Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Semithin Sections and Transmission Electron Microscopy (TEM)
2.3. Immunohistochemical Analysis
2.4. Morphometrical and Quantitative Studies
2.5. Digitally Colored TEM Images
3. Results
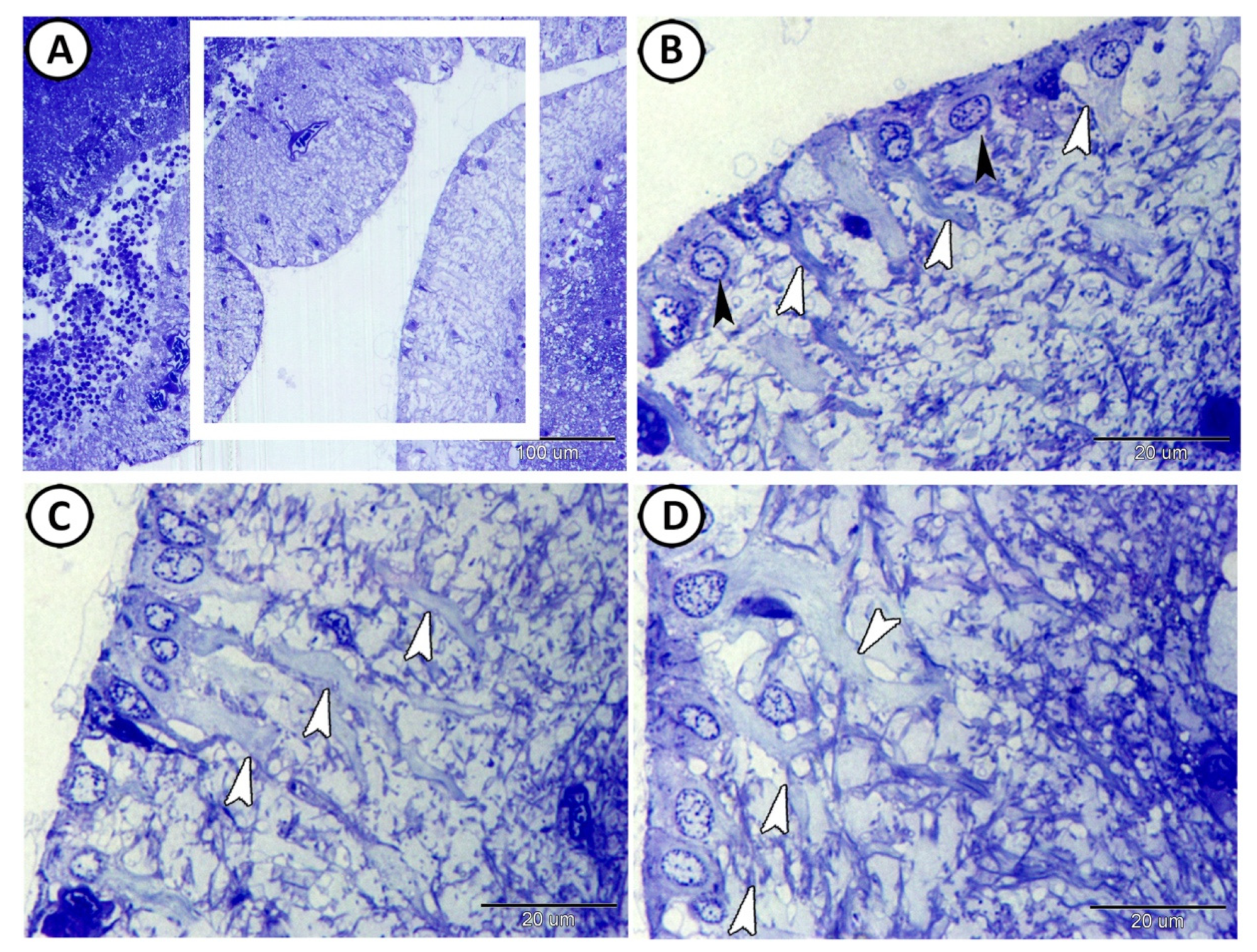
3.1. Histological Analysis
3.2. Immunohistochemistry
3.3. Transmission Electron Microscopy (TEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, R.K.A.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-García, A.; Ramírez-Herrejón, J.P.; Medina-Nava, M.; Hernández-Morales, R.; Domínguez-Domínguez, O. Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. J. Appl. Ichthyol. 2018, 34, 81–90. [Google Scholar] [CrossRef]
- Alda, F.; Reina, R.G.; Doadrio, I.; Bermingham, E. Phylogeny and biogeography of the Poecilia sphenops species complex (Actinopterygii, Poeciliidae) in Central America. Mol. Phylogenet. Evol. 2013, 66, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Tembo, R.N. The sublethal effects of low-pH exposure on the chemoreception of Poecilia sphenops. Arch. Environ. Contam. Toxicol. 2009, 57, 157–163. [Google Scholar] [CrossRef]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Johanson, C.E.; Duncan, J.A.; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef]
- Bulat, M.; Klarica, M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res. Rev. 2011, 65, 99–112. [Google Scholar] [CrossRef]
- Van Gennip, J.L.M.; Boswell, C.W.; Ciruna, B. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 2018, 4, eaav1781. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 2018, 50, 1666–1673. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef] [Green Version]
- Bedussi, B.; van Lier, M.G.; Bartstra, J.W.; de Vos, J.; Siebes, M.; VanBavel, E.; Bakker, E.N.T.P. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 2015, 12, 23. [Google Scholar] [CrossRef]
- Thouvenin, O.; Keiser, L.; Cantaut-Belarif, Y.; Carbo-Tano, M.; Verweij, F.; Jurisch-Yaksi, N.; Bardet, P.L.; van Niel, G.; Gallaire, F.; Wyart, C. Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. Elife 2020, 9, 47699. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Curr. Biol. 2020, 30, 2353–2362.e3. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef]
- Lalioti, M.E.; Kaplani, K.; Lokka, G.; Georgomanolis, T.; Kyrousi, C.; Dong, W.; Dunbar, A.; Parlapani, E.; Damianidou, E.; Spassky, N.; et al. GemC1 is a critical switch for neural stem cell generation in the postnatal brain. Glia 2019, 67, 2360–2373. [Google Scholar] [CrossRef]
- Kaneko, N.; Sawamoto, K. Adult neurogenesis and its alteration under pathological conditions. Neurosci. Res. 2009, 63, 155–164. [Google Scholar] [CrossRef]
