Sympathetic Innervation Modulates Mucosal Immune Homeostasis and Epithelial Host Defense
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemical Denervation Procedure (cSTX)
2.3. Flow Cytometry
2.4. RNA Sequencing
2.5. 16S Bacterial Sequencing
2.6. Hematoxylin and Eosin Staining
2.7. Immunofluorescence Staining
2.8. RNA Isolation and Quantitative RT-PCR from Mucosal Samples
2.9. In Vivo Murine Ileal Loop Model
2.10. Organoid Culture
2.11. Microarray Analysis
2.12. Quantitative RT-PCR from Organoids
2.13. Western Blot
2.14. Statistical Analysis
3. Results
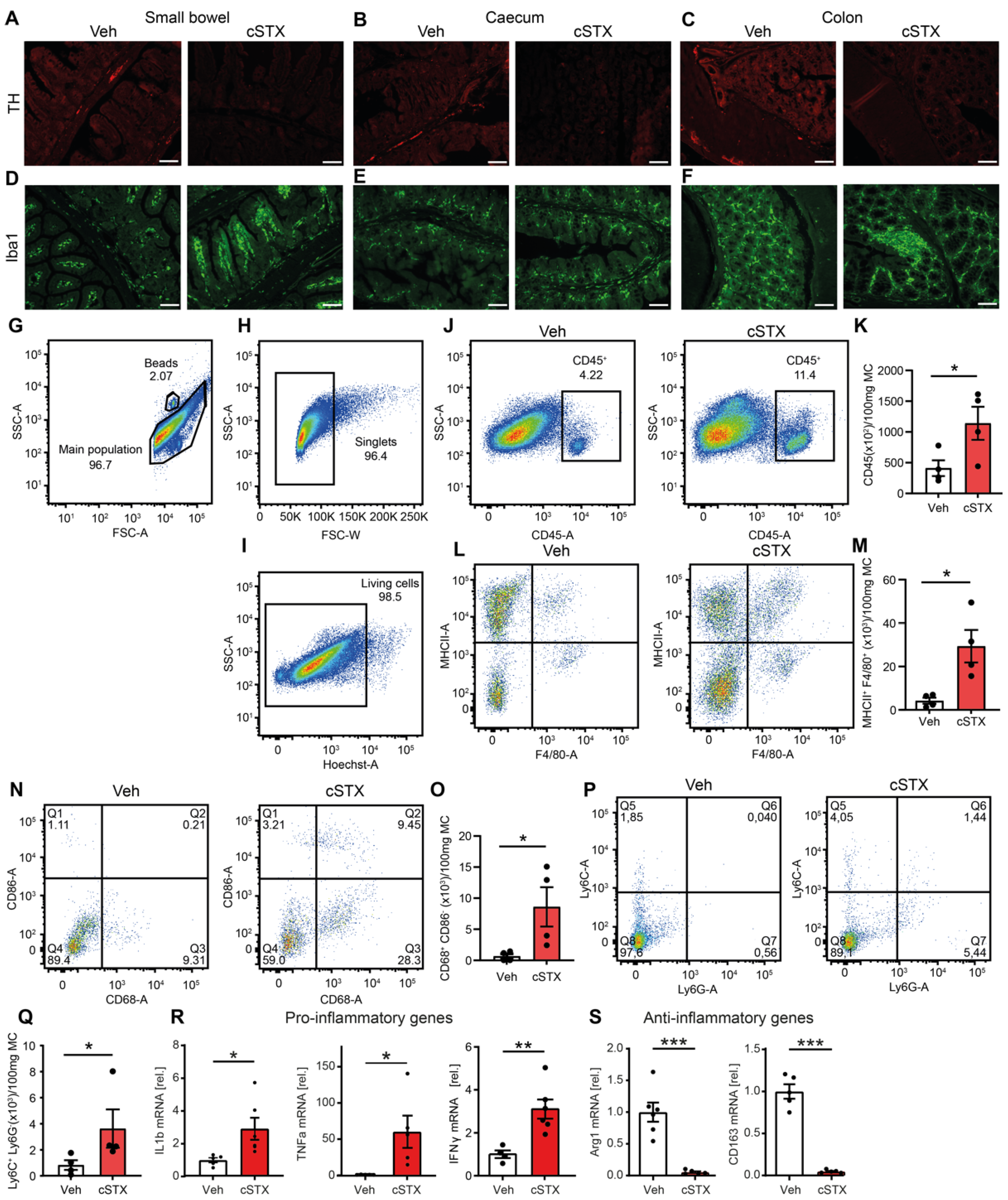
3.1. Sympathetic Denervation Leads to the Induction of Proinflammatory Genes in the Intestinal Mucosa
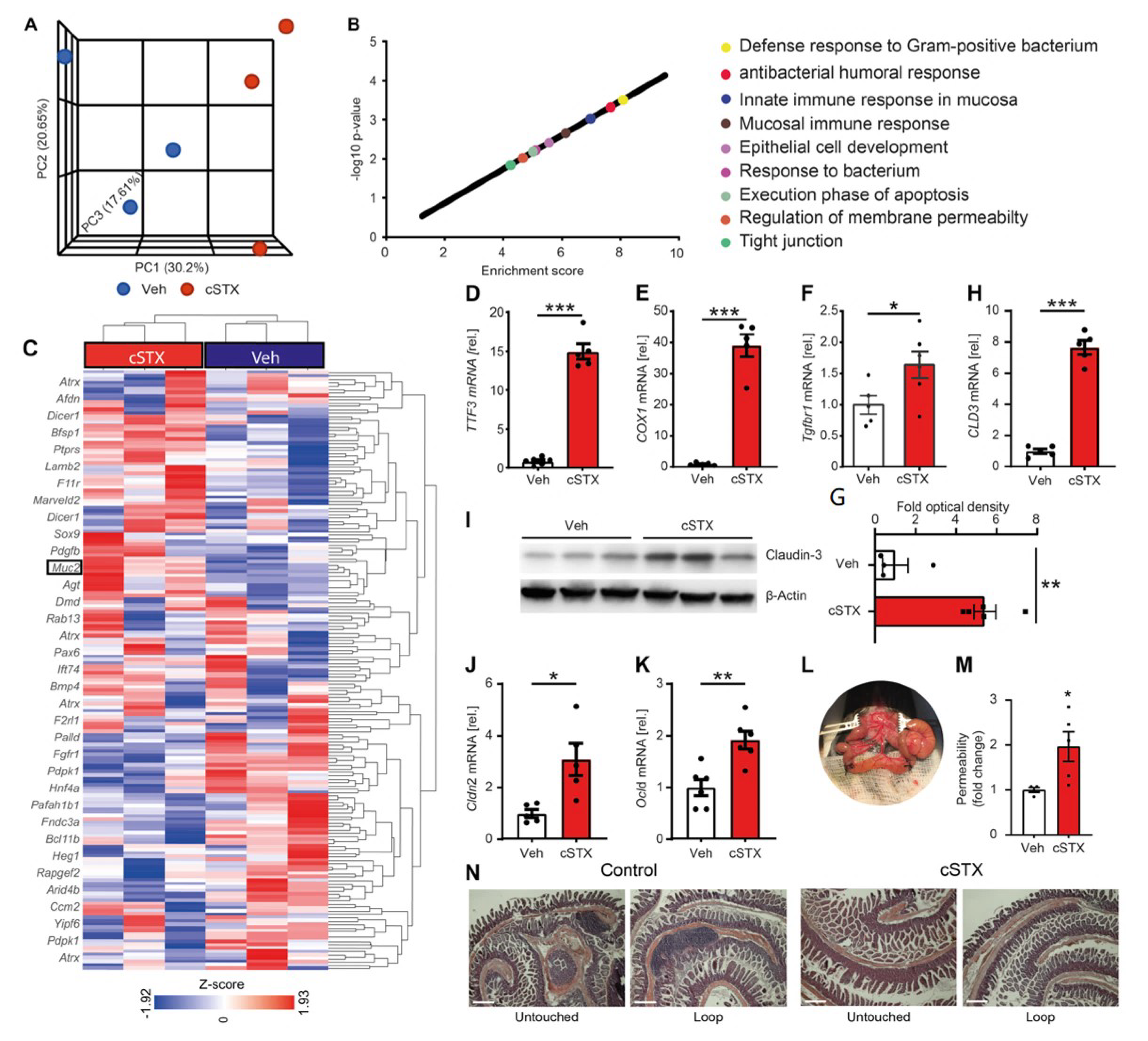
3.2. Sympathetic Neurons Modulate Epithelial Homeostasis
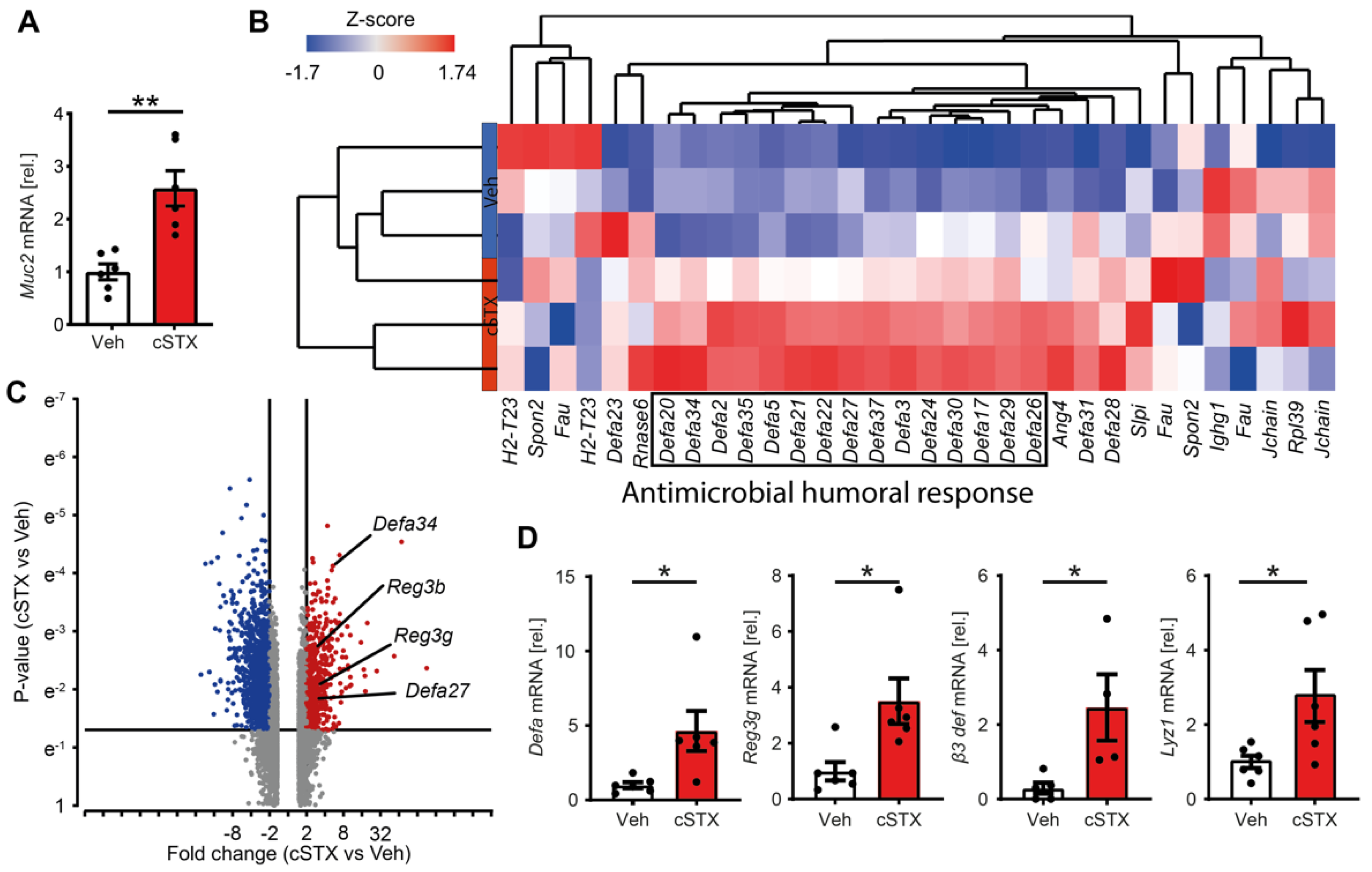
3.3. Sympathetic Denervation Induces Expression of Antimicrobial Defense Genes
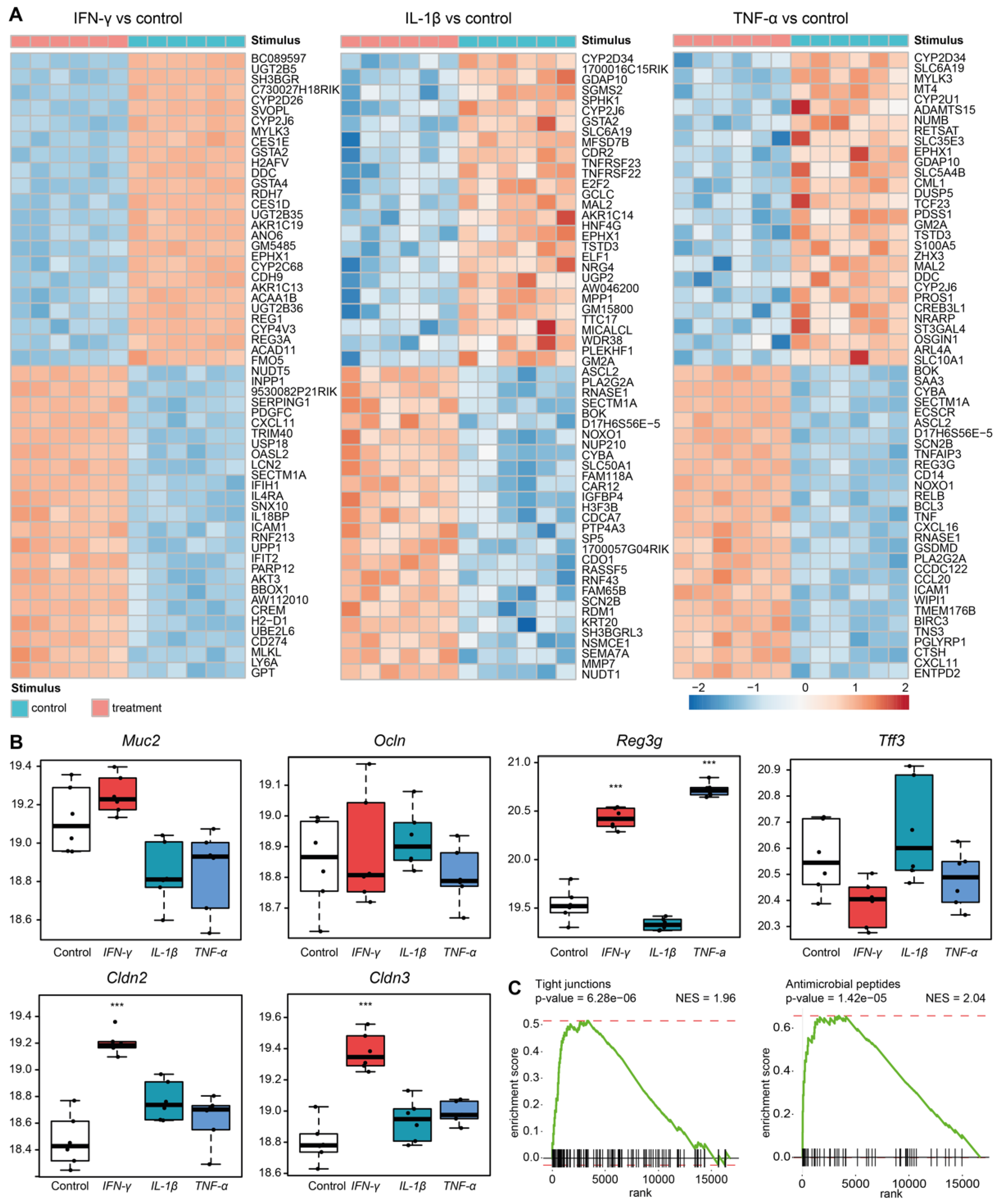
3.4. Sympathetic Neuronal Activity Impacts Epithelial Cell Functions Indirectly via Cytokines
3.5. Sympathetic Denervation Alters the Intestinal Microbial Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine hydrobromide |
| AMPs | Antimicrobial peptides |
| AR | Adrenergic receptors |
| Arg1 | Arginase 1 |
| cSTX | Chemical sympathectomy |
| Defa | Alpha defensins |
| FACS | Fluorescence-activated cell sorting |
| FDR | False-discovery rate |
| FITC | Fluorescein isothiocyanate |
| GO | Gene ontology |
| GSEA | Gene set enrichment analyses |
| IECs | Intestinal epithelial cells |
| IFN-γ | Interferon gamma |
| IL-1β | Interleukin 1 beta |
| NES | Normalized enrichment score |
| PCA | Principal component analysis |
| Reg3g | Regenerating islet-derived protein |
