Improvement of Bacterial Blight Resistance in Two Conventionally Cultivated Rice Varieties by Editing the Noncoding Region
Abstract
:1. Introduction
2. Materials and Methods
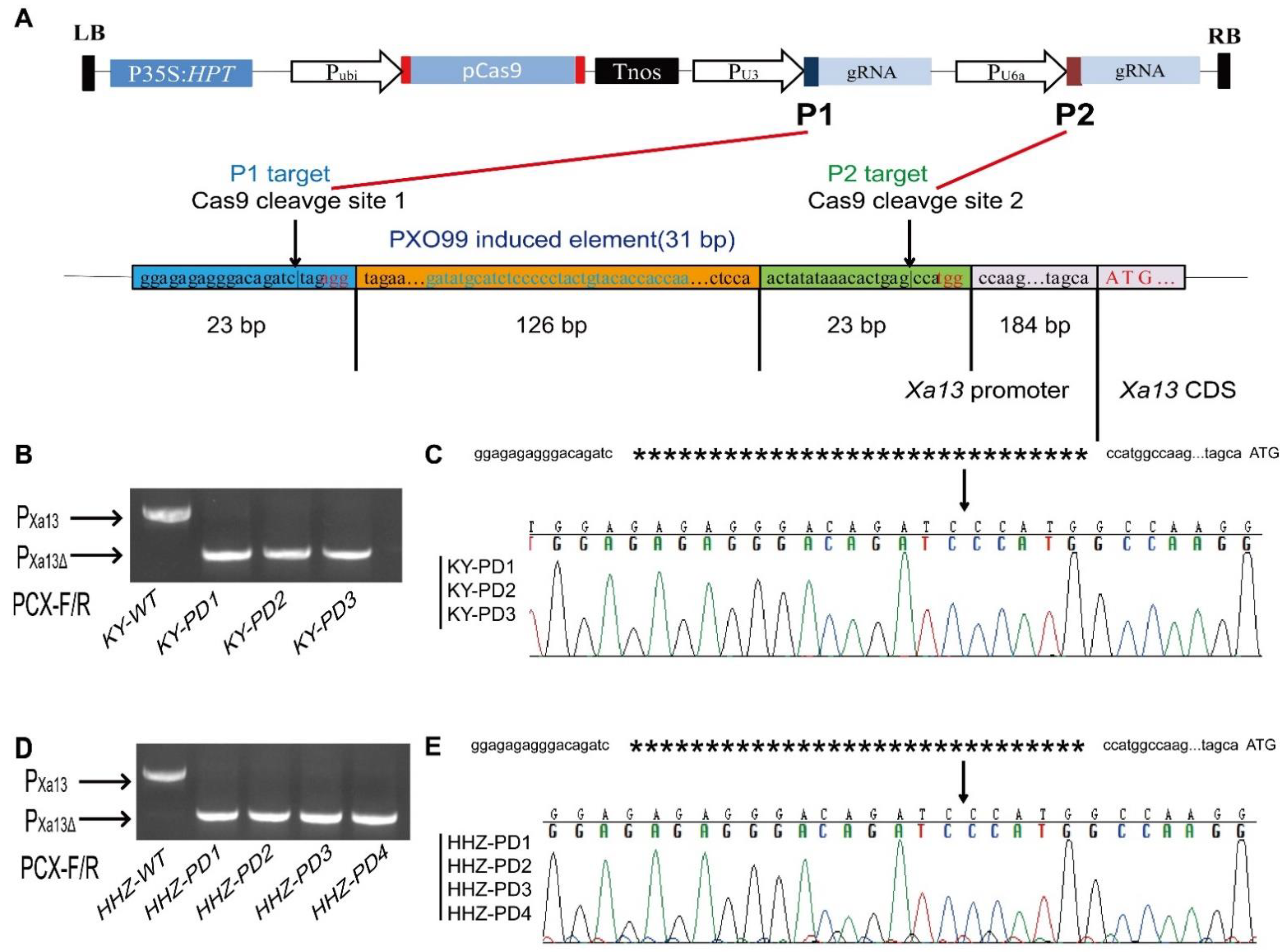
2.1. Selection of sgRNA Sites in the xa13 Gene Promoter
2.2. Cas9:P1+P2 Expression Vector Construction
2.3. Transformation of Rice Expression Vector
2.4. Transgenic Materials
2.5. PCR Detection of the Xa13 Promoter Editing Sites
2.6. RT-PCR and qRT-PCR
2.7. Evaluation of Disease Resistance of Transgenic Rice Plants
2.8. Evaluation of Fertility
2.9. Examination of Resistance Inheritance and Cas9-Segregation in T1 Progeny
2.10. Growth Curve of PXO99
3. Results
3.1. Cas9: P1+P2 Expression Vector Construction and Genotyping of Transgenic Plants
3.2. Detection of Xa13 Gene Expression in Transgenic Rice
3.3. Disease Resistance and Agronomic Traits of T0 Generation KY-PD1-3 and HHZ-PD1-4
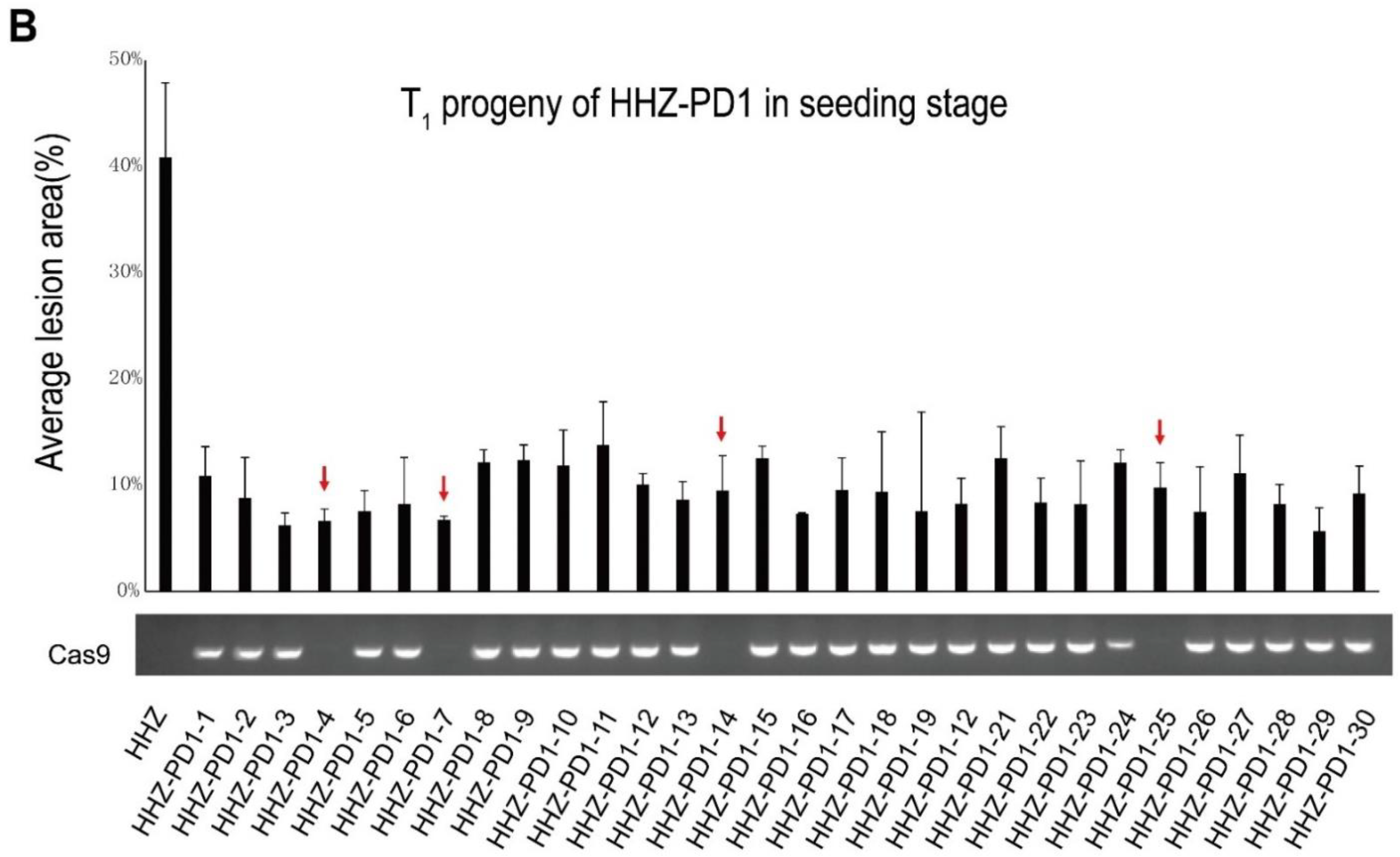
3.4. Screening of Cas9-Free Plants from the T1 Generation Single-Copy PD1 Family
3.5. Investigation of Important Agronomic Traits in the T2 Generation of Transgene-Free PD1 Family and Identification of the Bacterial Growth Curve
4. Discussion
4.1. Advantages and Limitations of Changing Target Gene Expression Pattern by Editing the Promoter Region
4.2. Advantages of CRISPR/cas9-Mediated Double-Target sgRNA Spot Deletion
4.3. Application of Recessive and Pleiotropic Gene xa13 in Rice Disease Resistance Breeding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Tibbit, C.; Ponting, C.P.; Liu, J.L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013, 4, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Savic, N.; Schwank, G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016, 168, 15–21. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin B. 2017, 7, 292–302. [Google Scholar] [CrossRef]
- Hussain, B.; Lucas, S.J.; Budak, H. CRISPR/Cas9 in plants: At play in the genome and at work for crop improvement. Brief Funct Genomics. 2018, 17, 319–328. [Google Scholar] [CrossRef]
