Regulation of APD and Force by the Na+/Ca2+ Exchanger in Human-Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue
Abstract
:1. Introduction
2. Methods
2.1. Rat Tissue
2.2. Human Tissue
2.3. Human-Induced Pluripotent stem cell-Derived Engineered Heart Tissue
2.4. Patch Clamp Measurements
2.5. Action Potential Measurements
2.6. Contractility Measurements
2.7. Computational Simulations with Rat, Human and hiPSC Cardiomyocyte Models
2.8. Drugs
2.9. Statistics
3. Results
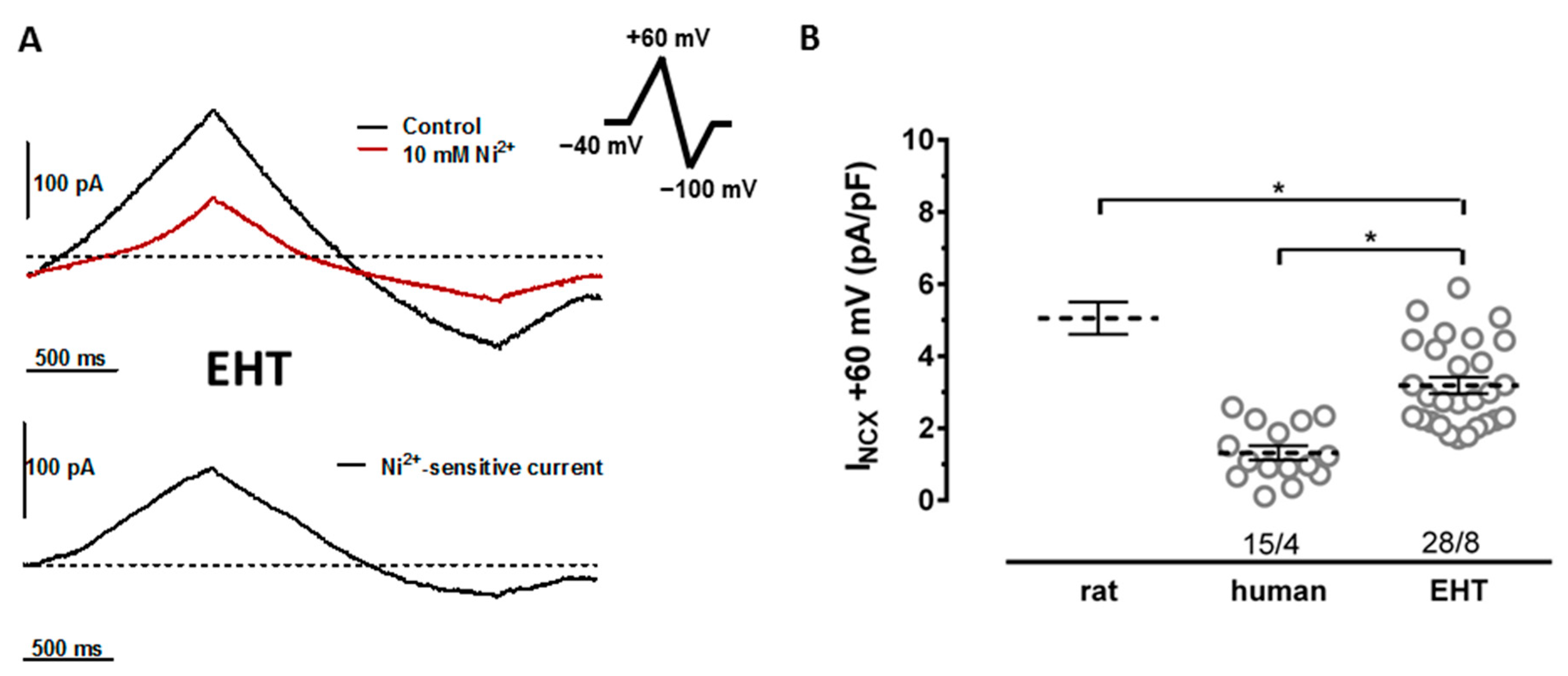
3.1. NCX Currents in hiPSC-CMs Are Not Smaller Than in Human Ventricular Cardiomyocytes
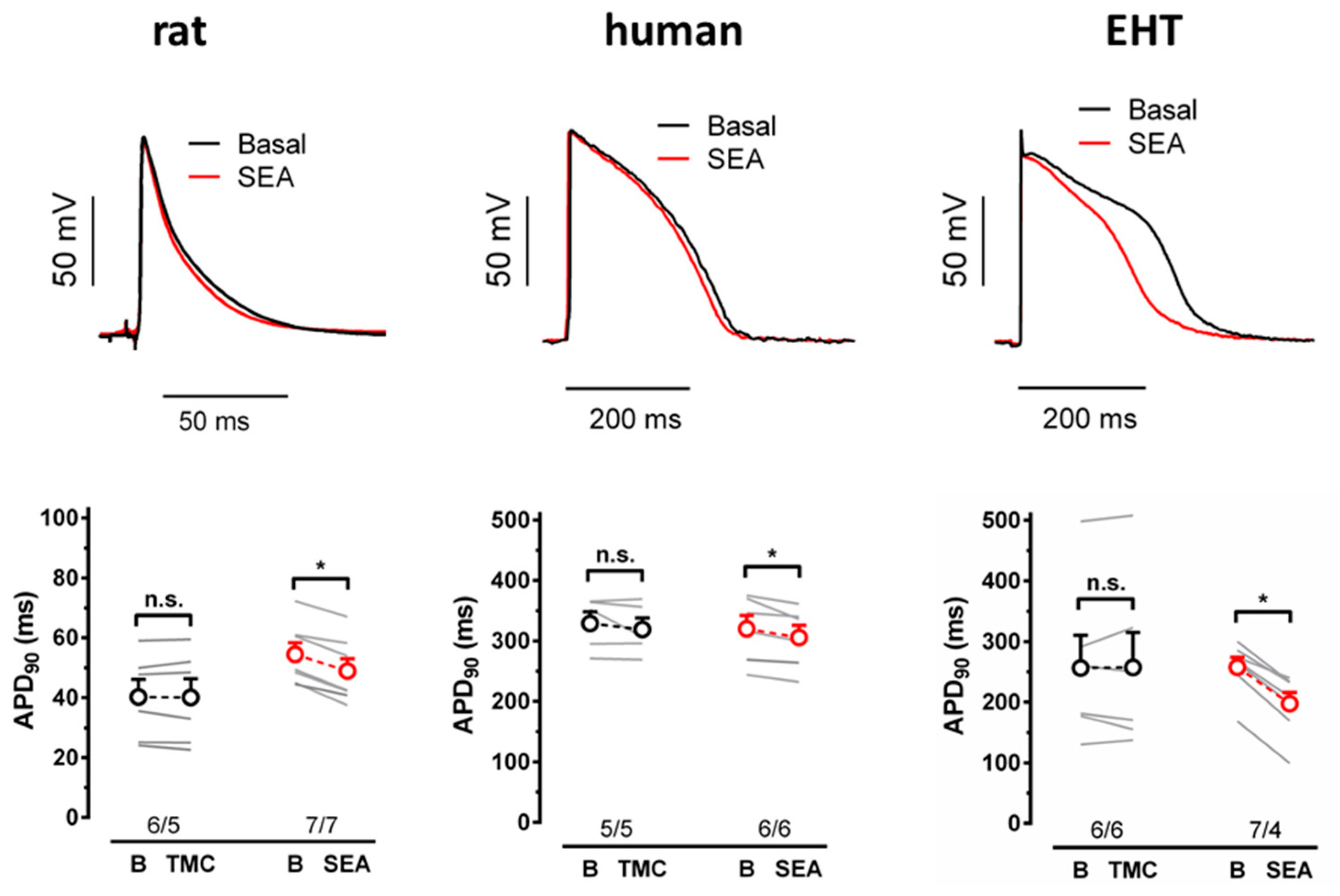
3.2. NCX Block Shortens the APD in EHT but Not in the Human Left Ventricle
3.3. SEA0400 Does Not Block Calcium Currents in hiPSC-CMs
3.4. NCX Block Increases Force in EHT but Not in the Human Left Ventricle
3.5. Effects of NCX Block on AP and Force in Comparison to Computational Model Predictions
4. Discussion
4.1. NCX Current Density
4.2. Contribution of NCX to the APD in Heart Muscle
4.3. Contribution of NCX to Force in Heart Muscle
4.4. Effect of NCX Block on the APD and Force Are Not Compromised by SEA0400’s Selectivity
4.5. Effects of NCX Block: Immature EHT vs. Mature LV
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APs | action potentials |
| APA | action potential amplitude |
| APD | action potential duration |
| APD90 | action potential duration at 90% repolarization |
| CaT | calcium transient |
| CM | cardiomyocyte |
| Cs | cell shortening |
| EHT | engineered heart tissue |
| hiPSC-CM | human-induced pluripotent stem cell-derived cardiomyocyte |
| ICa | calcium current |
| IK1 | inwardly rectifying potassium current density |
| IKr | rapidly activating potassium current |
| KO | knock-out |
| LV | left ventricle |
| NCX | sodium calcium exchanger |
| PMCA | plasma membrane Ca2+-ATPase |
| TMCs | time-matched controls |
References
- Eschenhagen, T.; Carrier, L. Cardiomyopathy phenotypes in human-induced pluripotent stem cell-derived cardiomyocytes–A systematic review. Pflugers Arch. 2019, 471, 755. [Google Scholar] [CrossRef] [Green Version]
- Paci, M.; Penttinen, K.; Pekkanen-Mattila, M.; Koivumäki, J.T. Arrhythmia Mechanisms in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Cardiovasc. Pharmacol. 2020, 77, 300–316. [Google Scholar] [CrossRef]
