The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Population and Tissue Collection
2.2. Immunohistochemistry Validation
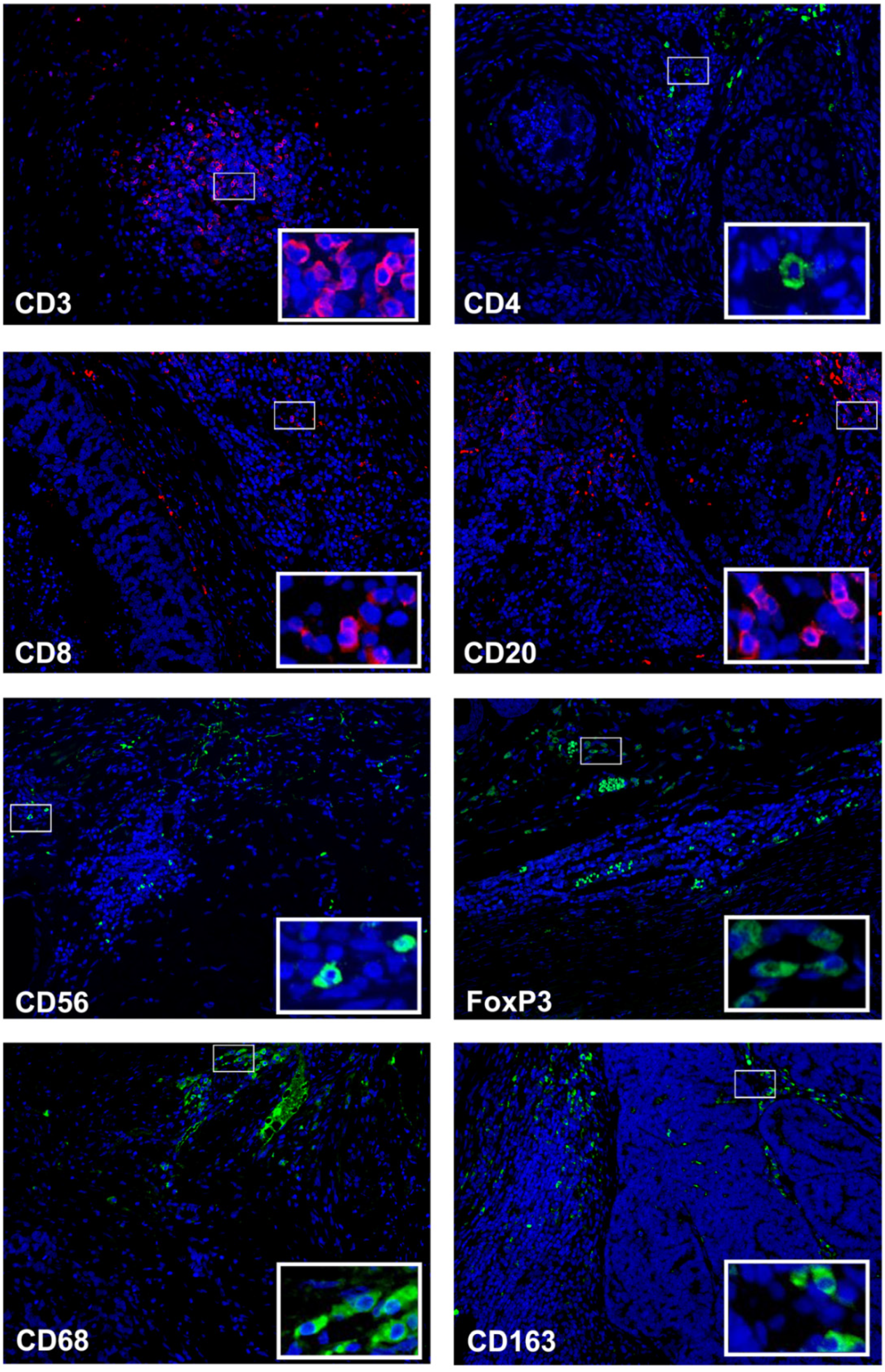
2.3. Immunofluorescence Staining, Image Collection and Evaluation
2.4. Statistical Analysis
3. Results
3.1. Clinical and Histopathological Characteristics
3.2. CD3+ T Cells Are the Predominant Infiltrating Cell Type in Feline Mammary Carcinoma
3.3. Stromal Densities of CD8+ Tumor-Infiltrating Lymphocytes Are Prognostic Markers for Feline Mammary Carcinoma
3.4. Tumor Infiltration by Stromal CD3+ T Cells, CD8+ T Lymphocytes and CD68+ Macrophages Is Increased in Triple Negative Normal-like Mammary Carcinoma Subtype
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- El Fatemi, H.; Chahbouni, S.; Jayi, S.; Moumna, K.; Melhouf, M.A.; Bannani, A.; Mesbahi, O.; Amarti, A. Luminal B tumors are the most frequent molecular subtype in breast cancer of North African women: An immunohistochemical profile study from Morocco. Diagn. Pathol. 2012, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, U.; Dar, A.H.; Wani, N.A.; Mir, M.A. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother. Pharmacol. 2021, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Badr, N.M.; Berditchevski, F.; Shaaban, A.M. The Immune Microenvironment in Breast Carcinoma: Predictive and Prognostic Role in the Neoadjuvant Setting. Pathobiology 2020, 87, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchiò, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Ahn, S.; Chung, Y.R.; Seo, A.N.; Kim, M.; Woo, J.W.; Park, S.Y. Changes and prognostic values of tumor-infiltrating lymphocyte subsets after primary systemic therapy in breast cancer. PLoS ONE 2020, 15, e0233037. [Google Scholar] [CrossRef]
- Jamiyan, T.; Kuroda, H.; Yamaguchi, R.; Nakazato, Y.; Noda, S.; Onozaki, M.; Abe, A.; Hayashi, M. Prognostic impact of a tumor-infiltrating lymphocyte subtype in triple negative cancer of the breast. Breast Cancer 2020, 27, 880–892. [Google Scholar] [CrossRef]
- Mi, H.; Gong, C.; Sulam, J.; Fertig, E.J.; Szalay, A.S.; Jaffee, E.M.; Stearns, V.; Emens, L.A.; Cimino-Mathews, A.M.; Popel, A.S. Digital Pathology Analysis Quantifies Spatial Heterogeneity of CD3, CD4, CD8, CD20, and FoxP3 Immune Markers in Triple-Negative Breast Cancer. Front. Physiol. 2020, 11, 583333. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Bianchini, G.; Ando, M.; Nozaki, F.; Kobayashi, D.; Criscitiello, C.; Curigliano, G.; Iwamoto, T.; Niikura, N.; Takei, H.; et al. Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer. Eur. J. Cancer 2019, 118, 41–48. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A Role for T-Lymphocytes in Human Breast Cancer and in Canine Mammary Tumors. BioMed Res. Int. 2014, 2014, 130894. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-positive breast cancer: State of the art and future perspectives. J. Hematol. Oncol. 2019, 12, 111. [Google Scholar] [CrossRef]
