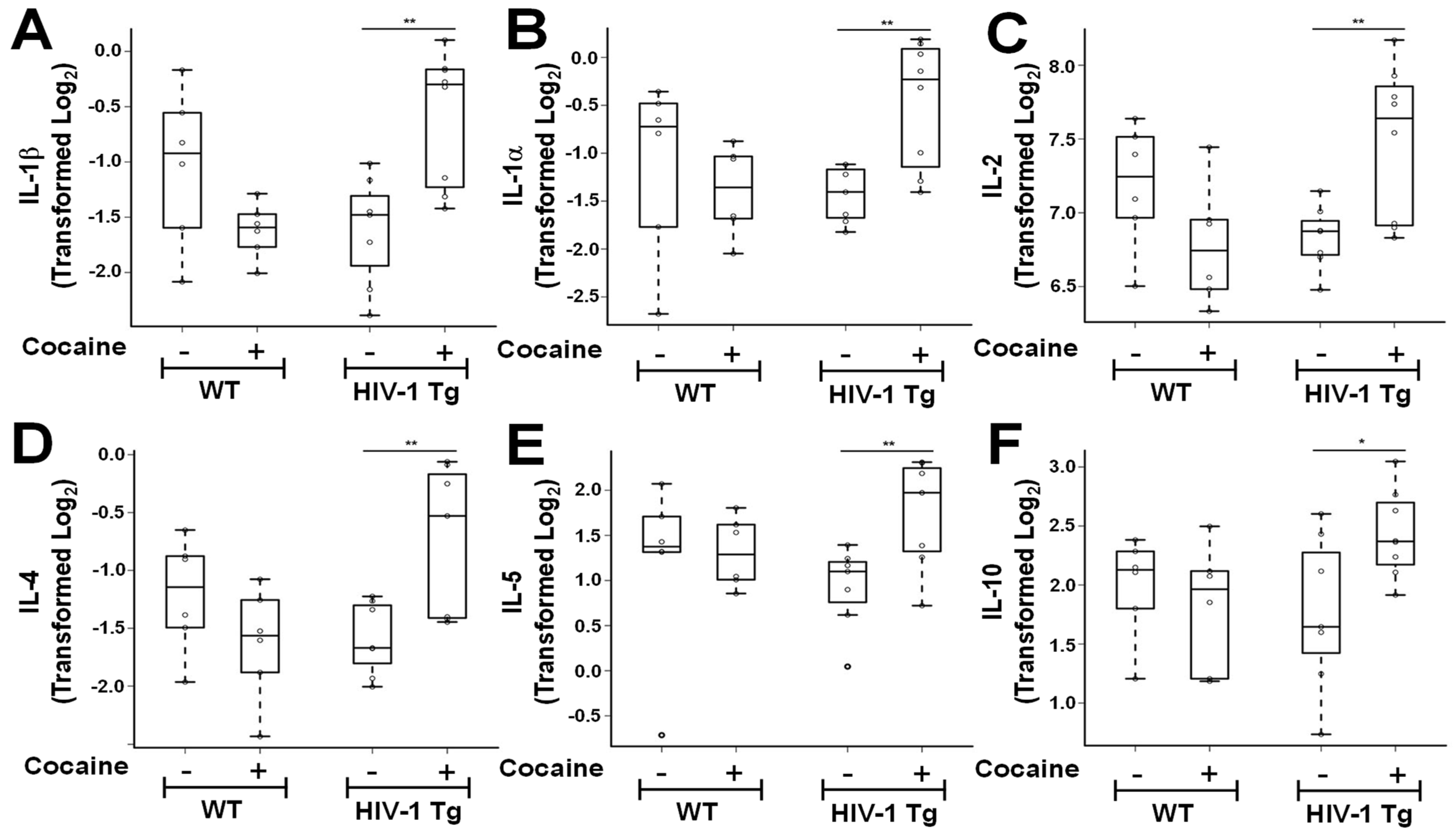
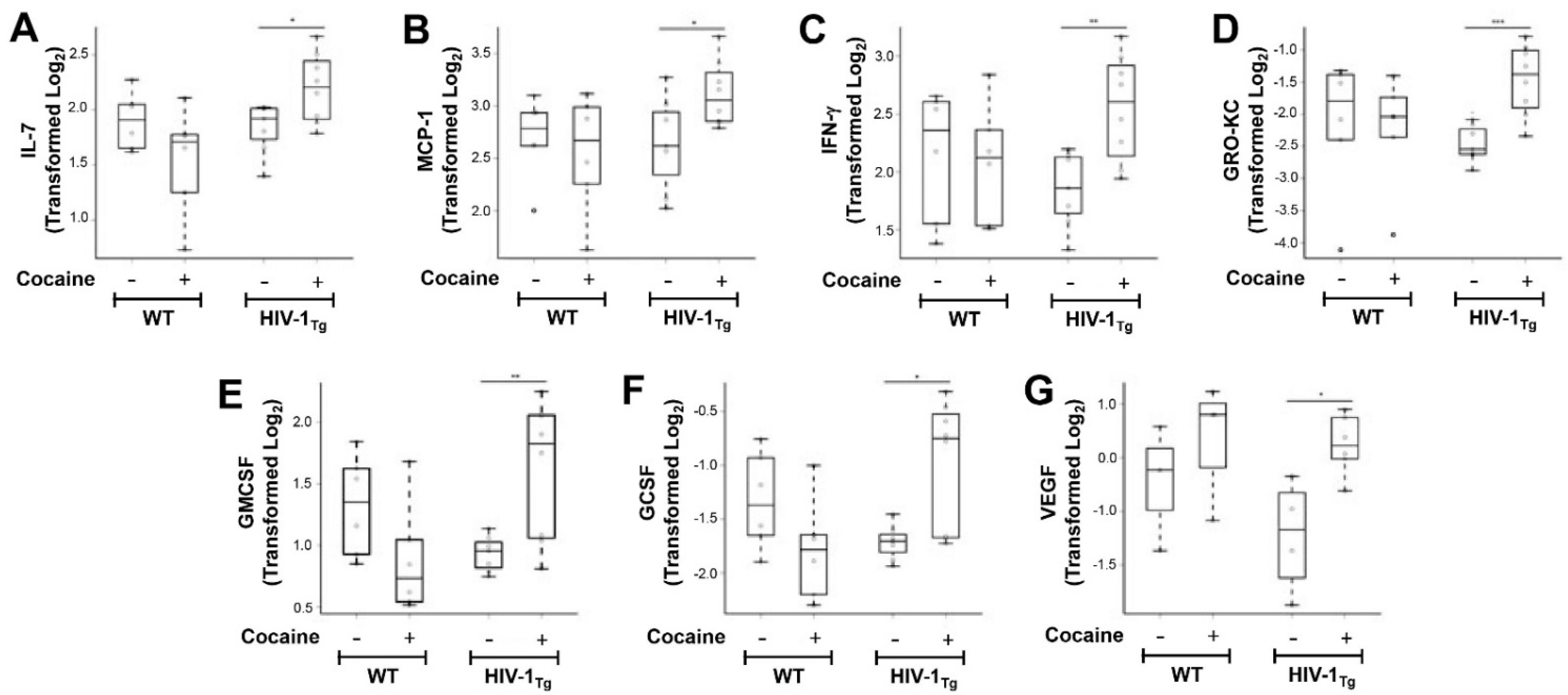
3.2. Cocaine Self-Administration Increases Pro- and Anti-Inflammatory Cytokine Generation in the fCTX of HIV-1Tg Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas−Celis, V.; Valiente−Echeverría, F.; Soto−Rifo, R.; Toro−Ascuy, D. New challenges of HIV−1 infection: How HIV−1 attacks and resides in the central nervous system. Cells 2019, 8, 1245. [Google Scholar] [CrossRef] [PubMed]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial cells: The main HIV−1 reservoir in the brain. Front. Cell. Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.B.; Kaul, M. Neuronal stress and injury caused by HIV−1, cART and drug abuse: Converging contributions to HAND. Brain Sci. 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Thangaraj, A.; Chivero, E.T.; Niu, F.; Kannan, M.; Liao, K.; Silverstein, P.S.; Periyasamy, P.; Buch, S. HIV−1 and drug abuse comorbidity: Lessons learned from the animal models of NeuroHIV. Neurosci. Lett. 2021, 754, 135863. [Google Scholar] [CrossRef] [PubMed]
- Larrat, E.P.; Zierler, S. Entangled Epidemics: Cocaine Use and HIV Disease. J. Psychoact. Drugs 1993, 25, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.H.; Bluthenthal, R.N.; E Booth, R.; Watters, J.K. HIV seroprevalence among street−recruited injection drug and crack cocaine users in 16 US municipalities. Am. J. Public Health 1998, 88, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Vignoles, M.; Avila, M.M.; Osimani, M.L.; de Los Angeles Pando, M.; Rossi, D.; Sheppard, H.; Sosa−Estani, S.; Benetucci, J.; Maulen, S.; Chiparelli, H.; et al. HIV seroincidence estimates among at−risk populations in Buenos Aires and Montevideo: Use of the serologic testing algorithm for recent HIV seroconversion. J. Acquir. Immune. Defic. Syndr. 2006, 42, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.K.; Gekker, G.; Chao, C.C.; Schut, R.; Molitor, T.W.; Balfour, H.H. Cocaine potentiates HIV−1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor−beta. J. Immunol. 1991, 146, 81–84. [Google Scholar] [PubMed]
- Peterson, P.K.; Gekker, G.; Chao, C.C.; Schut, R.; Verhoef, J.; Edelman, C.K.; Erice, A.; Balfour, H.H. Cocaine amplifies HIV−1 replication in cytomegalovirus−stimulated peripheral blood mononuclear cell cocultures. J. Immunol. 1992, 149, 676–680. [Google Scholar]