- Zhang, X.h.; Morad, M. Ca2+ signaling of human pluripotent stem cells-derived cardiomyocytes as compared to adult mammalian cardiomyocytes. Cell Calcium. 2020, 90, 102244. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kryshtal, D.O.; Feaster, T.K.; Sánchez-Freire, V.; Zhang, J.; Kamp, T.J.; Hong, C.C.; Wu, J.C.; Knollmann, B.C. Human induced pluripotent stem cell (hiPSC) derived cardiomyocytes to understand and test cardiac calcium handling: A glass half full. J. Mol. Cell. Cardiol. 2015, 89, 379–380. [Google Scholar] [CrossRef]
- Kane, C.; Couch, L.; Terracciano, C.M.N. Excitation–contraction coupling of human induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 2015, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jost, N.; Nagy, N.; Corici, C.; Kohajda, Z.; Horváth, A.; Acsai, K.; Biliczki, P.; Levijoki, J.; Pollesello, P.; Koskelainen, T.; et al. ORM-10103, a novel specific inhibitor of the Na+/Ca2+ exchanger, decreases early and delayed afterdepolarizations in the canine heart. Br. J. Pharmacol. 2013, 170, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Christ, T.; Kovács, P.P.; Acsai, K.; Knaut, M.; Eschenhagen, T.; Jost, N.; Varró, A.; Wettwer, E.; Ravens, U. Block of Na+/Ca2+ exchanger by SEA0400 in human right atrial preparations from patients in sinus rhythm and in atrial fibrillation. Eur. J. Pharmacol. 2016, 788, 286–293. [Google Scholar] [CrossRef]
- Dobrev, D.; Wettwer, E.; Himmel, H.M.; Kortner, A.; Kuhlisch, E.; Schüler, S.; Siffert, W.; Ravens, U. G-protein β3-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation 2000, 102, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Breckwoldt, K.; Letuffe-Brenière, D.; Mannhardt, I.; Schulze, T.; Ulmer, B.; Werner, T.; Benzin, A.; Klampe, B.; Reinsch, M.C.; Laufer, S.; et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017, 12, 1177–1190. [Google Scholar] [CrossRef]
- Lemoine, M.D.; Krause, T.; Koivumäki, J.T.; Prondzynski, M.; Schulze, M.L.; Girdauskas, E.; Willems, S.; Hansen, A.; Eschenhagen, T.; Christ, T. Human Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue as a Sensitive Test System for QT Prolongation and Arrhythmic Triggers. Circ. Arrhythmia Electrophysiol. 2018, 11, e006035. [Google Scholar] [CrossRef]
- Pecha, S.; Flenner, F.; Söhren, K.D.; Lorenz, K.; Eschenhagen, T.; Christ, T. β1 Adrenoceptor antagonistic effects of the supposedly selective β2 adrenoceptor antagonist ICI 118,551 on the positive inotropic effect of adrenaline in murine hearts. Pharmacol. Res. Perspect. 2015, 3, e00168. [Google Scholar] [CrossRef]