- Verma, R.; Hanby, A.M.; Horgan, K.; Verghese, E.T.; Volpato, M.; Carter, C.R.; Hughes, T.A. Levels of different subtypes of tumour-infiltrating lymphocytes correlate with each other, with matched circulating lymphocytes, and with survival in breast cancer. Breast Cancer Res. Treat. 2020, 183, 49–59. [Google Scholar] [CrossRef]
- Catacchio, I.; Silvestris, N.; Scarpi, E.; Schirosi, L.; Scattone, A.; Mangia, A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019, 12, 585–595. [Google Scholar] [CrossRef]
- Shou, J.; Zhang, Z.; Lai, Y.; Chen, Z.; Huang, J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: A systematic review and meta-analysis. BMC Cancer 2016, 16, 4–18. [Google Scholar] [CrossRef]
- Taouk, G.; Hussein, O.; Zekak, M.; Abouelghar, A.; Al-Sarraj, Y.; Abdelalim, E.M.; Karam, M. CD56 expression in breast cancer induces sensitivity to natural killer-mediated cytotoxicity by enhancing the formation of cytotoxic immunological synapse. Sci. Rep. 2019, 9, 8756. [Google Scholar] [CrossRef]
- Wu, M.; Mei, F.; Liu, W.; Jiang, J. Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed. Pharmacother. 2020, 121, 109637. [Google Scholar] [CrossRef]
- Ni, C.; Yang, L.; Xu, Q.; Yuan, H.; Wang, W.; Xia, W.; Gong, D.; Zhang, W.; Yu, K. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: A retrospective study and meta-analysis. J. Cancer 2019, 10, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.N.; dos Reis, D.C.; Salgado, B.S.; Cassali, G.D. Clinical significance and prognostic role of tumor-associated macrophages infiltration according to histologic location in canine mammary carcinomas. Res. Vet. Sci. 2021, 135, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Madeira, S.; Correia, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F. Molecular based subtyping of feline mammary carcinomas and clinicopathological characterization. Breast 2016, 27, 44–51. [Google Scholar] [CrossRef]
- Ferreira, D.; Soares, M.; Correia, J.; Adega, F.; Ferreira, F.; Chaves, R. Assessment of ER B B2 and TOP2α Gene Status and Expression Profile in f Eline m Ammary t Umors: Findings and Guidelines. Aging 2019, 11, 4688–4705. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Nascimento, C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Diagnostic Value of VEGF-A, VEGFR-1 and VEGFR-2 in Feline Mammary Carcinoma. Cancers 2021, 13, 117. [Google Scholar] [CrossRef]
- Zheng, J.; Wei, R.; Wang, Z.; Song, J.; Ge, Y.; Wu, R. Serum metabolomic analysis of feline mammary carcinomas based on LC-MS and MRM techniques. J. Vet. Res. 2020, 64, 581–588. [Google Scholar] [CrossRef]