- Webber, M.P.; Schoenbaum, E.E.; Gourevitch, M.N.; Buono, D.; Klein, R.S. A prospective study of HIV disease progression in female and male drug users. AIDS 1999, 13, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, L.; Callen, S.; Buch, S. Multiple faceted roles of cocaine in potentiation of HAND. Curr. HIV Res. 2016, 14, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.; Misra, V.; Cassol, E.; Ancuta, P.; Yan, Z.; Li, C.; Morgello, S.; Gabuzda, D. A Plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS ONE 2012, 7, e30881. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Chattopadhyay, P.; Brodie, T.M.; Qin, J.; Gu, W.; Mascola, J.R.; Michael, N.L.; Follmann, D.A.; Roederer, M. Immunologic and virologic events in early HIV infection predict subsequent rate of progression. J. Infect. Dis. 2010, 201, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; McCune, J.M.; Roland, M.; Page-Shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Lampiris, H.; et al. Relationship between T cell activation and CD4+T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 2008, 197, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Okay, G.; Koc, M.M.; Guler, E.M.; Yabaci, A.; Kocyigit, A.; Akkoyunlu, Y. The effect of antiretroviral therapy on IL−6, IL−1β, TNF−α, IFN−γ levels and their relationship with HIV−RNA and CD4+ T cells in HIV patients. Curr HIV Res 2020, 18, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Everall, I.; Vaida, F.; Khanlou, N.; Lazzaretto, D.; Achim, C.; Letendre, S.; Moore, D.; Ellis, R.; Cherner, M.; Gelman, B.; et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J. NeuroVirology 2009, 15, 360–370. [Google Scholar] [CrossRef]
- Brabers, N.A.C.H.; Nottet, H.S.L.M. Role of the pro−inflammatory cytokines TNF−alpha and IL−1beta in HIV−associated dementia. Eur. J. Clin. Investig. 2006, 36, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Wesselingh, S.L.; Takahashi, K.; Glass, J.D.; McArthur, J.C.; Griffin, J.W.; Griffin, D.E. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV−infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J. Neuroimmunol. 1997, 74, 1–8. [Google Scholar] [CrossRef]
- Nath, A.; Conant, K.; Chen, P.; Scott, C.; Major, E.O. Transient exposure to HIV−1 Tat protein results in cytokine production in macrophages and astrocytes. J. Biol. Chem. 1999, 274, 17098–17102. [Google Scholar] [CrossRef]
- Weiss, J.M.; Nath, A.; O Major, E.; Berman, J.W. HIV−1 Tat induces monocyte chemoattractant protein−1−mediated monocyte transmigration across a model of the human blood−brain barrier and up−regulates CCR5 expression on human monocytes. J. Immunol. 1999, 163, 2953–2959. [Google Scholar] [PubMed]
- Yang, Y.; Wu, J.; Lu, Y. Mechanism of HIV−1−TAT induction of interleukin−1beta from human monocytes: Involvement of the phospholipase C/protein kinase C signaling cascade. J. Med. Virol. 2010, 82, 735–746. [Google Scholar] [CrossRef]
- Kiebala, M.; Polesskaya, O.; Yao, Z.; Perry, S.W.; Maggirwar, S.B. Nuclear factor−kappa B family member RelB inhibits human immunodeficiency virus−1 Tat−induced tumor necrosis factor−alpha production. PLoS ONE 2010, 5, e11875. [Google Scholar] [CrossRef] [PubMed]
