Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs
Abstract
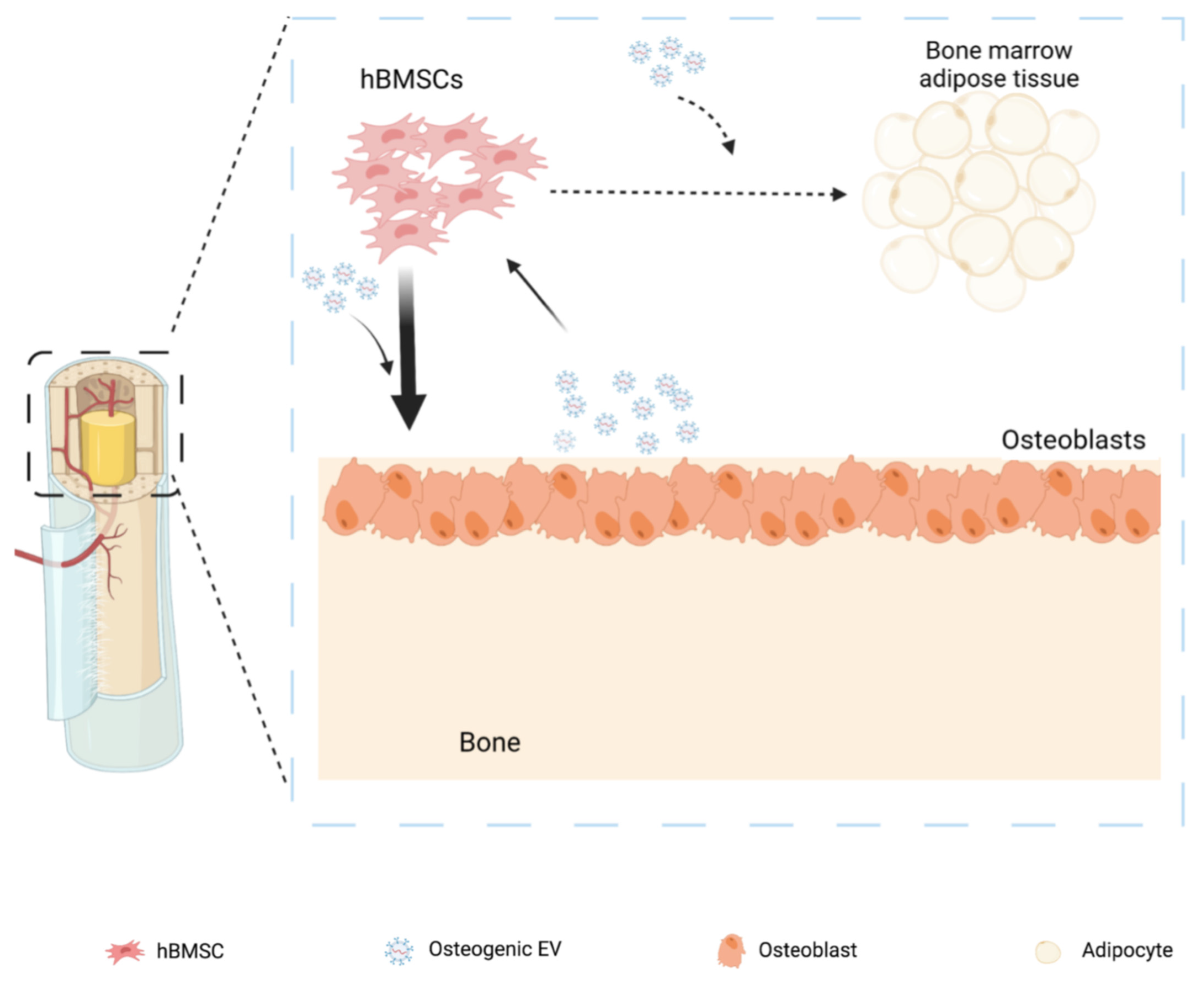
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Isolation and Culture of Human BMSCs
2.3. Osteogenic Differentiation of Human BMSCs
2.4. Adipogenic Differentiation of Human BMSCs
2.5. Generation of EVs-Depleted FCS
2.6. Collection of Conditioned Medium for EV Isolation
2.7. EV Isolation
2.8. Western Blot Analysis for Detection of EV Markers CD9, CD63 and CD81
2.9. EV Uptake Test
2.10. Nanoparticle Tracking Analysis (NTA)
2.11. Proliferation Assay
2.12. Apoptosis Assay
2.13. Viability Assay
2.14. Alkaline Phosphatase Assay
2.15. Alizarin Red Staining
2.16. Oil Red O Staining
2.17. RNA Isolation and Real-Time RT-PCR
2.18. Proteomics Analysis
2.18.1. Sample Preparation of EVs for Proteomics Analysis
2.18.2. Quantitative Proteomic Analysis by LC-MS/MS
2.18.3. Database Search and Bioinformatics Analysis
2.19. Statistical Analysis
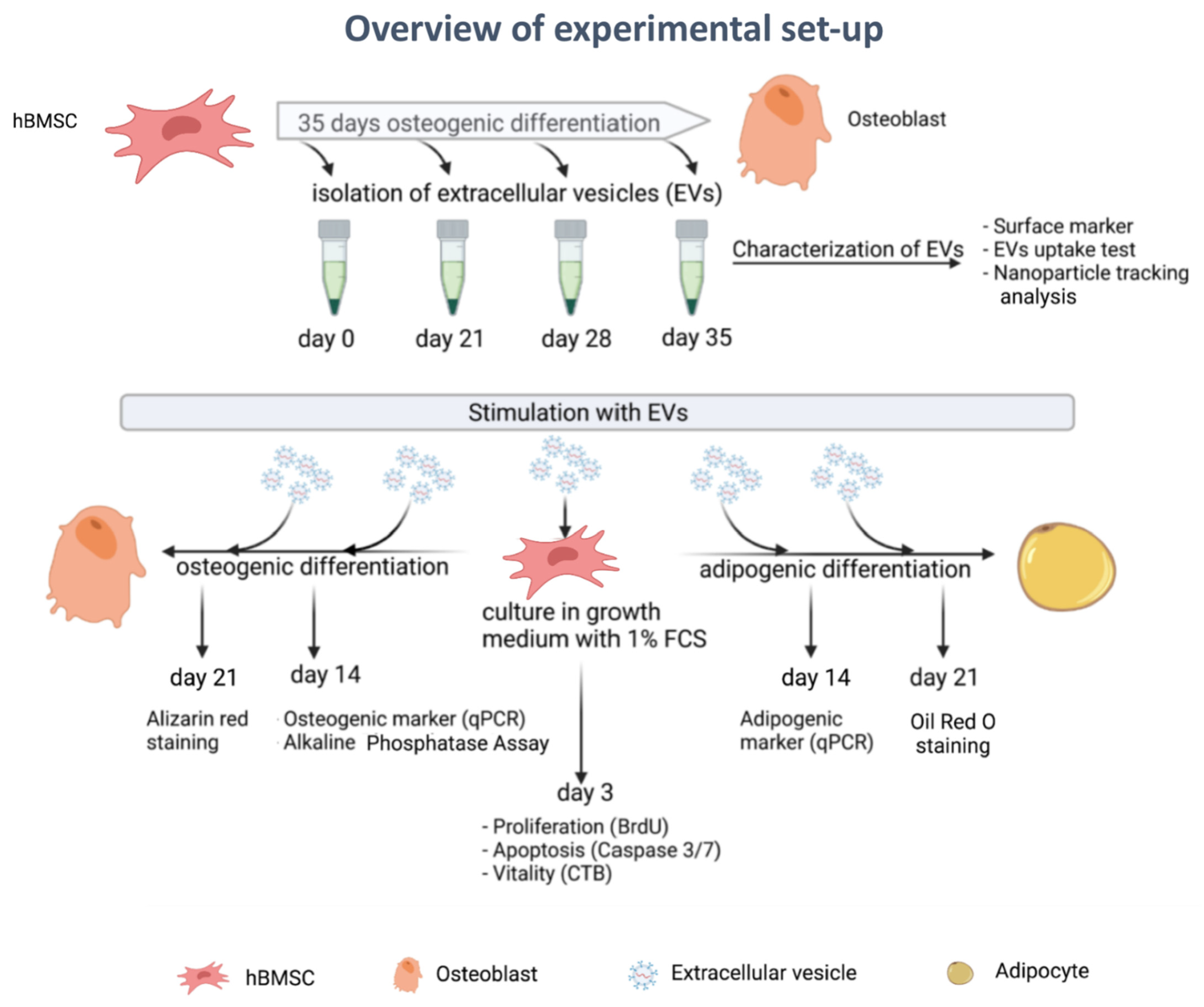
3. Results
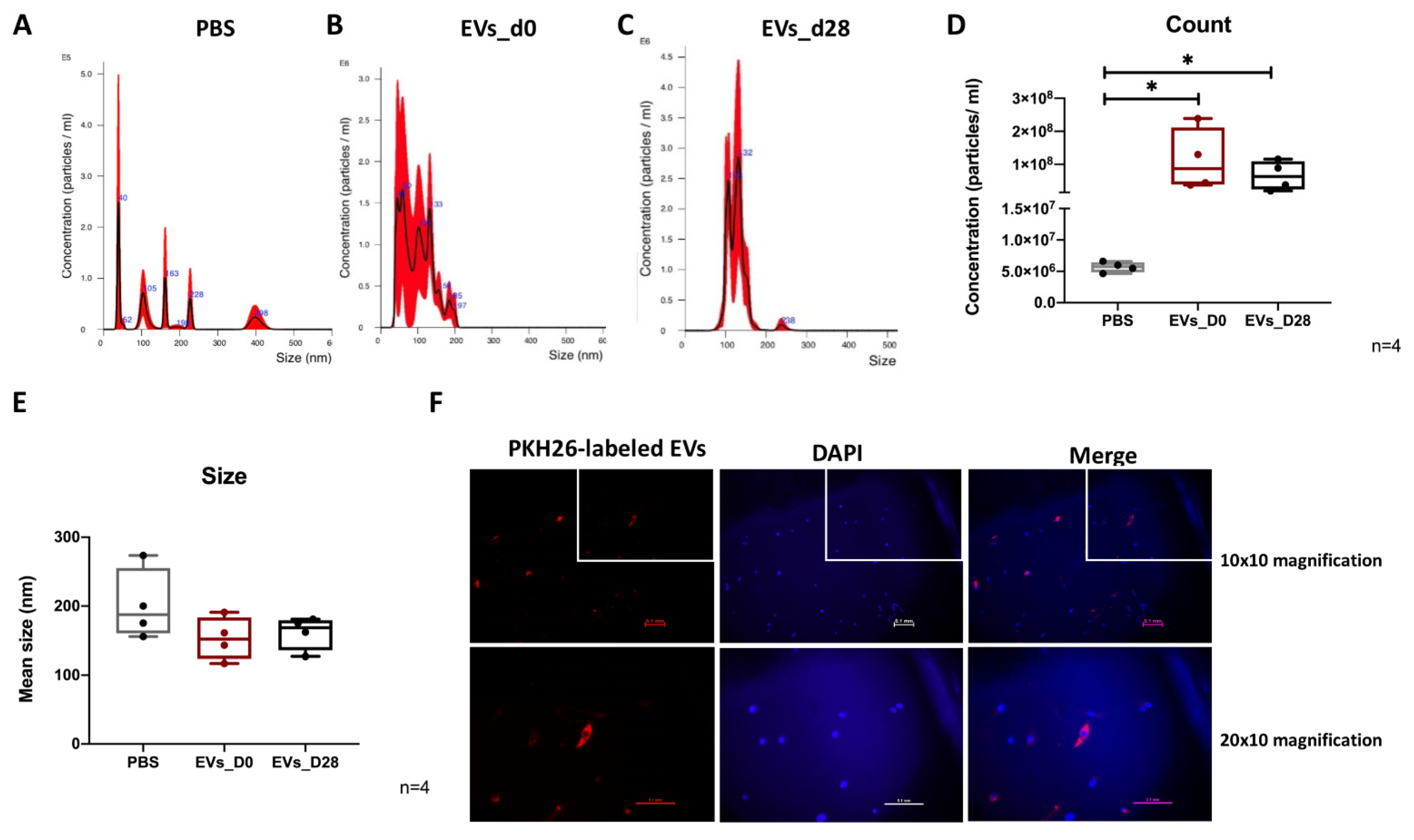
3.1. EV Isolation and Characterization
3.2. NTA Evaluation of EVs
3.3. Uptake of EVs by hBMSCs
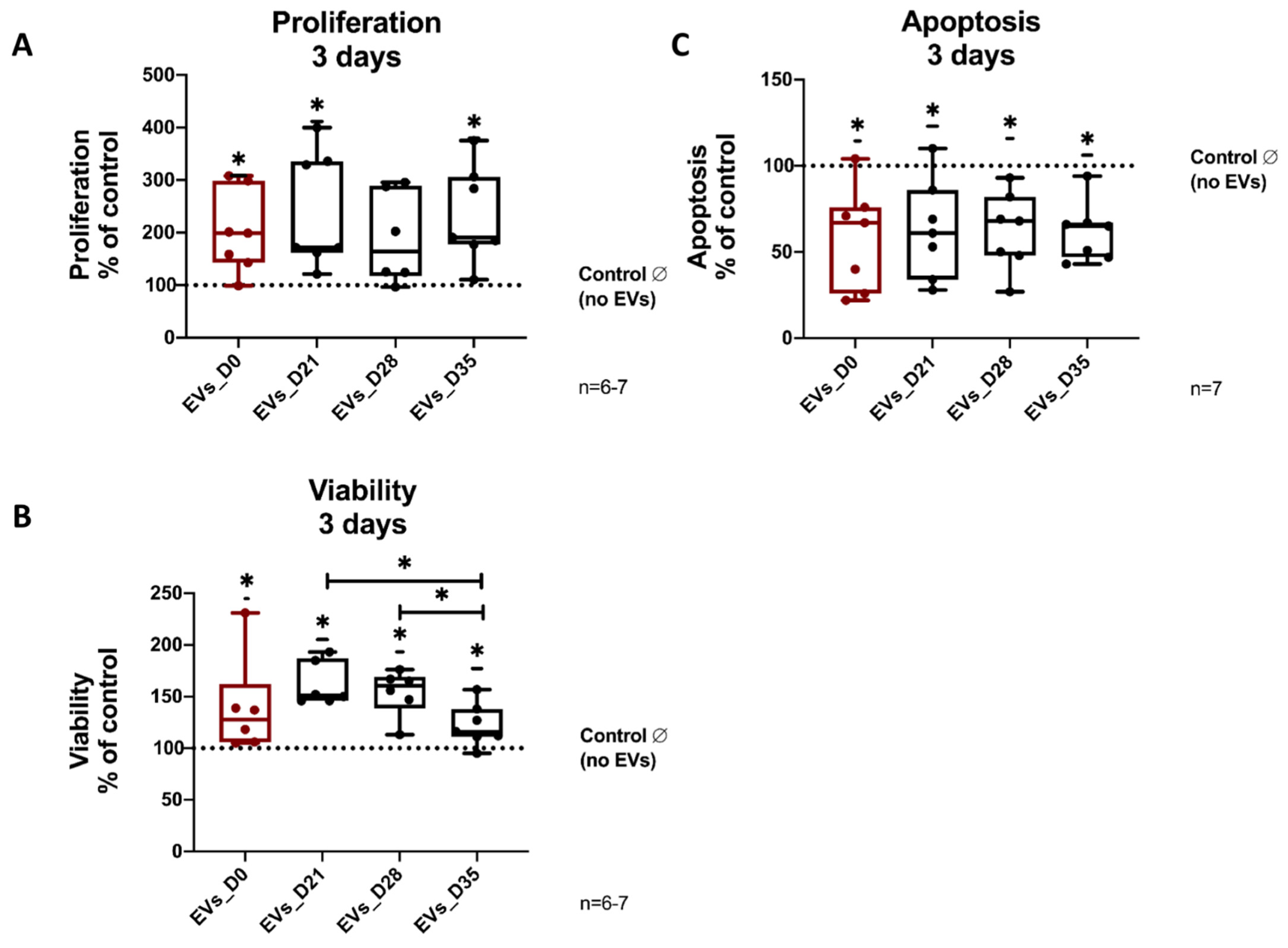
3.4. Effects of EVs Derived from Different Stages of Osteogenic Differentiating hBMSCs on Proliferation, Vitality and Apoptosis of Naïve hBMSCs
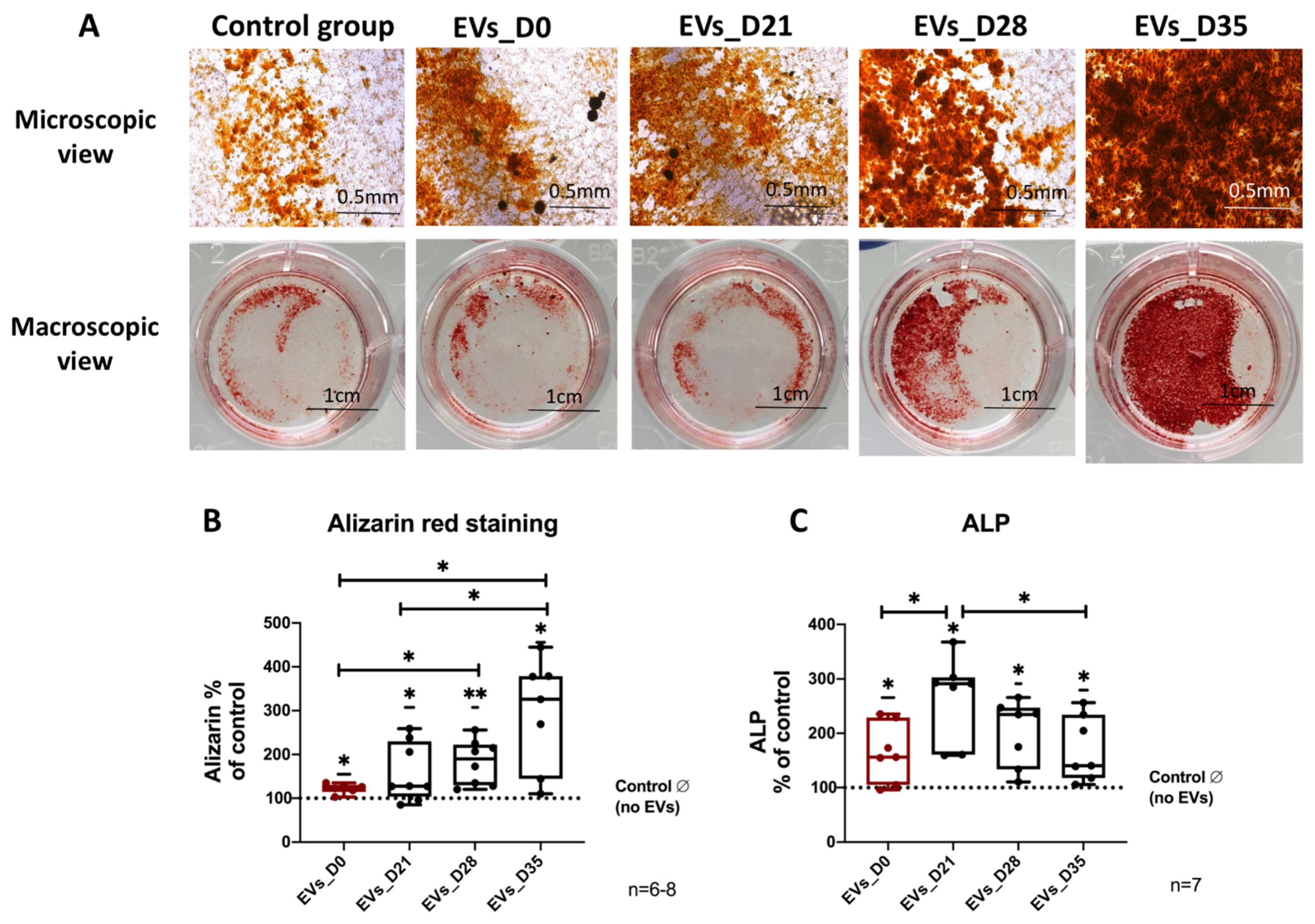
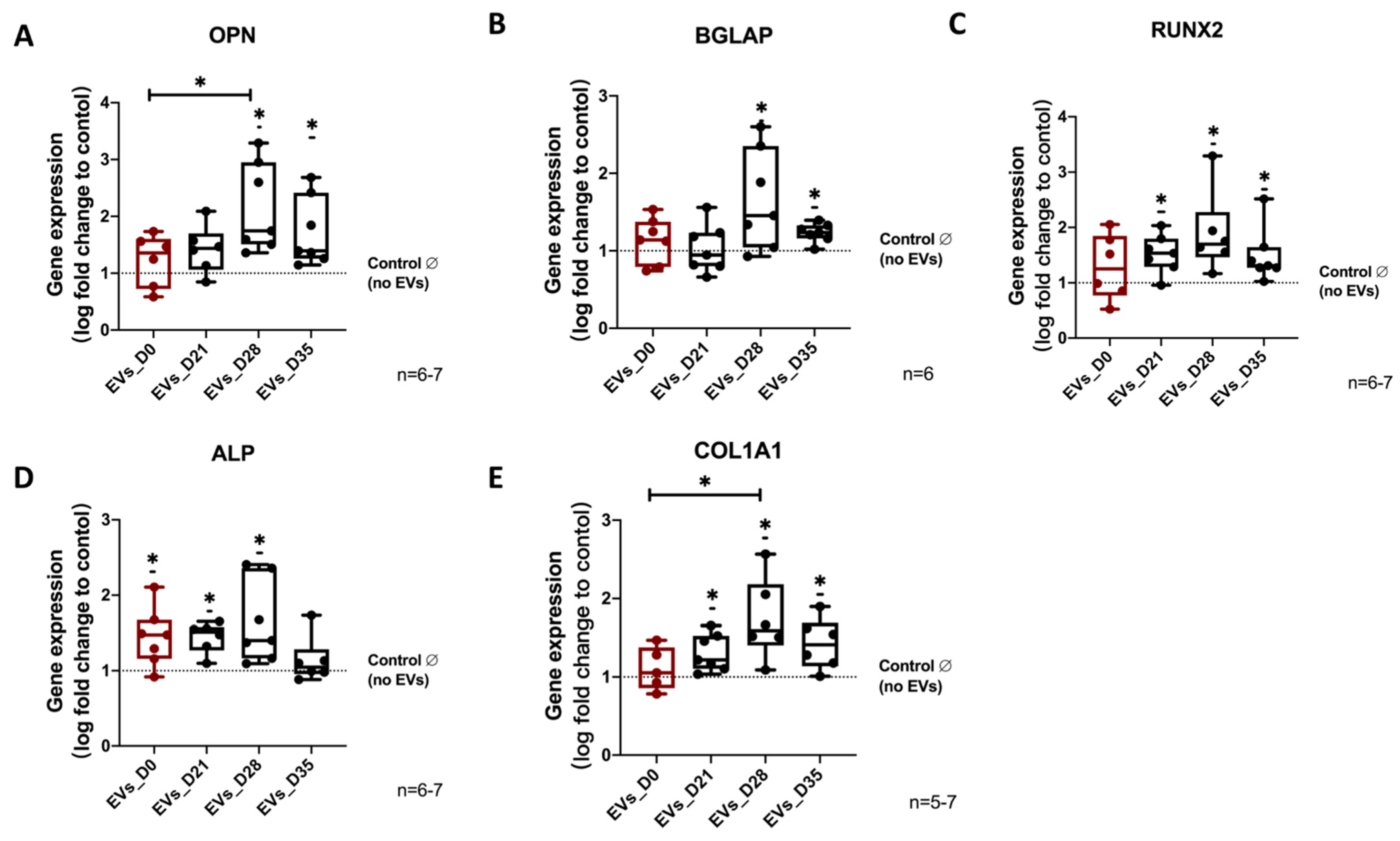
3.5. Effect of EVs Derived from Different Stages of Osteogenic Differentiated hBMSC on Osteogenic Differentiation of Naïve hBMSCs
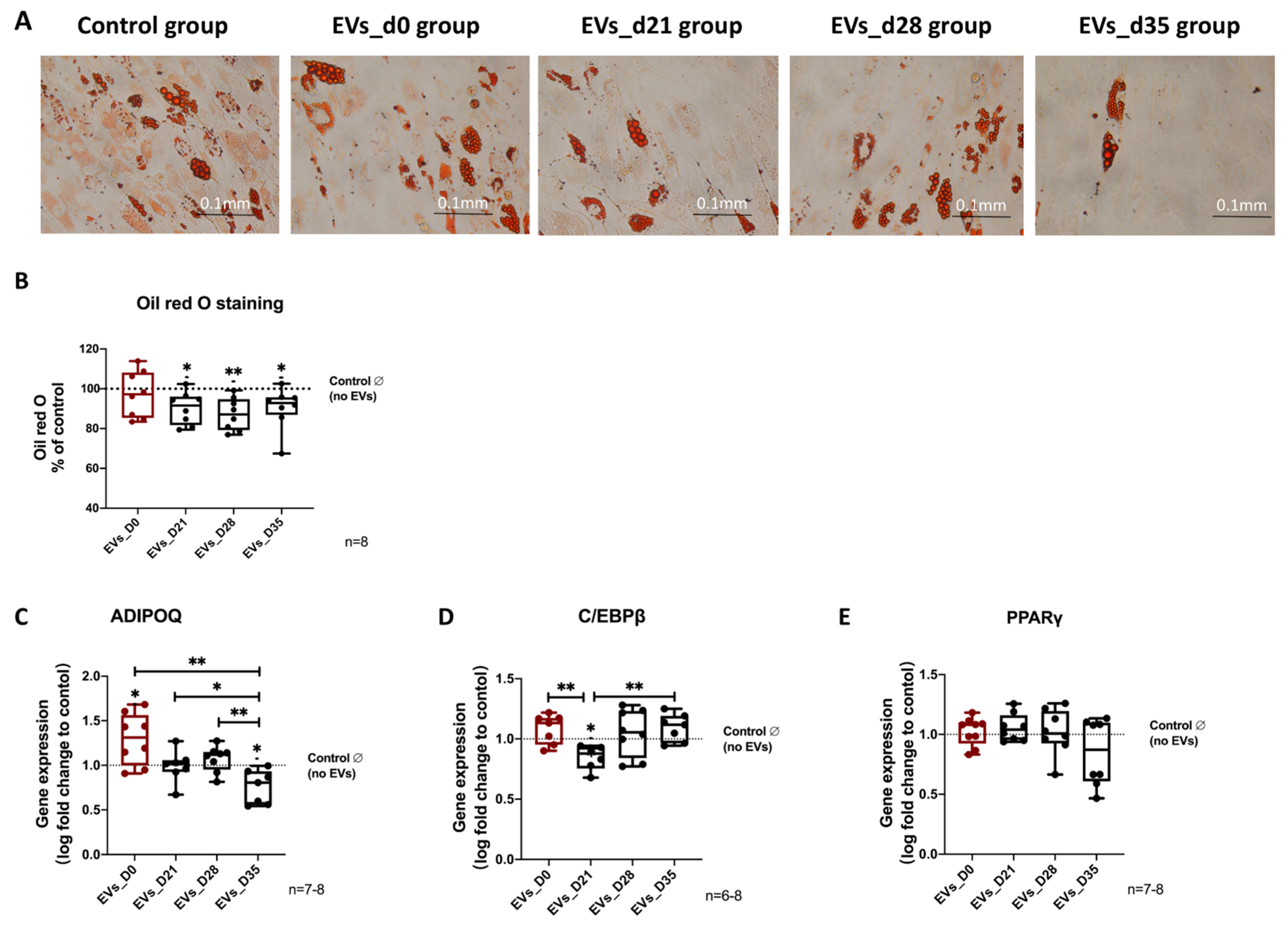
3.6. Effects of EVs Derived from Different Stages of Osteogenic Differentiated hBMSCs on Adipogenic Differentiation of Naïve hBMSCs
3.7. Proteomic Analysis of Osteogenic EVs and Naïve-EVs
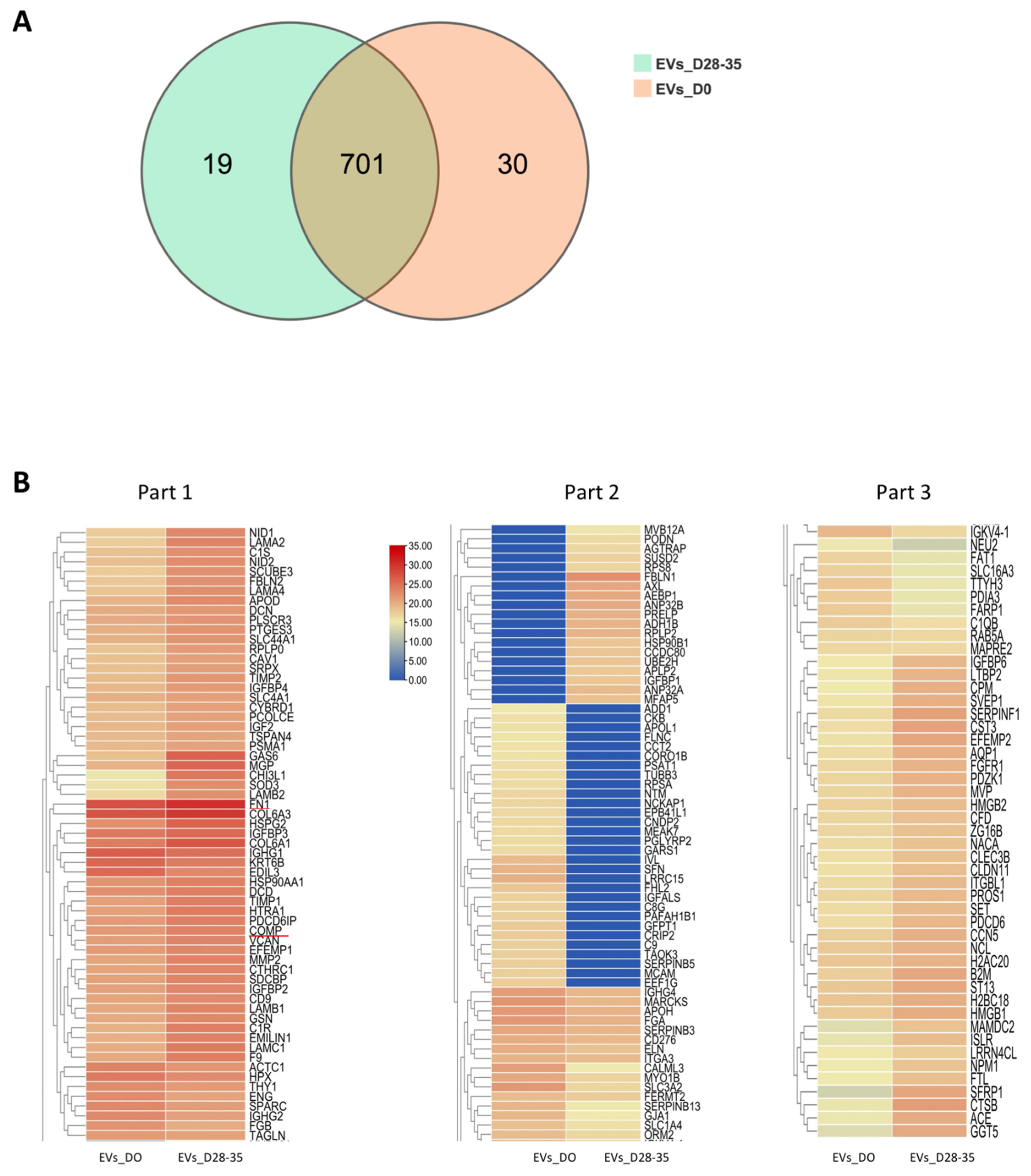
3.7.1. Summary of the Proteomic Profiles
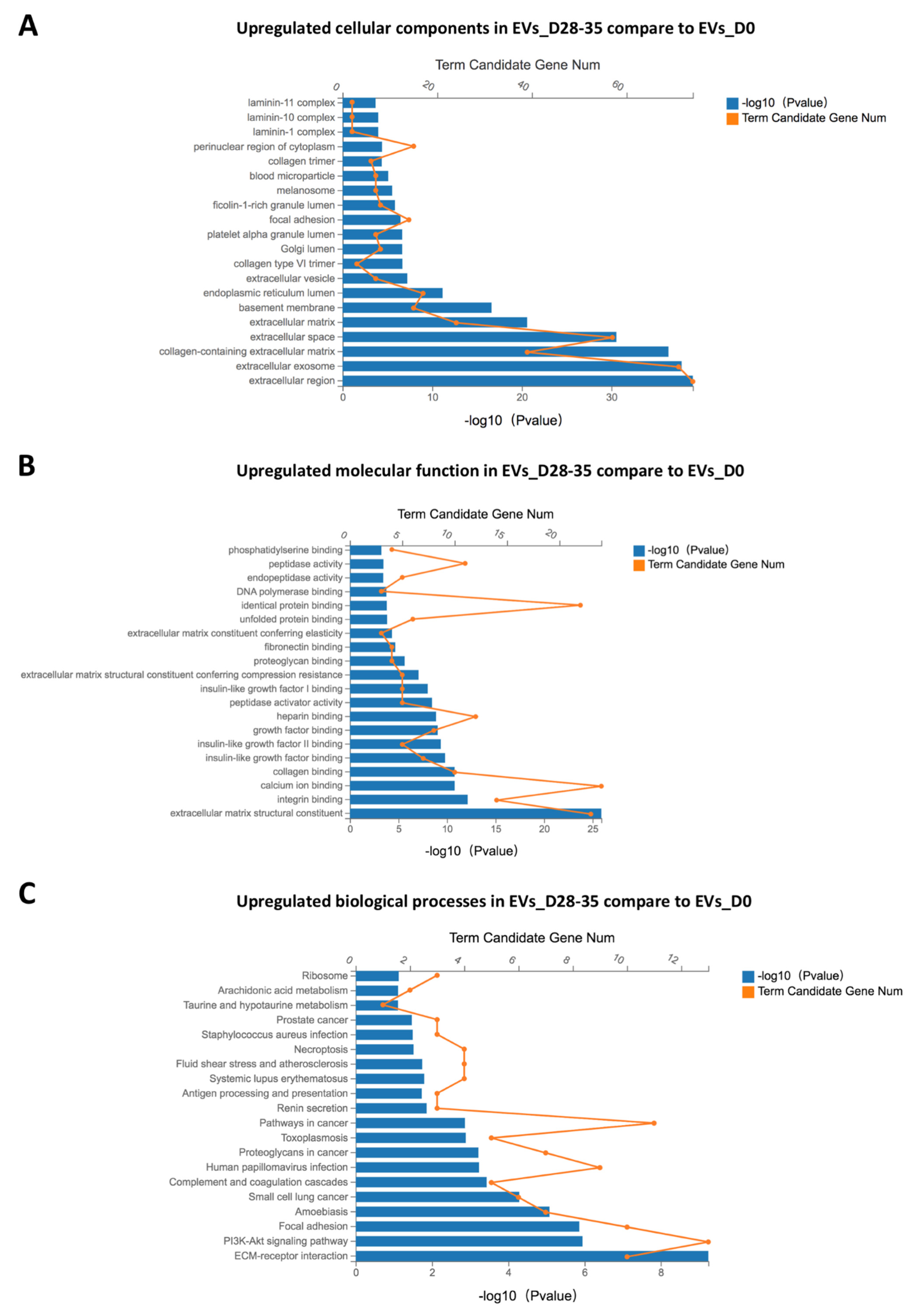
3.7.2. GO and KEGG Enrichment Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caplan, A.I. Mesenchymal Stem-Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Paula, F.J.A.; Rosen, C.J. Marrow Adipocytes: Origin, Structure, and Function. In Annual Review of Physiology; Nelson, M.T., Walsh, K., Eds.; Annual Review of Physiology; 2020; Volume 82, pp. 461–484. [Google Scholar]