| SNS | Sympathetic nervous system |
| STX | Sympathectomy |
| TFF3 | Trefoil factor 3 |
| TH | Tyrosine hydroxylase |
| TNF-α | Tumor Necrosis Factor |
References
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Genet. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Reimann, F.; Gribble, F. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Coopersmith, C.M. Redefining the gut as the motor of critical illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [Green Version]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The Antibacterial Lectin RegIIIγ Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; López-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of Intestinal α-Defensin Activation by the Metalloproteinase Matrilysin in Innate Host Defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Beck, P.L.; McKay, D.M. Neuroimmunophysiology of the gut: Advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 765–784. [Google Scholar] [CrossRef]
- Demonceau, C.; Piret, J.; Zorzi, D.; Thellin, O.; Heinen, E. Close interactions between sympathetic neural fibres and follicular dendritic cells network are not altered in Peyer’s patches and spleen of C57BL/6 mice during the preclinical stage of 139A scrapie infection. J. Neuroimmunol. 2014, 272, 1–9. [Google Scholar] [CrossRef]
- Chiocchetti, R.; Mazzuoli, G.M.-W.; Albanese, V.; Mazzoni, M.; Clavenzani, P.; Lalatta-Costerbosa, G.; Lucchi, M.L.; Di Guardo, G.; Marruchella, G.; Furness, J.B. Anatomical evidence for ileal Peyer’s patches innervation by enteric nervous system: A potential route for prion neuroinvasion? Cell Tissue Res. 2008, 332, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Fernandes, H.; Pachnis, V. Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol. 2017, 18, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Muller, P.; Feighery, L.; Oliveira, T.; Costa-Pinto, F.; Mucida, D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemze, R.A.; Welting, O.; Van Hamersveld, P.; Verseijden, C.; Nijhuis, L.E.; Hilbers, F.W.; Meijer, S.L.; Heesters, B.A.; Folgering, J.H.A.; Darwinkel, H.; et al. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Mol. Med. 2019, 25, 1. [Google Scholar] [CrossRef]
- You, X.-Y.; Zhang, H.-Y.; Han, X.; Wang, F.; Zhuang, P.-W.; Zhang, Y.-J. Intestinal Mucosal Barrier Is Regulated by Intestinal Tract Neuro-Immune Interplay. Front. Pharmacol. 2021, 12, 659716. [Google Scholar] [CrossRef]
- Mallesh, S.; Schneider, R.; Schneiker, B.; Lysson, M.; Efferz, P.; Lin, E.; de Jonge, W.; Wehner, S. Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice. Int. J. Mol. Sci. 2021, 22, 6872. [Google Scholar] [CrossRef]
- Hove, A.S.T.; Seppen, J.; de Jonge, W.J. Neuronal innervation of the intestinal crypt. Am. J. Physiol. Liver Physiol. 2021, 320, G193–G205. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Glinka, Y.; Gassen, M.; Youdim, M.B.H. Mechanism of 6-hydroxydopamine neurotoxicity. J. Neural Transm. Suppl. 1997, 50, 55–66. [Google Scholar] [CrossRef]
- Flemming, S.; Luissint, A.-C.; Nusrat, A.; Parkos, C.A. Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model. JCI Insight 2018, 3, e99722. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Farin, H.F.; Van Es, J.H.; Clevers, H. Redundant sources of wnt regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology 2012, 143, 1518–1529. [Google Scholar] [CrossRef]
- Matheis, F.; Muller, P.A.; Graves, C.L.; Gabanyi, I.; Kerner, Z.J.; Costa-Borges, D.; Ahrends, T.; Rosenstiel, P.; Mucida, D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020, 180, 64–78.e16. [Google Scholar] [CrossRef] [PubMed]