- Kiebala, M.; Maggirwar, S.B. Ibudilast, a pharmacologic phosphodiesterase inhibitor, prevents human immunodeficiency virus−1 Tat−mediated activation of microglial cells. PLoS ONE 2011, 6, e18633. [Google Scholar] [CrossRef]
- Mishra, R.; Chhatbar, C.; Singh, S.K. HIV−1 Tat C−mediated regulation of tumor necrosis factor receptor−associated factor−3 by microRNA 32 in human microglia. J. Neuroinflammation 2012, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Little, K.Y.; Ramssen, E.; Welchko, R.; Volberg, V.; Roland, C.J.; Cassin, B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009, 168, 173–180. [Google Scholar] [CrossRef]
- Moreira, F.P.; Medeiros, J.R.C.; Lhullier, A.C.; Souza, L.D.D.M.; Jansen, K.; Portela, L.V.; Lara, D.R.; da Silva, R.A.; Wiener, C.D.; Oses, J.P. Cocaine abuse and effects in the serum levels of cytokines IL−6 and IL−10. Drug Alcohol Depend. 2015, 158, 181–185. [Google Scholar] [CrossRef]
- Northcutt, A.; Hutchinson, M.; Wang, X.; Baratta, M.; Hiranita, T.; Cochran, T.; Pomrenze, M.B.; Galer, E.; A Kopajtic, T.; Li, C.; et al. DAT isn’t all that: Cocaine reward and reinforcement require Toll−like receptor 4 signaling. Mol. Psychiatry 2015, 20, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Watkins, L.R. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 2014, 76, 218–227. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Lutjens, R.; van der Stap, L.D.; Lekic, D.; Romano−Spica, V.; Morales, M.; Koob, G.F.; Repunte−Canonigo, V.; Sanna, P.P. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc. Natl. Acad. Sci. USA 2005, 102, 11533–11538. [Google Scholar] [CrossRef]
- Renthal, W.; Maze, I.; Krishnan, V.; Covington, H.E.; Xiao, G.; Kumar, A.; Russo, S.J.; Graham, A.; Tsankova, N.; Kippin, T.E.; et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 2007, 56, 517–529. [Google Scholar] [CrossRef]
- Piechota, M.; Korostynski, M.; Solecki, W.; Gieryk, A.; Slezak, M.; Bilecki, W.; Ziolkowska, B.; Kostrzewa, E.; Cymerman, I.; Swiech, L.; et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010, 11, R48. [Google Scholar] [CrossRef] [PubMed]
- Addai, A.B.; Pandhare, J.; Paromov, V.; Mantri, C.K.; Pratap, S.; Dash, C. Cocaine modulates HIV−1 integration in primary CD4+T cells: Implications in HIV−1 pathogenesis in drug−abusing patients. J. Leukoc. Biol. 2015, 97, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, J.B.; Dixit, D.; Rovner, R.; Zack, J.A.; Baldwin, G.C.; Vatakis, D.N. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J. Leukoc. Biol. 2013, 94, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Mantri, C.K.; Dash, J.P.; Mantri, J.V.; Dash, C. Correction: Cocaine enhances HIV−1 replication in CD4+ T cells by down−regulating MiR−125b. PLoS ONE 2018, 13, e0199338. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.N.; Chadha, K.C.; Hewitt, R.G.; Mahajan, S.; Sweet, A.; Schwartz, S.A.; Stoiser, B.; Thalhammer, F.; El−Menyawi, I.; Wilfing, A.; et al. Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1−infected patients. Clin. Diagn. Lab. Immunol. 2000, 7, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Napuri, J.; Pilakka−Kanthikeel, S.; Raymond, A.; Agudelo, M.; Yndart−Arias, A.; Saxena, S.K.; Nair, M. Cocaine enhances HIV−1 infectivity in monocyte derived dendritic cells by suppressing microRNA. PLoS ONE 2013, 8, e83682. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.L.; Mahajan, S.D.; Bindukumar, B.; Sykes, D.; Schwartz, S.A.; Nair, M.P.N. Proteomic analysis of the effects of cocaine on the enhancement of HIV−1 replication in normal human astrocytes (NHA). Brain Res. 2006, 1123, 226–236. [Google Scholar] [CrossRef]
- Liao, K.; Guo, M.; Niu, F.; Yang, L.; Callen, S.E.; Buch, S. Cocaine−mediated induction of microglial activation involves the ER stress−TLR2 axis. J. Neuroinflamm. 2016, 13, 33. [Google Scholar] [CrossRef]
- Guo, M.-L.; Liao, K.; Periyasamy, P.; Yang, L.; Cai, Y.; E Callen, S.; Buch, S. Cocaine−mediated microglial activation involves the ER stress−autophagy axis. Autophagy 2015, 11, 995–1009. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Lu, Y.; Wang, C.; Buch, S. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV−1) protein gp. PLoS ONE 2010, 5, e13427. [Google Scholar] [CrossRef]
- Yao, H.; E Allen, J.; Zhu, X.; Callen, S.; Buch, S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J. NeuroVirology 2009, 15, 164–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samikkannu, T.; Agudelo, M.; Gandhi, N.; Reddy, P.V.B.; Saiyed, Z.M.; Nwankwo, D.; Nair, M.P.N. Human immunodeficiency virus type 1 clade B and C gp120 differentially induce neurotoxin arachidonic acid in human astrocytes: Implications for neuroAIDS. J. NeuroVirology 2011, 17, 230–238. [Google Scholar] [CrossRef][Green Version]
- Tiwari, S.; Nair, M.P.; Saxena, S.K. Latest trends in drugs of abuse – HIV infection and neuroAIDS. Futur. Virol. 2013, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Israel, S.M.; Hassanzadeh-Behbahani, S.; Turkeltaub, P.E.; Moore, D.J.; Ellis, R.J.; Jiang, X. Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum. Brain Mapp. 2019, 40, 3010–3026. [Google Scholar] [CrossRef]
- Wiley, C.A.; Masliah, E.; Morey, M.; Lemere, C.; DeTeresa, R.; Grafe, M.; Hansen, L.; Terry, R. Neocortical damage during HIV infection. Ann. Neurol. 1991, 29, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Sporer, B.; Linke, R.; Seelos, K.; Paul, R.; Klopstock, T.; Pfister, H.-W. HIV−induced chorea: Evidence for basal ganglia dysregulation by SPECT. J. Neurol. 2005, 252, 356–358. [Google Scholar]