- Lim, S.H.; So, B.H.; Wang, J.C.; Song, E.S.; Park, Y.J.; Lee, B.M.; Kang, H.W. Functional analysis of pilQ gene in Xanthomanas oryzae pv. oryzae, bacterial blight pathogen of rice. J. Microbiol. 2008, 46, 214–220. [Google Scholar] [CrossRef]
- Chu, Z.; Yuan, M.; Yao, J.; Ge, X.; Yuan, B.; Xu, C.; Li, X.; Wang, S. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006, 20, 1250–1255. [Google Scholar] [CrossRef]
- Yuan, M.; Chu, Z.; Li, X.; Xu, C.; Wang, S. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 2010, 22, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Sanders, D. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Romer, P.; Recht, S.; Strauss, T.; Elsaesser, J.; Schornack, S.; Boch, J.; Wang, S.; Lahaye, T. Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae. New Phytol. 2010, 187, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Li, X.; Xiao, J.; Wang, S. Characterization of Xanthomonas oryzae-responsive cis-acting element in the promoter of rice race-specific susceptibility gene Xa13. Mol. Plant 2011, 4, 300–309. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Gong, Q.; Li, Z.; Li, Y.; Wang, S. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable tale-binding elements of multiple susceptibility genes in rice. Mol. Plant 2019, 12, 13. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Yang, B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Zhou, Z.; Chen, H.; Xie, C.; Lin, Y. A new rice breeding method: Crispr/cas9 system editing of the xa13 promoter to cultivate transgene-free bacterial blight-resistant rice. Plant Biotechnol. J. 2020, 18, 313–315. [Google Scholar] [CrossRef]
- Mew, T.W.; Cruz, V.; Medalla, E.S. Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Dis. 1992, 76, 1029–1032. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Liu, Y.G. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zhang, Q. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005, 23, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.E. An improved technique for evaluat-ing resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Li, C.; Wei, J.; Lin, Y.; Chen, H. Gene silencing using the recessive rice bacterial blight resistance gene xa13 as a new paradigm in plant breeding. Plant Cell Rep. 2012, 31, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Xa26, a gene conferring resistance toXanthomonas oryzaepv.oryzaein rice, encodes an LRR receptor kinase-like protein. Plant J. 2004, 37, 517–527. [Google Scholar] [CrossRef]
- Rodriguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Blanvillain-Baufume, S.; Reschke, M.; Sole, M.; Auguy, F.; Doucoure, H.; Szurek, B.; Meynard, D.; Szurek, B.; Koebnik, R. Targeted promoter editing for rice resistance to Xanthomonas oryzae pv. oryzae reveals differential activities for SWEET14-inducing TAL effectors. Plant Biotechnol. J. 2017, 15, 306–317. [Google Scholar] [CrossRef]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccDeltapthA4:dCsLOB1.3 infection. Plant Biotechnol. J. 2016, 14, 1291–1301. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Liu, D.R. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef]
- Zhang, H.; Si, X.; Ji, X.; Fan, R.; Liu, J.; Chen, K.; Wang, D. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018, 36, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Hajira, S.K.; Sundaram, R.M.; Laha, G.S.; Yugander, A.; Balachandran, S.M.; Viraktamath, B.C.; Sujatha, K.; Rekha, G. A Single-Tube, Functional Marker-Based Multiplex PCR Assay for Simultaneous Detection of Major Bacterial Blight Resistance Genes Xa21, xa13 and xa5 in Rice. Rice Sci. 2016, 23, 144–151. [Google Scholar] [CrossRef]




| Primer | Primer Sequence (5’-3’) | Function |
|---|---|---|
| P2U6a-F | GCCGgagagagggacagatctag | vector construction |
| P2U6a-R | AAACctagatctgtccctctctc | vector construction |
| P1U3-F | GGCActatataaacactgagcca | vector construction |
| P1U3-R | AAACtggctcagtgtttatatag | vector construction |
| gRctga-B2 | AGCGTGggtctcGtcagGGTCCATCCACTCCAAGCTC | vector construction |