- Kloth, B.; Pecha, S.; Moritz, E.; Schneeberger, Y.; Söhren, K.D.; Schwedhelm, E.; Reichenspurner, H.; Eschenhagen, T.; Böger, R.H.; Christ, T.; et al. AkrinorTM, a cafedrine/theodrenaline mixture (20:1), increases force of contraction of human atrial myocardium but does not constrict internal mammary artery in vitro. Front. Pharmacol. 2017, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Pecha, S.; Koivumäki, J.; Geelhoed, B.; Kempe, R.; Berk, E.; Engel, A.; Reichenspurner, H.; Eschenhagen, T.; Ravens, U.; Kaumann, A.; et al. Normalization of force to muscle cross-sectional area: A helpful attempt to reduce data scattering in contractility studies? Acta Physiol. 2018, 224, e13202. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.; Eder, A.; Bonstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schworer, A.; Uebeler, J.; Eschenhagen, T.; et al. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Gattoni, S.; Røe, Å.T.; Frisk, M.; Louch, W.E.; Niederer, S.A.; Smith, N.P. The calcium–frequency response in the rat ventricular myocyte: An experimental and modelling study. J. Physiol. 2016, 594, 4193–4224. [Google Scholar] [CrossRef] [Green Version]
- Margara, F.; Wang, Z.J.; Levrero-Florencio, F.; Santiago, A.; Vázquez, M.; Bueno-Orovio, A.; Rodriguez, B. In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment. Prog. Biophys. Mol. Biol. 2021, 159, 58–74. [Google Scholar] [CrossRef]
- Prondzynski, M.; Lemoine, M.D.; Zech, A.T.; Horváth, A.; di Mauro, V.; Koivumäki, J.T.; Kresin, N.; Busch, J.; Krause, T.; Krämer, E.; et al. Disease modeling of a mutation in α-actinin 2 guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mol. Med. 2019, 11, e11115. [Google Scholar] [CrossRef]
- Otsomaa, L.; Levijoki, J.; Wohlfahrt, G.; Chapman, H.; Koivisto, A.-P.; Syrjanen, K.; Koskelainen, T.; Peltokorpi, S.-E.; Finckenberg, P.; Heikkila, A.; et al. Discovery and characterization of ORM-11372, a unique and positively inotropic sodium-calcium exchanger/inhibitor. Br. J. Pharmacol. 2020, 177, 5534–5554. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium–Calcium exchangers (NCX)–Structure, function, and regulation in health and disease. Mol. Aspects Med. 2013, 34, 220–235. [Google Scholar] [CrossRef]
- Shattock, M.J.; Ottolia, M.; Bers, D.M.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.B.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 2015, 593, 1361–1382. [Google Scholar] [CrossRef] [Green Version]
- Uzun, A.U.; Mannhardt, I.; Breckwoldt, K.; Horváth, A.; Johannsen, S.S.; Hansen, A.; Eschenhagen, T.; Christ, T. Ca2+-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front. Pharmacol. 2016, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Bers, D.M. Cardiac excitation contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Negretti, N.; O’Neill, S.C.; Eisner, D.A. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc. Res. 1993, 27, 1826–1830. [Google Scholar] [CrossRef]