- Wang, G.-J.; Chang, L.; Volkow, N.D.; Telang, F.; Logan, J.; Ernst, T.; Fowler, J.S. Decreased brain dopaminergic transporters in HIV−associated dementia patients. Brain 2004, 127, 2452–2458. [Google Scholar] [CrossRef]
- Kumar, A.M.; Ownby, R.L.; Waldrop−Valverde, D.; Fernandez, B.; Kumar, M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. J. NeuroVirology 2010, 17, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Vigorito, M.; Liu, X.; Zhou, D.; Wu, X.; Chang, S.L. The HIV−1 transgenic rat as a model for HIV−1 infected individuals on HAART. J. Neuroimmunol. 2010, 218, 94–101. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.; Sexton, T.; Pattison, L.P.; Childers, S.R.; Hemby, S.E. Increased sensitivity to cocaine self−administration in HIV−1 transgenic rats is associated with changes in striatal dopamine transporter binding. J. Neuroimmune Pharmacol. 2015, 10, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Wayman, W.N.; Chen, L.; Hu, X.−T.; Napier, T.C. HIV−1 transgenic rat prefrontal cortex hyper−excitability is enhanced by cocaine self−administration. Neuropsychopharmacology 2015, 41, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Wayman, W.N.; Chen, L.; Persons, A.L.; Napier, T.C. Cortical consequences of HIV−1 Tat exposure in rats are enhanced by chronic cocaine. Curr. HIV Res. 2015, 13, 80–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ezeomah, C.; Cunningham, K.A.; Stutz, S.J.; Fox, R.G.; Bukreyeva, N.; Dineley, K.T.; Paessler, S.; Cisneros, I.E. Fentanyl self−administration impacts brain immune responses in male Sprague−Dawley rats. Brain Behav. Immun. 2020, 87, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Persons, A.L.; Bradaric, B.; Dodiya, H.B.; Ohene−Nyako, M.; Forsyth, C.B.; Keshavarzian, A.; Shaikh, M.; Napier, T.C. Colon dysregulation in methamphetamine self−administering HIV−1 transgenic rats. PLoS ONE 2018, 13, e0190078. [Google Scholar] [CrossRef] [PubMed]
- Lashomb, A.L.; Vigorito, M.; Chang, S.L. Further characterization of the spatial learning deficit in the human immunodeficiency virus−1 transgenic rat. J. NeuroVirology 2009, 15, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.M.; Booze, R.; Mactutus, C.F. Modeling deficits in attention, inhibition, and flexibility in HAND. J. Neuroimmune Pharmacol. 2014, 9, 508–521. [Google Scholar] [CrossRef]
- Vigorito, M.; Connaghan, K.P.; Chang, S.L. The HIV−1 transgenic rat model of neuroHIV. Brain Behav. Immun. 2015, 48, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Dagur, R.; Liao, K.; Sil, S.; Niu, F.; Sun, Z.; Lyubchenko, Y.L.; Peeples, E.S.; Hu, G.; Buch, S. Neuronal-derived extracellular vesicles are enriched in the brain and serum of HIV-1 transgenic rats. J. Extracell. Vesicles 2019, 9, 1703249. [Google Scholar] [CrossRef]