| Uctga-B2′ | TTCAGAggtctcTctgaCACTGGAATCGGCAGCAAAGG | vector construction |
| gRcggt-BL | AGCGTGggtctcGaccgACGCGTCCATCCACTCCAAGCTC | vector construction |
| U-F | CTCCGTTTTACCTGTGGAATCG | vector construction |
| gRNA-R | CGGAGGAAAATTCCATCCAC | vector construction |
| PT1 | 5’-TTCAGAggtctcTctcgACTAGTGGAATCGGCAGCAAAGG-3’/5’-AGCGTGggtctcGtcagGGTCCATCCACTCCAAGCTC-3’ | vector construction |
| PT2L | 5’-TTCAGAggtctcTctgaCACTGGAATCGGCAGCAAAGG-3’/5’-AGCGTGggtctcGaccgACGCGTCCATCCACTCCAAGCTC-3’ | vector construction |
| Primer | Primer Sequence (5’-3’) | Function |
|---|---|---|
| PCX-F | GCATCATTGTCCATGGTTG | genotype test |
| PCX-R | GCTAGAGAGGAAGGCTTAAG | genotype test |
| GAPDH-F | CTGCAACTCAGAAGACCGTTG | qRT-PCR test |
| GAPDH-R | CCTGTTGTCACCCTGGAAGTC | qRT-PCR test |
| X13-Qt1F | AGTCGACGGGAGGGTACAG | qRT-PCR test |
| X13-Qt1R | GACGAGGTAGAGGGTGGTGA | qRT-PCR test |
| PCas9-F | ATGACTCTCTTACCTTCA | genotype test |
| PCas9-R | TAGTTCTTCATCTTCTTG | genotype test |
| hpt-F | AGAATCTCGTGCTTTCAGCTTCGA | genotype test |
| hpt-R | TCAAGACCAATGCGGAGCATATAC | genotype test |
| Line | n | Lesion Length (cm) a | Lesion Area (%) a | Seeding Setting Rate (%) |
|---|---|---|---|---|
| KY131 | 50 b1 | 16.99 ± 4.63 | 62.46 ± 12.90 | 77.5 ± 10.3 c |
| KY-PD1 | 6 | 1.25 ± 0.53 | 5.32 ± 1.93 | 74.6 |
| KY-PD2 | 6 | 1.33 ± 0.68 | 6.46 ± 3.67 | 67.4 |
| KY-PD3 | 6 | 0.83 ± 0.52 | 3.00 ± 2.08 | 81.1 |
| HHZ | 50 b2 | 17.92 ± 4.73 | 65.94 ±13.60 | 89.6 ± 3.7 c |
| HHZ-PD1 | 6 | 1.75 ± 0.42 | 7.56 ± 1.65 | 86.38 |
| HHZ-PD2 | 6 | 1.58 ± 0.38 | 7.36 ± 2.36 | 88.28 |
| HHZ-PD3 | 6 | 1.50 ± 0.32 | 5.30 ± 1.67 | 93.46 |
| HHZ-PD4 | 6 | 2.00 ± 0.32 | 7.03 ± 1.46 | 89.83 |
| Line | n | Heading Stage (d) | Plant Height (cm) | Panicles per Plant | Panicle Length (cm) | Seeding Setting Rate (%) | Yield per Plant (g) |
|---|---|---|---|---|---|---|---|
| KY131 | 30 | 64.40 ± 1.35aA | 81.73 ± 1.60aA | 9.73 ± 1.64aA | 19.32 ± 0.62aA | 79.25 ± 5.09aA | 20.44 ± 1.00aA |