- Owen, L.N. TNM Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Althobiti, M.; Aleskandarany, M.A.; Joseph, C.; Toss, M.; Mongan, N.; Diez-Rodriguez, M.; Nolan, C.C.; Ashankyty, I.; Ellis, I.O.; Green, A.R.; et al. Heterogeneity of tumour-infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology 2018, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Vihervuori, H.; Autere, T.A.; Repo, H.; Kurki, S.; Kallio, L.; Lintunen, M.M.; Talvinen, K.; Kronqvist, P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3105–3114. [Google Scholar] [CrossRef]
- Nascimento, C.; Ferreira, F. Tumor microenvironment of human breast cancer, and feline mammary carcinoma as a potential study model. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188587. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, G.N.; Henriques, J.; Lobo, L.; Vilhena, H.; Figueira, A.C.; Canadas-Sousa, A.; Dias-Pereira, P.; Prada, J.; Pires, I.; Queiroga, F.L. Adjuvant doxorubicin vs metronomic cyclophosphamide and meloxicam vs surgery alone for cats with mammary carcinomas: A retrospective study of 137 cases. Vet. Comp. Oncol. 2020, 19, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Koletsa, T.; Kotoula, V.; Koliou, G.-A.; Manousou, K.; Chrisafi, S.; Zagouri, F.; Sotiropoulou, M.; Pentheroudakis, G.; Papoudou-Bai, A.; Christodoulou, C.; et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: Do they add information over stromal tumor-infiltrating lymphocyte density? Cancer Immunol. Immunother. 2020, 69, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Millar, E.; Browne, L.; Slapetova, I.; Shang, F.; Ren, Y.; Bradshaw, R.; Brauer, H.A.; O’Toole, S.; Beretov, J.; Whan, R.; et al. TILs Immunophenotype in Breast Cancer Predicts Local Failure and Overall Survival: Analysis in a Large Radiotherapy Trial with Long-Term Follow-Up. Cancers 2020, 12, 2365. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Dias, M.; Prada, J.; Gregorio, H.; Lobo, L.; Queiroga, F. Intratumoral CD3+ T-Lymphocytes Immunoexpression and Its Association with c-Kit, Angiogenesis, and Overall Survival in Malignant Canine Mammary Tumors. Anal. Cell. Pathol. 2015, 2015, 920409. [Google Scholar] [CrossRef]
- Ann Fincham, R.E.; Delvecchio, F.R.; Goulart, M.R.; Sheng Yeong, J.P.; Kocher, H.M. Natural killer cells in pancreatic cancer stroma. World J. Gastroenterol. 2021, 27, 3483–3501. [Google Scholar] [CrossRef]