- Choi, H.S.; Eisner, D.A. The effects of inhibition of the sarcolemmal Ca-ATPase on systolic calcium fluxes and intracellular calcium concentration in rat ventricular myocytes. Pflügers Arch. 1999, 437, 966–971. [Google Scholar] [CrossRef]
- Choi, H.S.; Eisner, D.A. The role of sarcolemmal Ca2+-ATPase in the regulation of resting calcium concentration in rat ventricular myocytes. J. Physiol. 1999, 515, 109–118. [Google Scholar] [CrossRef]
- Ismaili, D.; Geelhoed, B.; Christ, T. Ca2+ currents in cardiomyocytes: How to improve interpretation of patch clamp data? Prog. Biophys. Mol. Biol. 2020, 157, 33–39. [Google Scholar] [CrossRef]
- Christ, T.; Galindo-Tovar, A.; Thoms, M.; Ravens, U.; Kaumann, A.J. Inotropy and L-type Ca2+ current, activated by β 1-and β 2 -adrenoceptors, are differently controlled by phosphodiesterases 3 and 4 in rat heart. Br. J. Pharmacol. 2009, 156, 62–83. [Google Scholar] [CrossRef] [Green Version]
- Nagy, N.; Kormos, A.; Kohajda, Z.; Szebeni, Á.; Szepesi, J.; Pollesello, P.; Levijoki, J.; Acsai, K.; Virág, L.; Nánási, P.P.; et al. Selective Na+/Ca2+ exchanger inhibition prevents Ca2+ overload-induced triggered arrhythmias. Br. J. Pharmacol. 2014, 171, 5665–5681. [Google Scholar] [CrossRef] [Green Version]
- Kohajda, Z.; Farkas-Morvay, N.; Jost, N.; Nagy, N.; Geramipour, A.; Horváth, A.; Varga, R.S.; Hornyik, T.; Corici, C.; Acsai, K.; et al. The Effect of a Novel Highly Selective Inhibitor of the Sodium/Calcium Exchanger (NCX) on Cardiac Arrhythmias in In Vitro and In Vivo Experiments. PLoS ONE 2016, 11, e0166041. [Google Scholar] [CrossRef]
- Geramipour, A.; Kohajda, Z.; Corici, C.; Prorok, J.; Szakonyi, Z.; Oravecz, K.; Márton, Z.; Nagy, N.; Tóth, A.; Acsai, K.; et al. The investigation of the cellular electrophysiological and antiarrhythmic effects of a novel selective sodium–calcium exchanger inhibitor, GYKB-6635, in canine and guinea-pig hearts. Can. J. Physiol. Pharmacol. 2016, 94, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Philipson, K.D.; Goldhaber, J.I. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: Reduced transsarcolemmal Ca2+ flux. Circ. Res. 2005, 97, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szentandrássy, N.; Birinyi, P.; Szigeti, G.; Farkas, A.; Magyar, J.; Tóth, A.; Csernoch, L.; Varró, A.; Nánási, P.P. SEA0400 fails to alter the magnitude of intracellular Ca2+ transients and contractions in Langendorff-perfused guinea pig heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Nakamura, H.; Shimada, H.; Tanaka, H.; Shigenobu, K. Cardioprotection without cardiosuppression by SEA0400, a novel inhibitor of Na+-Ca2+ exchanger, during ischemia and reperfusion in guinea-pig myocardium. Life Sci. 2005, 77, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shimada, H.; Namekata, I.; Kawanishi, T.; Iida-Tanaka, N.; Shigenobu, K. Involvement of the Na+/Ca2+ Exchanger in Ouabain-Induced Inotropy and Arrhythmogenesis in Guinea-Pig Myocardium as Revealed by SEA0400. J. Pharmacol. Sci. 2007, 103, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, U.; Ismaili, D.; Mannhardt, I.; Pinnschmidt, H.; Schulze, T.; Christ, T.; Eschenhagen, T.; Hansen, A. Regulation of ICa,L and force by PDEs in human-induced pluripotent stem cell-derived cardiomyocytes. Br. J. Pharmacol. 2020, 177, 3036–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birinyi, P.; Tóth, A.; Jóna, I.; Acsai, K.; Almássy, J.; Nagy, N.; Prorok, J.; Gherasim, I.; Papp, Z.; Hertelendi, Z.; et al. The Na+/Ca2+ exchange blocker SEA0400 fails to enhance cytosolic Ca2+ transient and contractility in canine ventricular cardiomyocytes. Cardiovasc. Res. 2008, 78, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Birinyi, P.; Acsai, K.; Bányász, T.; Tóth, A.; Horváth, B.; Virág, L.; Szentandrássy, N.; Magyar, J.; Varró, A.; Fülöp, F.; et al. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2005, 372, 63–70. [Google Scholar] [CrossRef]
- Acsai, K.; Kun, A.; Farkas, A.S.; Fülöp, F.; Nagy, N.; Balázs, M.; Szentandrássy, N.; Nánási, P.P.; Papp, J.G.; Varró, A.; et al. Effect of partial blockade of the Na+/Ca2+-exchanger on Ca2+ handling in isolated rat ventricular myocytes. Eur. J. Pharmacol. 2007, 576, 1–6. [Google Scholar] [CrossRef]
- Bourgonje, V.J.A.; Vos, M.A.; Ozdemir, S.; Doisne, N.; Acsai, K.; Varro, A.; Sztojkov-Ivanov, A.; Zupko, I.; Rauch, E.; Kattner, L.; et al. Combined Na+/Ca2+ Exchanger and L-Type Calcium Channel Block as a Potential Strategy to Suppress Arrhythmias and Maintain Ventricular Function. Circ. Arrhythmia Electrophysiol. 2013, 6, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Z.A.; Virág, L.; Tóth, A.; Biliczki, P.; Acsai, K.; Bányász, T.; Nánási, P.; Papp, J.G.; Varró, A. Selective inhibition of sodium–calcium exchanger by SEA-0400 decreases early and delayed afterdepolarization in canine heart. Br. J. Pharmacol. 2004, 143, 827–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oravecz, K.; Kormos, A.; Gruber, A.; Márton, Z.; Kohajda, Z.; Mirzaei, L.; Jost, N.; Levijoki, J.; Pollesello, P.; Koskelainen, T.; et al. Inotropic effect of NCX inhibition depends on the relative activity of the reverse NCX assessed by a novel inhibitor ORM-10962 on canine ventricular myocytes. Eur. J. Pharmacol. 2018, 818, 278–286. [Google Scholar] [CrossRef]