- Saiwai, H.; Kumamaru, H.; Ohkawa, Y.; Kubota, K.; Kobayakawa, K.; Yamada, H.; Yokomizo, T.; Iwamoto, Y.; Okada, S. Ly6C+Ly6G−Myeloid-derived suppressor cells play a critical role in the resolution of acute inflammation and the subsequent tissue repair process after spinal cord injury. J. Neurochem. 2013, 125, 74–88. [Google Scholar] [CrossRef]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Li, J.; Tan, S.; Xu, M.; Tao, J.; Jiang, J.; Liu, H.; Wu, B. COX-1/PGE2/EP4 alleviates mucosal injury by upregulating β-arr1-mediated Akt signaling in colitis. Sci. Rep. 2017, 7, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Goto, Y. Epithelial Cells as a Transmitter of Signals from Commensal Bacteria and Host Immune Cells. Front. Immunol. 2019, 10, 2057. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, G.; Matar, C.; Perdigon, G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-Like Receptors. Clin. Vaccine Immunol. 2005, 12, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.D.; Smythies, L.E.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflügers Arch. Eur. J. Physiol. 2017, 469, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Burkard, N.; Ungewiß, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.-T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankertz, J.; Schulzke, J.-D. Altered permeability in inflammatory bowel disease: Pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Taupin, D.R.; Kinoshita, K.; Podolsky, D.K. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Büller, H.A.; Dekker, J.; VAN Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Bosmans, J.W.A.M.; Jongen, A.C.H.M.; Birchenough, G.M.H.; Nyström, E.E.L.; Gijbels, M.J.J.; Derikx, J.P.M.; Bouvy, N.D.; Hansson, G.C. Functional mucous layer and healing of proximal colonic anastomoses in an experimental model. Br. J. Surg. 2017, 104, 619–630. [Google Scholar] [CrossRef]