- Gómez-Ambrosi, J.; Rodríguez, A.; Catalán, V.; Frühbeck, G. The Bone-Adipose Axis in Obesity and Weight Loss. Obes. Surg. 2008, 18, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Yang, Q.W.; Chen, G.Y.; Du, Z.W.; Ren, M.; Wang, A.; Zhao, H.Y.; Li, Z.Y.; Zhang, G.Z.; Song, Y. LncRNA expression profiling of BMSCs in osteonecrosis of the femoral head associated with increased adipogenic and decreased osteogenic differentiation. Sci. Rep. 2018, 8, 9127. [Google Scholar] [CrossRef]
- Motomura, G.; Yamamoto, T.; Miyanishi, K.; Yamashita, A.; Sueishi, K.; Iwamoto, Y. Bone marrow fat-cell enlargement in early steroid-induced osteonecrosis—A histomorphometric study of autopsy cases. Pathol. Res. Pract. 2005, 200, 807–811. [Google Scholar] [CrossRef]
- Griffith, J.F.; Kumta, S.M.; Huang, Y. Hard arteries, weak bones. Skelet. Radiol. 2011, 40, 517–521. [Google Scholar] [CrossRef][Green Version]
- Ding, W.G.; Wei, Z.X.; Liu, J.B. Reduced local blood supply to the tibial metaphysis is associated with ovariectomy-induced osteoporosis in mice. Connect. Tissue Res. 2011, 52, 25–29. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. In Annual Review of Cell and Developmental Biology; Schekman, R., Lehmann, R., Eds.; Annual Review of Cell and Developmental Biology; 2014; Volume 30, pp. 255–289. [Google Scholar]
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 46. [Google Scholar] [CrossRef]
- Herrmann, M.; Diederichs, S.; Melnik, S.; Riegger, J.; Trivanovic, D.; Li, S.; Jenei-Lanzl, Z.; Brenner, R.E.; Huber-Lang, M.; Zaucke, F.; et al. Extracellular Vesicles in Musculoskeletal Pathologies and Regeneration. Front. Bioeng. Biotechnol. 2021, 8, 624096. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.H.; Li, Y.F.; Chen, P. Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Des. Dev. Ther. 2019, 13, 45–55. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, Q.; Niu, X.; Hu, B.; Chen, S.B.; Song, W.Q.; Ding, J.; Zhang, C.Q.; Wang, Y. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int. J. Biol. Sci. 2017, 13, 232–244. [Google Scholar] [CrossRef]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekstrom, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Qin, Y.H.; Sun, R.X.; Wu, C.L.; Wang, L.; Zhang, C.Q. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Wei, F.; Li, Z.M.; Crawford, R.; Xiao, Y.; Zhou, Y.H. Immunoregulatory role of exosomes derived from differentiating mesenchymal stromal cells on inflammation and osteogenesis. J. Tissue Eng. Regen. Med. 2019, 13, 1978–1991. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Leyh, M.; Seitz, A.; Duerselen, L.; Schaumburger, J.; Ignatius, A.; Grifka, J.; Graessel, S. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res. Ther. 2014, 16, 453. [Google Scholar] [CrossRef]
- Leyh, M.; Seitz, A.; Durselen, L.; Springorum, H.R.; Angele, P.; Ignatius, A.; Grifka, J.; Grassel, S. Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res. Ther. 2014, 5, 77. [Google Scholar] [CrossRef]
- Grassel, S.; Stockl, S.; Jenei-Lanzl, Z. Isolation, culture, and osteogenic/chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Methods Mol. Biol. 2012, 879, 203–267. [Google Scholar] [CrossRef] [PubMed]
- Niedermair, T.; Lukas, C.; Li, S.; Stoeckl, S.; Craiovan, B.; Brochhausen, C.; Federlin, M.; Herrmann, M.; Graessel, S. Influence of Extracellular Vesicles Isolated From Osteoblasts of Patients With Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs. Front. Bioeng. Biotechnol. 2020, 8, 615520. [Google Scholar] [CrossRef] [PubMed]
- Stoeckl, S.; Bauer, R.J.; Bosserhoff, A.K.; Goettl, C.; Grifka, J.; Graessel, S. Sox9 modulates cell survival and adipogenic differentiation of multipotent adult rat mesenchymal stem cells. J. Cell Sci. 2013, 126, 2890–2902. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stoeckl, S.; Lukas, C.; Goetz, J.; Herrmann, M.; Federlin, M.; Graessel, S. hBMSC-Derived Extracellular Vesicles Attenuate IL-1 beta-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways. Front. Bioeng. Biotechnol. 