- Ozdemir, S.; Bito, V.; Holemans, P.; Vinet, L.; Mercadier, J.-J.; Varro, A.; Sipido, K.R. Pharmacological Inhibition of Na/Ca Exchange Results in Increased Cellular Ca2+ Load Attributable to the Predominance of Forward Mode Block. Circ. Res. 2008, 102, 1398–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bögeholz, N.; Schulte, J.S.; Kaese, S.; Bauer, B.K.; Pauls, P.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Kirchhefer, U.; Eckardt, L.; et al. The Effects of SEA0400 on Ca2+ Transient Amplitude and Proarrhythmia Depend on the Na+/Ca2+ Exchanger Expression Level in Murine Models. Front. Pharmacol. 2017, 8, 649. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Namekata, I.; Takeda, K.; Kazama, A.; Shimizu, Y.; Moriwaki, R.; Hirayama, W.; Sato, A.; Kawanishi, T.; Shigenobu, K. Unique excitation–contraction characteristics of mouse myocardium as revealed by SEA0400, a specific inhibitor of Na+–Ca2+ exchanger. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2005, 371, 526–534. [Google Scholar] [CrossRef]
- Amran, M.S.; Hashimoto, K.; Homma, N. Effects of Sodium-Calcium Exchange Inhibitors, KB-R7943 and SEA0400, on Aconitine-Induced Arrhythmias in Guinea Pigs in Vivo, in Vitro, and in Computer Simulation Studies. J. Pharmacol. Exp. Ther. 2004, 310, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogwizd, S.M.; Schlotthauer, K.; Li, L.; Yuan, W.; Bers, D.M. Arrhythmogenesis and Contractile Dysfunction in Heart Failure. Circ. Res. 2001, 88, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Studer, R.; Reinecke, H.; Bilger, J.; Eschenhagen, T.; Böhm, M.; Hasenfuß, G.; Just, H.; Holtz, J.; Drexler, H. Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ. Res. 1994, 75, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Carafoli, E. Calcium pump of the plasma membrane. Physiol. Rev. 1991, 71, 129–153. [Google Scholar] [CrossRef]
- Farkas, A.S.; Acsai, K.; Nagy, N.; Tóth, A.; Fülöp, F.; Seprényi, G.; Birinyi, P.; Nánási, P.P.; Forster, T.; Csanády, M.; et al. Na+/Ca2+ exchanger inhibition exerts a positive inotropic effect in the rat heart, but fails to influence the contractility of the rabbit heart. Br. J. Pharmacol. 2008, 154, 93–104. [Google Scholar] [CrossRef] [Green Version]