- D’Aversa, T.; Eugenin, E.; Berman, J. NeuroAIDS: Contributions of the human immunodeficiency virus−1 proteins tat and gp120 as well as CD40 to microglial activation. J. Neurosci. Res. 2005, 81, 436–446. [Google Scholar] [CrossRef]
- Repunte−Canonigo, V.; Lefebvre, C.; George, O.; Kawamura, T.; Morales, M.; Koob, G.F.; Califano, A.; Masliah, E.; Sanna, P.P. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV−1 transgenic rats. Mol. Neurodegener. 2014, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, C.L.; Glasper, E.R.; Harrell, C.S.; Malviya, S.A.; Otis, J.S.; Neigh, G.N. Meloxicam blocks neuroinflammation, but not depressive−like behaviors, in HIV−1 transgenic female rats. PLoS ONE 2014, 9, e108399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reid, W.C.; Ibrahim, W.G.; Kim, S.J.; Denaro, F.; Casas, R.; Lee, D.E.; Maric, D.; Hammoud, D.A. Characterization of neuropathology in the HIV−1 transgenic rat at different ages. J. Neuroimmunol. 2016, 292, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Yue, X.; Ibrahim, W.G.; Lentz, M.R.; Peterson, K.L.; Jagoda, E.M.; Kassiou, M.; Maric, D.; Reid, W.C.; Hammoud, D.A. Lack of neuroinflammation in the HIV−1 transgenic rat: An [18F]−DPA714 PET imaging study. J. Neuroinflammation 2015, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Balasubramaniam, M.; Villalta, F.; Dash, C.; Pandhare, J. Impact of cocaine abuse on HIV pathogenesis. Front. Microbiol. 2015, 6, 1111. [Google Scholar] [CrossRef] [PubMed]
- Coller, J.K.; Hutchinson, M.R. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol. Ther. 2012, 134, 219–245. [Google Scholar] [CrossRef]
- Marasco, C.; Goodwin, C.R.; Winder, D.G.; Schramm−Sapyta, N.L.; McLean, J.A.; Wikswo, J.P. Systems−level view of cocaine addiction: The interconnection of the immune and nervous systems. Exp. Biol. Med. 2014, 239, 1433–1442. [Google Scholar] [CrossRef]
- Stamatovich, S.N.; Lopez−Gamundi, P.; Suchting, R.; Colpo, G.D.; Walss−Bass, C.; Lane, S.D.; Schmitz, J.M.; Wardle, M.C. Plasma pro− and anti−inflammatory cytokines may relate to cocaine use, cognitive functioning, and depressive symptoms in cocaine use disorder. Am. J. Drug Alcohol Abus. 2020, 47, 52–64. [Google Scholar] [CrossRef]
- Levandowski, M.L.; Hess, A.R.B.; Grassi−Oliveira, R.; de Almeida, R.M.M. Plasma interleukin−6 and executive function in crack cocaine−dependent women. Neurosci. Lett. 2016, 628, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; D’Sa, C.; Kimmerling, A.; Siedlarz, K.M.; Tuit, K.L.; Stowe, R.; Sinha, R. Immune system inflammation in cocaine dependent individuals: Implications for medications development. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 156–166. [Google Scholar] [CrossRef]