2020, 8, 603598. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Li, S.S.; Stockl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; Konig, M.A.; Johnstone, B.; Grassel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1 beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Pirkmajer, S.; Chibalin, A.V. Serum starvation: Caveat emptor. Am. J. Physiol.-Cell Physiol. 2011, 301, C272–C279. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Gu, X.S.; Ding, F. Neuroprotective Effects of Salidroside in the PC12 Cell Model Exposed to Hypoglycemia and Serum Limitation. Cell. Mol. Neurobiol. 2008, 28, 1067–1078. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Zhao, X.N.; Chen, X.N.; Wei, Y.Z.; Du, W.; Wang, Y.B.; Liu, L.A.; Zhao, W.A.; Han, Z.B.; Kong, D.L.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.H.; Wang, L.; Gao, Z.L.; Chen, G.Y.; Zhang, C.Q. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.; Takahashi, A.; Yanagiya, A.; Yamaguchi, T.; Abe, T.; Kureha, T.; Kuba, K.; Kanegae, Y.; Furuta, Y.; Yamamoto, T.; et al. Essential functions of the CNOT7/8 catalytic subunits of the CCR4-NOT complex in mRNA regulation and cell viability. RNA Biol. 2020, 17, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.Y.; Georgiou, A.I.; MacFarlane, R.J.; Klontzas, M.E.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Fibronectin stimulates the osteogenic differentiation of murine embryonic stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 1929–1940. [Google Scholar] [CrossRef]
- Ishida, K.; Acharya, C.; Christiansen, B.A.; Yik, J.H.N.; DiCesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013, 55, 23–35. [Google Scholar] [CrossRef]
- Majeska, R.J.; Rodan, G.A. The effect of 1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J. Biol. Chem. 1982, 257, 3362–3365. [Google Scholar] [CrossRef]
- Induction of Osteogenic Differentiation in Human Mesenchymal Stem Cells by Crosstalk with Osteoblasts. BioResearch 2015, 4, 121–130. [CrossRef]