| Species | Cell Type | Substance | Concentration (in µM) | APD90 | Peak CaT | Cs | Force | Reference |
|---|---|---|---|---|---|---|---|---|
| Dog | Ventricle | SEA0400 | 1 | — | ↔ | — | — | Nagy et al. [41] |
| SEA0400 | 1 | — | ↔ | ↔ | — | Birinyi et al. [37] | ||
| SEA0400 | 1 | ↓ | ↔ | — | — | Bourgonje et al. [40] | ||
| SEA0400 | 1 | ↔ | ↔ | ↔ | — | Nagy et al. [29] | ||
| ORM-10103 | 10 | ↔ | ↔ | ↔ | — | |||
| GYKB-6635 | 1 | ↔ | — | — | — | Geramipouretal. [31] | ||
| ORM-10962 | 1 | ↔ | ↑ | ↑ | — | Kohajda et al. [30] | ||
| ORM-10962 | 1 | — | ↑ | ↑ | — | Oravecz et al. [42] | ||
| Human | Ventricle | ORM-11372 | 10 | ↔ | — | — | — | Otsomaa et al. [19] |
| Atrium | SEA0400 | 10 | ↔ | — | — | ↔ | Christ et al. [7] | |
| HiPSC | ORM-11372 | 0.1; 0.3 | ↓ | — | — | — | Otsomaa et al. [19] | |
| Rat | Ventricle | SEA0400 | 0.3 | — | ↑ | ↑ | — | Acsai et al. [39] |
| SEA0400 | 1 | — | ↑ | — | — | Szentandrássy et al. [33] | ||
| Mouse | Ventricle | SEA0400 | 0.3; 1 | — | ↑ | ↑ | — | Ozdemir et al. [43] |
| SEA0400 | 1 | — | ↑ | — | — | Bögeholz et al. [44] | ||
| SEA0400 | 1; 10 | ↓ | ↑ | ↑ | ↑ | Tanaka et al. [45] | ||
| Guinea pig | Ventricle | SEA0400 | 1 | — | ↔ | — | — | Szentandrássy et al. [33] |
| SEA0400 | 1 | — | — | — | ↔ | Tanaka et al. [35] | ||
| SEA0400 | 1; 10; 100 | ↔ | — | — | — | Amran et al. [46] | ||
| SEA0400 | 1 | ↔ | — | — | ↔ | Namekata et al. [34] | ||
| Pig | ventricle | SEA0400 | 0.3; 1 | — | ↑ | ↑ | — | Ozdemir et al. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaili, D.; Gurr, K.; Horváth, A.; Yuan, L.; Lemoine, M.D.; Schulz, C.; Sani, J.; Petersen, J.; Reichenspurner, H.; Kirchhof, P.; et al. Regulation of APD and Force by the Na+/Ca2+ Exchanger in Human-Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue. Cells 2022, 11, 2424. https://doi.org/10.3390/cells11152424
Ismaili D, Gurr K, Horváth A, Yuan L, Lemoine MD, Schulz C, Sani J, Petersen J, Reichenspurner H, Kirchhof P, et al. Regulation of APD and Force by the Na+/Ca2+ Exchanger in Human-Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue. Cells. 2022; 11(15):2424. https://doi.org/10.3390/cells11152424
Chicago/Turabian StyleIsmaili, Djemail, Katrin Gurr, András Horváth, Lei Yuan, Marc D. Lemoine, Carl Schulz, Jascha Sani, Johannes Petersen, Hermann Reichenspurner, Paulus Kirchhof, and et al. 2022. "Regulation of APD and Force by the Na+/Ca2+ Exchanger in Human-Induced Pluripotent Stem Cell-Derived Engineered Heart Tissue" Cells 11, no. 15: 2424. https://doi.org/10.3390/cells11152424