- Brown, K.T.; Levis, S.C.; O’Neill, C.E.; Northcutt, A.L.; Fabisiak, T.J.; Watkins, L.R.; Bachtell, R.K. Innate immune signaling in the ventral tegmental area contributes to drug−primed reinstatement of cocaine seeking. Brain, Behav. Immun. 2017, 67, 130–138. [Google Scholar] [CrossRef]
- Haar, C.V.; Ferland, J.−M.; Kaur, S.; Riparip, L.; Rosi, S.; Winstanley, C.A. Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. Eur. J. Neurosci. 2018, 50, 2134–2145. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.M.; Murray, J.; Byrd, D.A.; Hurd, Y.L.; Morgello, S. HIV−related cognitive impairment shows bi−directional association with dopamine receptor DRD1 and DRD2 polymorphisms in substance−dependent and substance−independent populations. J. NeuroVirology 2013, 19, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
| WT | HIV-1Tg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine (log2 Transformed) | Saline | Cocaine | 95% CI | p-Value | Adjusted p-Value | Saline | Cocaine | 95% CI | p-Value | Adjusted p-Value |
| Mean (SD) | Mean (SD) | (Bonferroni) | Mean (SD) | Mean (SD) | (Bonferroni) | |||||
| G−CSF | −1.33 (0.45) | −1.79 (0.47) | [0.218, 1.23] | 0.114 | 0.110 | −1.71 (0.16) | −0.99 (0.60) | [−1.04, 0.131] | 0.011 | 0.011 |
| GM−CSF | 1.32 (0.40) | 0.88 (0.44) | [0.216, 1.55] | 0.097 | 0.097 | 0.93 (0.14) | 1.62 (0.56) | [−1.80, 0.097] | 0.01 | 0.010 |
| GRO/KC | −2.14 (1.06) | −2.24 (0.86) | [0.505, 1.48] | 0.852 | 0.850 | −2.46 (0.29) | −1.46 (0.55) | [−1.35, 1.14] | 0.001 | 0.001 |
| IFN−γ | 2.15 (0.56) | 2.08 (0.51) | [0.266, 1.15] | 0.828 | 0.830 | 1.85 (0.33) | 2.55 (0.46) | [−0.758, 0.620] | 0.004 | 0.040 |
| IL−1α | −1.12 (0.91) | −1.39 (0.47) | [0.386, 1.53] | 0.537 | 0.540 | −1.43 (0.29) | −0.47 (0.66) | [−1.23, 0.707] | 0.004 | 0.004 |
| IL−1β | −1.04 (0.70) | −1.62 (0.25) | [0.421, 1.65] | 0.102 | 0.100 | −1.62 (0.50) | −0.59 (0.60) | [−1.31, 0.155] | 0.003 | 0.003 |
| IL−2 | 7.19 (0.42) | 6.78 (0.41) | [0.194, 1.10] | 0.123 | 0.120 | 6.83 (0.22) | 7.48 (0.52) | [−0.933, 0.130] | 0.01 | 0.010 |
| IL−4 | −1.21 (0.49) | −1.63 (0.48) | [0.327, 1.42] | 0.169 | 0.170 | −1.59 (0.32) | −0.72 (0.61) | [−1.04, 0.209] | 0.005 | 0.005 |
| IL−5 | 1.19 (0.98) | 1.31 (0.39) | [0.259, 1.42] | 0.784 | 0.780 | 0.92 (0.46) | 1.76 (0.58) | [−0.906, 1.15] | 0.008 | 0.008 |
| IL−6 | 0.84 (0.88) | 0.87 (0.65) | [−0.275, 1.30] | 0.95 | 0.950 | 0.71 (0.77) | 1.22 (0.59) | [−0.978, 1.04] | 0.18 | 0.180 |
| IL−7 | 1.90 (0.26) | 1.55 (0.49) | [0.058, 0.673] | 0.155 | 0.150 | 1.83 (0.23) | 2.20 (0.31) | [−0.879, 0.168] | 0.023 | 0.023 |
| IL−10 | 1.99 (0.43) | 1.82 (0.53) | [0.024, 1.30] | 0.565 | 0.560 | 1.77 (0.66) | 2.43 (0.37) | [−0.790, 0.458] | 0.044 | 0.044 |