- Seung, B.-J.; Lim, H.-Y.; Shin, J.-I.; Kim, H.-W.; Cho, S.-H.; Kim, S.-H.; Sur, J.-H. CD204-Expressing Tumor-Associated Macrophages Are Associated With Malignant, High-Grade, and Hormone Receptor—Negative Canine Mammary Gland Tumors. Vet. Pathol. 2018, 55, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Tesi, M.; Millanta, F.; Gnocchi, M.; Poli, A. M1 and M2 tumour-associated macrophages subsets in canine malignant mammary tumours: An immunohistochemical study. Res. Vet. Sci. 2021, 136, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.-M.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Ormandy, L.A.; Heimesaat, M.M.; Kirschning, C.J.; Manns, M.P.; Korangy, F.; Greten, T.F. Primary sterile necrotic cells fail to cross-prime CD8+T cells. OncoImmunology 2012, 1, 1017–1026. [Google Scholar] [CrossRef]
- Nagarajan, D.; McArdle, S.E.B. Immune Landscape of Breast Cancers. Biomedicines 2018, 6, 20. [Google Scholar] [CrossRef]
- Gruosso, T.; Gigoux, M.; Manem, V.S.K.; Bertos, N.; Zuo, D.; Perlitch, I.; Saleh, S.M.I.; Zhao, H.; Souleimanova, M.; Johnson, R.M.; et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 2019, 129, 1785–1800. [Google Scholar] [CrossRef]
- Wang, J.; Browne, L.; Slapetova, I.; Shang, F.; Lee, K.; Lynch, J.; Beretov, J.; Whan, R.; Graham, P.H.; Millar, E.K.A. Multiplexed immunofluorescence identifies high stromal CD68+PD-L1+ macrophages as a predictor of improved survival in triple negative breast cancer. Sci. Rep. 2021, 11, 21608. [Google Scholar] [CrossRef]


| Clinicopathological Feature | Number of Animals (%) | Clinicopathological Feature | Number of Animals (%) |
|---|---|---|---|
| Age | Tumor malignancy grade | ||
| <8 years old | 2 (2.7%) | I | 2 (2.7%) |
| 8–12 years old | 38 (52.1%) | II | 13 (17.8%) |
| >12 years old | 30 (41.1%) | III | 58 (79.5%) |
| Unknown | 3 (4.1%) | ||
| Spayed | Tumor necrosis | ||
| No | 42 (57.5%) | No | 18 (24.7%) |
| Yes | 28 (38.4%) | Yes | 55 (75.3%) |
| Unknown | 3 (4.1%) | ||
| Contraceptive administration | Lymphocytic infiltration | ||
| No | 25 (34.2%) | No | 15 (20.5%) |
| Yes | 28 (38.4%) | Yes | 57 (78.1%) |
| Unknown | 20 (27.4%) | Unknown | 1 (1.4%) |
| Multiple tumors | Tumor ulceration | ||
| No | 40 (54.8%) | No | 61 (83.6%) |
| Yes | 33 (45.2%) | Yes | 12 (16.4%) |
| Lymph node status | HP classification | ||
| Negative | 39 (53.4%) | Tubulopapillary carcinoma | 25 (34.2%) |
| Positive | 26 (35%) | Solid carcinoma | 11 (15.1%) |
| Unknown | 8 (11%) | Cribriform carcinoma | 9 (12.3%) |
| Tumor size | Papillary-cystic carcinoma | 5 (6.8%) | |
| <2 cm | 16 (21.9%) | Tubular carcinoma | 17 (23.3%) |
| ≥2 cm | 57 (78.1%) | Mucinous carcinoma | 6 (8.2%) |
| TNM classification | Molecular subtype | ||
| I | 19 (26%) | Luminal A | 13 (17.8%) |
| II | 13 (17.8%) | Luminal B | 15 (20.5%) |
| III | 34 (46.6%) | HER2-positive | 15 (20.5%) |
| IV | 7 (9.6%) | Triple Negative Normal-like | 15 (20.5%) |
| Triple Negative Basal-like | 15 (20.5%) |