- Wang, L.; Llorente, C.; Hartmann, P.; Yang, A.-M.; Chen, P.; Schnabl, B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 2015, 421, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Yokoi, Y.; Fukaya, R.; Ohira, S.; Shinozaki, R.; Nishida, T.; Kikuchi, M.; Ayabe, T. Expression and Localization of Paneth Cells and Their α-Defensins in the Small Intestine of Adult Mouse. Front. Immunol. 2020, 11, 570296. [Google Scholar] [CrossRef] [PubMed]
- Meade, K.G.; O’Farrelly, C. β-Defensins: Farming the Microbiome for Homeostasis and Health. Front. Immunol. 2019, 9, 3072. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; De Palma, G.; Willemze, R.; Hilbers, F.; Verseijden, C.; Luyer, M.; Nuding, S.; Wehkamp, J.; Souwer, Y.; de Jong, E.; et al. Acetylcholine-producing t cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G920–G933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolls, J.K., Jr.; McCarry, P.B.; Chan, Y.R. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 2008, 8, 829–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, C.; McLean, M.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Eriguchi, Y.; Nakamura, K.; Yokoi, Y.; Sugimoto, R.; Takahashi, S.; Hashimoto, D.; Teshima, T.; Ayabe, T.; Selsted, M.E.; Ouellette, A.J. Essential role of IFN-γ in T cell–associated intestinal inflammation. JCI Insight 2018, 3, e121886. [Google Scholar] [CrossRef]