| IL−12 | 0.14 (0.77) | 0.05 (0.88) | [−0.117, 1.40] | 0.857 | 0.860 | 0.16 (0.80) | 0.81 (0.40) | [−1.16, 0.978] | 0.088 | 0.088 |
| IL−18 | 2.57 (0.08) | 2.65 (0.38) | [−0.671, 1.31] | 0.711 | 0.710 | 2.44 (0.81) | 2.76 (0.40) | [−0.510, 0.667] | 0.449 | 0.450 |
| M−CSF | −2.97 (0.35) | −3.15 (0.39) | [−0.261, 0.418] | 0.425 | 0.430 | −2.98 (0.34) | −2.90 (0.24) | [−0.656, 0.300] | 0.618 | 0.620 |
| MCP−1 | 2.70 (0.39) | 2.56 (0.56) | [0.018, 0.927] | 0.608 | 0.610 | 2.64 (0.46) | 3.11 (0.31) | [−0.781, 0.484] | 0.043 | 0.043 |
| MIP−3α | −1.28 (0.27) | −1.81 (0.52) | [−0.188, 0.614] | 0.059 | 0.059 | −1.24 (0.34) | −1.02 (0.37) | [−1.08, 0.026] | 0.272 | 0.270 |
| TNF−α | 5.23 (0.16) | 4.95 (0.39) | [−0.032, 0.477] | 0.137 | 0.140 | 5.19 (0.18) | 5.41 (0.27) | [−0.697, 0.120] | 0.081 | 0.081 |
| VEGF | −0.46 (1.17) | 0.41 (1.08) | [0.144, 3.24] | 0.366 | 0.370 | −1.45 (1.04) | 0.24 (0.56) | [−1.48, 3.24] | 0.038 | 0.038 |
| Variable (log2 Transformed) | HIV-1Tg Cocaine Interaction (WT: Saline Referent) Estimate | p-Value | 95% CI |
|---|---|---|---|
| G−CSF | 1.18 | 0.003 | [0.45, 1.91] |
| GM−CSF | 1.14 | 0.002 | [0.46, 1.81] |
| GRO/KC | 1.1 | 0.06 | [−0.05, 2.25] |
| IFN−γ | 0.78 | 0.042 | [0.03, 1.52] |
| IL−1α | 1.23 | 0.017 | [0.24, 2.22] |
| IL−1β | 1.62 | 0.001 | [0.74, 2.49] |
| IL−2 | 1.05 | 0.003 | [0.39, 1.71] |
| IL−4 | 1.29 | 0.003 | [0.50, 2.08] |
| IL−5 | 0.72 | 0.155 | [−0.29, 1.78] |
| IL−6 | 0.48 | 0.397 | [−0.67, 1.64] |
| IL−7 | 0.72 | 0.01 | [0.19, 1.25] |
| IL−10 | 0.83 | 0.046 | [0.01, 1.64] |
| IL−12 | 0.73 | 0.201 | [−0.42, 1.88] |
| IL−18 | 0.24 | 0.6 | [−0.71, 1.19] |
| M−CSF | 0.26 | 0.322 | [−0.27, 0.78] |
| MCP−1 | 0.62 | 0.076 | [−0.07, 1.31] |
| MIP−3α | 0.74 | 0.021 | [0.12, 1.36] |
| TNF−α | 0.51 | 0.019 | [0.09, 0.93] |
| VEGF | 0.81 | 0.393 | [−1.17, 2.80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezeomah, C.; Fongsaran, C.; Persons, A.L.; Napier, T.C.; Cisneros, I.E. Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats. Cells 2022, 11, 2405. https://doi.org/10.3390/cells11152405
Ezeomah C, Fongsaran C, Persons AL, Napier TC, Cisneros IE. Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats. Cells. 2022; 11(15):2405. https://doi.org/10.3390/cells11152405
Chicago/Turabian StyleEzeomah, Chiomah, Chanida Fongsaran, Amanda L. Persons, T. Celeste Napier, and Irma E. Cisneros. 2022. "Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats" Cells 11, no. 15: 2405. https://doi.org/10.3390/cells11152405
APA StyleEzeomah, C., Fongsaran, C., Persons, A. L., Napier, T. C., & Cisneros, I. E. (2022). Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats. Cells, 11(15), 2405. https://doi.org/10.3390/cells11152405