- Pham, L.B.H.; Yoo, Y.R.; Park, S.H.; Back, S.A.; Kim, S.W.; Bjorge, I.; Mano, J.; Jang, J.H. Investigating the effect of fibulin-1 on the differentiation of human nasal inferior turbinate-derived mesenchymal stem cells into osteoblasts. J. Biomed. Mater. Res. Part A 2017, 105, 2291–2298. [Google Scholar] [CrossRef]
- Li, H.Y.; Cui, Y.Z.; Luan, J.; Zhang, X.M.; Li, C.Z.; Zhou, X.Y.; Shi, L.; Wang, H.X.; Han, J.X. PRELP (proline/arginine-rich end leucine-rich repeat protein) promotes osteoblastic differentiation of preosteoblastic MC3T3-E1 cells by regulating the beta-catenin pathway. Biochem. Biophys. Res. Commun. 2016, 470, 558–562. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.R.; Yan, K.; Wang, Y.; Yang, Y.; Wu, X. Matrix Gla Protein Promotes the Bone Formation by Up-Regulating Wnt/beta-Catenin Signaling Pathway. Front. Endocrinol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Gu, J.G.; Fujibayashi, A.; Yamada, K.M.; Sekiguchi, K. Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J. Biol. Chem. 2002, 277, 19922–19928. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A.R. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Chua, I.L.S.; Kim, H.W.; Lee, J.H. Signaling of extracellular matrices for tissue regeneration and therapeutics. Tissue Eng. Regen. Med. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The Key Regulatory Roles of the PI3K/Akt Signaling Pathway in the Functionalities of Mesenchymal Stem Cells and Applications in Tissue Regeneration. Tissue Eng. Part B-Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef]
- Cao, Z.D.; Umek, R.M.; McKnight, S.L. Regulated expression of 3 C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Ro, H.S.; Zhang, L.; Majdalawieh, A.; Kim, S.W.; Wu, X.; Lyons, P.J.; Webber, C.; Ma, H.; Reidy, S.P.; Boudreau, A.; et al. Adipocyte enhancer-binding protein 1 modulates adiposity and energy homeostasis. Obesity 2007, 15, 288–302. [Google Scholar] [CrossRef]
- Tremblay, F.; Revett, T.; Huard, C.; Zhang, Y.; Tobin, J.F.; Martinez, R.V.; Gimeno, R.E. Bidirectional Modulation of Adipogenesis by the Secreted Protein Ccdc80/DRO1/URB. J. Biol. Chem. 2009, 284, 8136–8147. [Google Scholar] [CrossRef]