- Song, H.-L.; Lu, S.; Liu, P. Tumor necrosis factor-alpha induces apoptosis of enterocytes in mice with fulminant hepatic failure. World J. Gastroenterol. 2005, 11, 3701–3709. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Santisteban, M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Craven, M.; Egan, C.E.; Dowd, S.; McDonough, S.P.; Dogan, B.; Denkers, E.Y.; Bowman, D.; Scherl, E.J.; Simpson, K.W. Inflammation Drives Dysbiosis and Bacterial Invasion in Murine Models of Ileal Crohn’s Disease. PLoS ONE 2012, 7, e41594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Dey, N.; Soergel, D.A.W.; Repo, S.; Brenner, S.E. Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol. 2013, 13, 131. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallesh, S.; Ten Hove, A.S.; Schneider, R.; Schneiker, B.; Efferz, P.; Kalff, J.C.; de Jonge, W.J.; Wehner, S. Sympathetic Innervation Modulates Mucosal Immune Homeostasis and Epithelial Host Defense. Cells 2022, 11, 2606. https://doi.org/10.3390/cells11162606
Mallesh S, Ten Hove AS, Schneider R, Schneiker B, Efferz P, Kalff JC, de Jonge WJ, Wehner S. Sympathetic Innervation Modulates Mucosal Immune Homeostasis and Epithelial Host Defense. Cells. 2022; 11(16):2606. https://doi.org/10.3390/cells11162606
Chicago/Turabian StyleMallesh, Shilpashree, Anne S. Ten Hove, Reiner Schneider, Bianca Schneiker, Patrik Efferz, Jörg C. Kalff, Wouter J. de Jonge, and Sven Wehner. 2022. "Sympathetic Innervation Modulates Mucosal Immune Homeostasis and Epithelial Host Defense" Cells 11, no. 16: 2606. https://doi.org/10.3390/cells11162606
APA StyleMallesh, S., Ten Hove, A. S., Schneider, R., Schneiker, B., Efferz, P., Kalff, J. C., de Jonge, W. J., & Wehner, S. (2022). Sympathetic Innervation Modulates Mucosal Immune Homeostasis and Epithelial Host Defense. Cells, 11(16), 2606. https://doi.org/10.3390/cells11162606