| Immune Cells | Mean of Positive Cells (%) ± SEM | Median of Positive Cells (%) ± SE |
|---|---|---|
| Total CD3+ | 17.6 ± 1.2 | 16.3 ± 10.3 |
| sCD3+ | 9.3 ± 0.9 | 7.4 ± 7.8 |
| iCD3+ | 8.3 ± 1.2 | 4 ± 10.4 |
| Total CD4+ | 2.7 ± 0.2 | 2.1 ± 1.9 |
| sCD4+ | 1.5 ± 0.2 | 1.2 ± 1.4 |
| iCD4+ | 1.2 ± 0.2 | 0.5 ± 1.6 |
| Total CD8+ | 3.9 ± 0.4 | 2.9 ± 3.5 |
| sCD8+ | 1.4 ± 0.3 | 0.5 ± 2.1 |
| iCD8+ | 2.6 ± 0.4 | 1.4 ± 3.2 |
| Total CD20+ | 14.4 ± 1.5 | 10.9 ± 12.5 |
| sCD20+ | 8.1 ± 1.2 | 4.8 ± 10.0 |
| iCD20+ | 6.5 ± 0.9 | 3.7 ± 7.6 |
| Total CD56+ | 2.6 ± 0.4 | 1.6 ± 3.4 |
| sCD56+ | 1.9 ± 0.4 | 0.9 ± 3 |
| iCD56+ | 0.7 ± 1.2 | 0.1 ± 1 |
| Total FoxP3+ | 1.5 ± 0.2 | 0.8 ± 1.7 |
| sFoxP3+ | 1.2 ± 0.2 | 0.7 ± 1.4 |
| iFoxP3+ | 0.3 ± 0.1 | 0 ± 0.7 |
| Total CD68+ | 4.4 ± 0.9 | 1 ± 8 |
| sCD68+ | 1.4 ± 0.5 | 0 ± 4.6 |
| iCD68+ | 2.9 ± 0.8 | 0.3 ± 6.9 |
| Total CD163+ | 1.4 ± 0.3 | 0.6 ± 2.3 |
| sCD163+ | 1 ± 0.2 | 0.3 ± 2.1 |
| iCD163+ | 0.4 ± 0.1 | 0 ± 0.7 |
| Immune Cell Phenotype | Disease Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Total CD3+ | 0.539 (0.268–1.085) | 0.083 | 1.145 (0.491–2.671) | 0.753 |
| sCD3+ | 1.090 (0.511–2.322) | 0.824 | 1.459 (0.611–3.483) | 0.395 |
| iCD3+ | 2.587 (0.903–7.411) | 0.077 | 1.065 (0.429–2.644) | 0.893 |
| Total CD4+ | 1.086 (0.500–2.357) | 0.835 | 1.286 (0.542–3.052) | 0.586 |
| sCD4+ | 0.771 (0.363–1.637) | 0.499 | 1.189 (0.502–2.818) | 0.693 |
| iCD4+ | 0.959 (0.461–1.995) | 0.911 | 1.262 (0.549–2.902) | 0.584 |
| Total CD8+ | 0.864 (0.387–1.929) | 0.721 | 1.031 (0.413–2.575) | 0.948 |
| sCD8+ | 0.514 (0.256–1.031) | 0.061 | 0.421 (0.197–0.900) | 0.026 |
| iCD8+ | 0.771 (0.364–1.632) | 0.497 | 1.199 (0.481–2.991) | 0.697 |
| Total CD20+ | 0.954 (0.449–2.027) | 0.904 | 1.425 (0.570–3.558) | 0.449 |
| sCD20+ | 1.150 (0.539–2.451) | 0.718 | 2.691 (0.929–7.793) | 0.068 |
| iCD20+ | 0.901 (0.396–2.053) | 0.804 | 0.891 (0.355–2.236) | 0.806 |
| Total CD56+ | 1.448 (0.595–3.525) | 0.415 | 1.670 (0.577–4.833) | 0.344 |
| sCD56+ | 1.838 (0.706–4.783) | 0.212 | 1.861 (0.642–5.393) | 0.252 |
| iCD56+ | 0.983 (0.488–1.980) | 0.961 | 1.673 (0.746–3.750) | 0.212 |
| Total FoxP3+ | 2.039 (0.714–5.824) | 0.183 | 1.466 (0.505–4.258) | 0.482 |
| sFoxP3+ | 2.371 (0.830–6.771) | 0.107 | 1.311 (0.493–3.482) | 0.588 |
| iFoxP3+ | 0.915 (0.446–1.876) | 0.809 | 0.901 (0.412–1.970) | 0.794 |
| Total CD68+ | 1.571 (0.678–3.641) | 0.292 | 1.091 (0.477–2.496) | 0.836 |
| sCD68+ | 0.693 (0.310–1.548) | 0.371 | 0.643 (0.258–1.601) | 0.343 |
| iCD68+ | 1.922 (0.860–4.292) | 0.111 | 1.362 (0.605–3.069) | 0.456 |
| Total CD163+ | 0.683 (0.314–1.486) | 0.337 | 0.935 (0.375–2.332) | 0.886 |
| sCD163+ | 0.704 (0.337–1.472) | 0.351 | 1.322 (0.528–3.310) | 0.550 |