- Perdomo, G.; Kim, D.H.; Zhang, T.; Qu, S.; Thomas, E.A.; Toledo, F.G.S.; Slusher, S.; Fan, Y.; Kelley, D.E.; Dong, H.H. A role of apolipoprotein D in triglyceride metabolism. J. Lipid Res. 2010, 51, 1298–1311. [Google Scholar] [CrossRef]
- Inokawa, A.; Inuzuka, T.; Takahara, T.; Shibata, H.; Maki, M. Tubby-like protein superfamily member PLSCR3 functions as a negative regulator of adipogenesis in mouse 3T3-L1 preadipocytes by suppressing induction of late differentiation stage transcription factors. Biosci. Rep. 2016, 36, e00287. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.R.; Hui, S.K.; Xian, C.J. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am. J. Stem Cells 2012, 1, 205–224. [Google Scholar] [PubMed]
- Devlin, M.J.; Rosen, C.J. The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015, 3, 141–147. [Google Scholar] [CrossRef]
| Gene | Primer Sequences (5′–3′) |
|---|---|
| GAPDH | Fwd, CTGACTTCAACAGCGACACC |
| Rev, CCCTGTTGCTGTAGCCAAAT | |
| TBP | Fwd, TTGTACCGCAGCTGCAAAAT |
| Rev, TATATTCGGCGTTTCGGGCA | |
| ALP | Fwd, CCTCCTCGGAAGACACTCTG |
| Rev, CCTCCTCGGAAGACACTCTG | |
| BGLAP | Fwd, GTGCAGAGTCCAGCAAAGGT |
| Rev, TCAGCCAACTCGTCACAGTC | |
| COL1A1 | Fwd, ACGTCCTGGTGAAGTTGGTC |
| Rev, ACCAGGGAAGCCTCTCTCTC | |
| RUNX2 | Fwd, CGGAATGCCTCTGCTGTTATG |
| Rev, GCTTCTGTCTGTGCCTTCTG | |
| OPN | Fwd, TGAAACGAGTCAGCTGGATG |
| Rev, TGAAATTCATGGCTGTGGAA | |
| C/EBPβ | Fwd, AACCTGGAGACGCAGCACAA |
| Rev, GAACAAGTTCCGCAGGGTGC | |
| PPARγ | Fwd, GACCAAAGCAAAGGCGAGGG |
| Rev, CCCTGAAAGATGCGGATGGC | |
| ADIPOQ | Fwd, AAGGAGATCCAGGTCTTATTGGTC |
| Rev, CGAATGGGCATGTTGGGGAT |
| UniProt | Protein Names | Gene Names | EVs_D28-35 /EVs_D0 Ratio |
|---|---|---|---|
| P23142 | Fibulin-1 | FBLN1 | 100 |
| P51888 | Prolargin | PRELP | 100 |
| P08493 | Matrix Gla protein | MGP | 63.867 |
| P01034 | Cystatin-C | CST3 | 7.567 |
| Q8IX30 | Signal peptide, CUB and EGF-like domain-containing protein 3 | SCUBE3 | 7.149 |
| P11362 | Fibroblast growth factor receptor 1 | FGFR1 | 6.795 |
| O76076 | WNT1-inducible-signaling pathway protein 2 | CCN5 | 6.312 |