| KY-PD1 transgenic lines | 30 | 65.13 ± 1.65aA | 82.23 ± 1.41aA | 9.57 ± 1.61aA | 19.08 ± 0.48aA | 78.66 ± 5.65aA | 20.17 ± 0.91abA |
| KY-PD1 transgenic-free lines | 30 | 65.13 ± 1.93aA | 82.17 ± 1.60aA | 10.13 ± 0.90aA | 19.01 ± 0.62aA | 79.13 ± 4.14aA | 20.72 ± 0.97bA |
| Line | n | Heading Stage (d) | Plant Height (cm) | Panicles per Plant | Panicle Length (cm) | Seeding Setting Rate (%) | Yield per Plant (g) |
|---|---|---|---|---|---|---|---|
| HHZ | 30 | 93.60 ± 1.81aA | 101.90 ± 2.35aA | 16.67 ± 1.63aA | 22.42 ± 0.70aA | 84.71 ± 5.29aA | 36.26 ± 1.20aA |
| HHZ-PD1 transgenic lines | 30 | 93.10 ± 2.87aA | 101.00 ± 2.79aA | 16.57 ± 1.61aA | 22.30 ± 0.46aA | 84.29 ± 5.64aA | 36.07 ± 0.81abA |
| HHZ-PD1 transgenic-free lines | 30 | 92.27 ± 3.30aA | 101.57 ± 1.92aA | 16.93 ± 1.05aA | 22.25 ± 0.49aA | 85.01 ± 4.15aA | 36.65 ± 0.87bA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhou, L.; Wu, B.; Li, S.; Zha, W.; Li, W.; Zhou, Z.; Yang, L.; Shi, L.; Lin, Y.; et al. Improvement of Bacterial Blight Resistance in Two Conventionally Cultivated Rice Varieties by Editing the Noncoding Region. Cells 2022, 11, 2535. https://doi.org/10.3390/cells11162535
Li C, Zhou L, Wu B, Li S, Zha W, Li W, Zhou Z, Yang L, Shi L, Lin Y, et al. Improvement of Bacterial Blight Resistance in Two Conventionally Cultivated Rice Varieties by Editing the Noncoding Region. Cells. 2022; 11(16):2535. https://doi.org/10.3390/cells11162535
Chicago/Turabian StyleLi, Changyan, Lei Zhou, Bian Wu, Sanhe Li, Wenjun Zha, Wei Li, Zaihui Zhou, Linfeng Yang, Lei Shi, Yongjun Lin, and et al. 2022. "Improvement of Bacterial Blight Resistance in Two Conventionally Cultivated Rice Varieties by Editing the Noncoding Region" Cells 11, no. 16: 2535. https://doi.org/10.3390/cells11162535
APA StyleLi, C., Zhou, L., Wu, B., Li, S., Zha, W., Li, W., Zhou, Z., Yang, L., Shi, L., Lin, Y., & You, A. (2022). Improvement of Bacterial Blight Resistance in Two Conventionally Cultivated Rice Varieties by Editing the Noncoding Region. Cells, 11(16), 2535. https://doi.org/10.3390/cells11162535