| iCD163+ | 0.852 (0.407–1.786) | 0.672 | 1.232 (0.566–2.683) | 0.600 |
| Immune Cells | Luminal A and B | HER2-Positive | Triple Negative Basal-like | Triple Negative Normal-like | p-Value |
|---|---|---|---|---|---|
| Total CD3+ | 15.3 ± 12.9 | 20.1 ± 9.5 | 17.9 ± 8.3 | 14.8 ± 6.5 | 0.697 |
| sCD3+ | 3.9 ± 8.9 | 6 ± 6.8 | 9.6 ± 7.8 | 12.3 ± 5.5 | 0.033 |
| iCD3+ | 6 ± 12.4 | 12.5 ± 11.2 | 1.7 ± 7.7 | 1.1 ± 3 | 0.150 |
| Total CD4+ | 1.7 ± 1.9 | 2.2 ± 1.7 | 1.8 ± 1.9 | 3.7 ± 2 | 0.115 |
| sCD4+ | 1.3 ± 1.3 | 0.8 ± 1.2 | 1 ± 1.5 | 1.9 ± 1.7 | 0.319 |
| iCD4+ | 0.2 ± 1.5 | 0.5 ± 1.6 | 0.6 ± 1.8 | 0.6 ± 1.8 | 0.505 |
| Total CD8+ | 2.7 ± 4 | 2.7 ± 2.1 | 3.3 ± 2.7 | 6.1 ± 3.9 | 0.095 |
| sCD8+ | 0.8 ± 1.5 | 0.1 ± 1.1 | 0.4 ± 0.9 | 1.6 ± 3.6 | 0.044 |
| iCD8+ | 1 ± 3.7 | 1.5 ± 2.2 | 2.5 ± 2.7 | 1.4 ± 3.6 | 0.763 |
| Total CD20+ | 10.8 ± 11.1 | 7.3 ± 6.1 | 12.4 ± 17.6 | 15 ± 12 | 0.125 |
| sCD20+ | 5.1 ± 6.7 | 1.9 ± 4.6 | 5.9 ± 15.2 | 8.6 ± 11.1 | 0.129 |
| iCD20+ | 3.2 ± 7.4 | 4.2 ± 4.3 | 2.9 ± 11.5 | 9.3 ± 6.4 | 0.908 |
| Total CD56+ | 1.6 ± 4.2 | 1.7 ± 2.8 | 0.7 ± 3.4 | 2.3 ± 2.8 | 0.366 |
| sCD56+ | 1.2 ± 3.9 | 1.2 ± 2.6 | 0.3 ± 2.7 | 1.4 ± 1.8 | 0.133 |
| iCD56+ | 0.1 ± 0.9 | 0.3 ± 0.9 | 0 ± 1.3 | 0.2 ± 1.3 | 0.785 |
| Total FoxP3+ | 0.6 ± 1.3 | 0.8 ± 1.3 | 0.5 ± 2 | 1.3 ± 1.9 | 0.341 |
| sFoxP3+ | 0.4 ± 1.1 | 0.6 ± 0.7 | 0.4 ± 2 | 1 ± 1.6 | 0.189 |
| iFoxP3+ | 0 ± 0.6 | 0 ± 0.8 | 0 ± 0.1 | 0 ± 0.5 | 0.366 |
| Total CD68+ | 0.9 ± 10.5 | 0.6 ± 3.8 | 0.9 ± 4.6 | 4 ± 9 | 0.344 |
| sCD68+ | 0 ± 2.2 | 0 ± 1.7 | 0 ± 1.3 | 1 ± 9.3 | 0.023 |
| iCD68+ | 0.6 ± 10.3 | 0.3 ± 3.3 | 0 ± 4.5 | 0.3 ± 2.7 | 0.876 |
| Total CD163+ | 0.3 ± 1.2 | 1.6 ± 4 | 0.8 ± 2.3 | 0.7 ± 1.1 | 0.149 |
| sCD163+ | 0.2 ± 1 | 1.2 ± 3.6 | 0.4 ± 2.3 | 0.3 ± 0.8 | 0.195 |
| iCD163+ | 0 ± 0.5 | 0.5 ± 1 | 0 ± 0.6 | 0 ± 0.6 | 0.485 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, C.; Gameiro, A.; Correia, J.; Ferreira, J.; Ferreira, F. The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications. Cells 2022, 11, 2578. https://doi.org/10.3390/cells11162578
Nascimento C, Gameiro A, Correia J, Ferreira J, Ferreira F. The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications. Cells. 2022; 11(16):2578. https://doi.org/10.3390/cells11162578
Chicago/Turabian StyleNascimento, Catarina, Andreia Gameiro, Jorge Correia, João Ferreira, and Fernando Ferreira. 2022. "The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications" Cells 11, no. 16: 2578. https://doi.org/10.3390/cells11162578
APA StyleNascimento, C., Gameiro, A., Correia, J., Ferreira, J., & Ferreira, F. (2022). The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications. Cells, 11(16), 2578. https://doi.org/10.3390/cells11162578