| P02751 | IGFBP4 | FN1 | 6.29 |
| Q96CG8 | Collagen triple helix repeat-containing protein 1 | CTHRC1 | 5.782 |
| P22692 | Insulin-like growth factor-binding protein 4 | IGFBP4 | 4.895 |
| P24592 | Insulin-like growth factor-binding protein 6 | IGFBP6 | 4.756 |
| P07585 | Decorin | DCN | 4.365 |
| P05452 | Tetranectin | CLEC3B | 4.298 |
| P08253 | 72 kDa type IV collagenase | MMP2 | 4.06 |
| P01344 | Insulin-like growth factor II | IGF2 | 4.017 |
| P18065 | Insulin-like growth factor-binding protein 2 | IGFBP2 | 3.611 |
| P17936 | Insulin-like growth factor-binding protein 3 | IGFBP3 | 3.582 |
| P09429 | High mobility group protein B1 | HMGB1 | 3.223 |
| P12109 | Collagen alpha-1(VI) chain | COL6A1 | 3.163 |
| P13611 | Versican core protein | VCAN | 3.093 |
| P49747 | Cartilage oligomeric matrix protein | COMP | 2.569 |
| Q4LDE5 | Sushi, von Willebrand factor type A, EGF and pentraxin domain-containing protein 1 | SVEP1 | 2.515 |
| UniProt | Protein Names | Gene Names | EVs_D28-35 /EVs_D0 Ratio |
|---|---|---|---|
| Q8IUX7 | Adipocyte enhancer-binding protein 1 | AEBP1 | 100 |
| Q76M96 | Coiled-coil domain-containing protein 80 | CCDC80 | 100 |
| P05090 | Apolipoprotein D | APOD | 8.375 |
| Q9NRY6 | Phospholipid scramblase 3 | PLSCR3 | 2.635 |
| UniProt | Protein Names | Gene Names | EVs_D28-35 /EVs_D0 Ratio |
|---|---|---|---|
| P55268 | Laminin subunit beta-2 | LAMB2 | 6.053 |
| Q9Y6C2 | EMILIN-1 | EMILIN1 | 5.021 |
| P07942 | Laminin subunit beta-1 | LAMB1 | 4.939 |
| P98095 | Fibulin-2 | FBLN2 | 4.838 |
| Q14112 | Nidogen-2 | NID2 | 3.652 |
| Q16363 | Laminin subunit alpha-4 | LAMA4 | 3.344 |
| Q15113 | ProcollagenC-endopeptidase enhancer 1 | PCOLCE | 2.897 |
| P12111 | Collagen alpha-3(VI) chain | COL6A3 | 2.492 |
| GO-Term ID | Term Description | Observed Gene Count | False Discovery Rate (p Value) | Candidate Gene Name |
|---|---|---|---|---|
| GO:0008284 | Positive regulation of cell population proliferation | 22 | 8.71 × 10−6 | TIMP1, MMP2, SFRP1, COMP, LAMB1, IGFBP2, LAMC1, SDCBP, PDCD6, HMGB2, NPM1, AQP1, CTHRC1, FBLN1, GAS6, HMGB1, FN1, HTRA1, CST3, IGF2, FGFR1, NACA |
| GO:0008285 | Negative regulation of cell population proliferation | 13 | 0.0125 | SFRP1, SERPINF1, TIMP2, NPM1, IGFBP6, PODN, FBLN1, CAV1, APOD, SRPX, IGFBP3, CD9, B2M |
| GO:0060548 | Negative regulation of cell death | 17 | 0.0047 | TIMP1, SFRP1, COMP, SERPINF1, HMGB2, NPM1, HSP90B1, AXL, AQP1, GAS6, CAV1, FN1, SET, WISP2, HSPG2, CST3, NACA |
| GO:0031324 | Negative regulation of cellular metabolic processes | 28 | 0.0293 | TIMP1, SFRP1, AEBP1, SERPINF1, SDCBP, SLC4A1, TIMP2, APLP2, COL6A3, HMGB2, NPM1, AQP1, NCL, FBLN1, GAS6, HIST2H2AC, CAV1, APOD, HMGB1, SET, EMILIN1, IGFBP3, PROS1, MVP, CST3, IGF2, FGFR1, NACA |
| GO:0043085 | Positive regulation of catalytic activity | 21 | 0.0070 | SFRP1, PCOLCE, CHI3L1, TIMP2, PDCD6, ACE, HMGB2, AXL, IGFBP6, FBLN1, GAS6, HSP90AA1, CAV1, HMGB1, ANP32B, FN1, GSN, IGFBP3, IGF2, FGFR1, PTGES3 |
| GO:0043086 | Negative regulation of catalytic activity | 15 | 0.0051 | TIMP1, SFRP1, SERPINF1, SLC4A1, TIMP2, APLP2, COL6A3, NPM1, AQP1, GAS6, CAV1, SET, PROS1, MVP, CST3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Stöckl, S.; Li, S.; Herrmann, M.; Lukas, C.; Reinders, Y.; Sickmann, A.; Grässel, S. Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs. Cells 2022, 11, 2491. https://doi.org/10.3390/cells11162491
Wang C, Stöckl S, Li S, Herrmann M, Lukas C, Reinders Y, Sickmann A, Grässel S. Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs. Cells. 2022; 11(16):2491. https://doi.org/10.3390/cells11162491
Chicago/Turabian StyleWang, Chenglong, Sabine Stöckl, Shushan Li, Marietta Herrmann, Christoph Lukas, Yvonne Reinders, Albert Sickmann, and Susanne Grässel. 2022. "Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs" Cells 11, no. 16: 2491. https://doi.org/10.3390/cells11162491
APA StyleWang, C., Stöckl, S., Li, S., Herrmann, M., Lukas, C., Reinders, Y., Sickmann, A., & Grässel, S. (2022). Effects of Extracellular Vesicles from Osteogenic Differentiated Human BMSCs on Osteogenic and Adipogenic Differentiation Capacity of Naïve Human BMSCs. Cells, 11(16), 2491. https://doi.org/10.3390/cells11162491