- Ogino, T.; Sawada, M.; Takase, H.; Nakai, C.; Herranz-Pérez, V.; Cebrián-Silla, A.; Kaneko, N.; García-Verdugo, J.M.; Sawamoto, K. Characterization of multiciliated ependymal cells that emerge in the neurogenic niche of the aged zebrafish brain. J. Comp. Neurol. 2016, 524, 2982–2992. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Hall, Z.J.; Heuzé, A.; Joly, J.S.; Tropepe, V.; Kaslin, J. The role of neuro-epithelial-like and radial-glial stem and progenitor cells in development, plasticity, and repair. Prog. Neurobiol. 2018, 170, 99–114. [Google Scholar] [CrossRef]
- Ito, Y.; Tanaka, H.; Okamoto, H.; Ohshima, T. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 2010, 342, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Kruger, L.; Maxwell, D.S. Comparative fine structure of vertebrate neuroglia: Teleosts and reptiles. J. Comp. Neurol. 1967, 129, 115–141. [Google Scholar] [CrossRef]
- Stevenson, J.A.; Yoon, M.G. Morphology of radial glia, ependymal cells, and periventricular neurons in the optic tectum of goldfish (Carassius auratus). J. Comp. Neurol. 1982, 205, 128–138. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; Taylor and Francis: Abingdon, UK, 2017; pp. 1–246. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural Stem Cells Confer Unique Pinwheel Architecture to the Ventricular Surface in Neurogenic Regions of the Adult Brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol 1965, 27, 1A–149A. [Google Scholar]
- Icardo, J.M.; Colvee, E.; Lauriano, E.R.; Capillo, G.; Guerrera, M.C.; Zaccone, G. The structure of the gas bladder of the spotted gar, Lepisosteus oculatus. J. Morphol. 2015, 276, 90–101. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; Di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Organ. 2016, 119, 85–89. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Albano, M.; Savoca, S.; Di Bella, G.; Albergamo, A.; Koutkova, Z.; Sandova, M.; Velisek, J.; Fabrello, J.; et al. Effects of long-term exposure of Mytilus galloprovincialis to thiacloprid: A multibiomarker approach. Environ. Pollut. 2021, 289, 117892. [Google Scholar] [CrossRef]
- Pagano, M.; Savoca, S.; Impellitteri, F.; Albano, M.; Capillo, G.; Faggio, C. Toxicological Evaluation of Acetylsalicylic Acid in Non-Target Organisms: Chronic Exposure on Mytilus galloprovincialis (Lamarck, 1819). Front. Physiol. 2022, 1165. [Google Scholar] [CrossRef]
- Karlsbakk, E.; Kristmundsson, Á.; Albano, M.; Brown, P.; Freeman, M.A. Redescription and phylogenetic position of Myxobolus ‘eglefini’ and Myxobolus platessae n. comb. (Myxosporea), parasites in the cartilage of some North Atlantic marine fishes, with notes on the phylogeny and classification of the Platysporina. Parasitol. Int. 2017, 66, 952–959. [Google Scholar] [CrossRef] [Green Version]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.N.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Fame, R.M.; Lehtinen, M.K. Emergence and Developmental Roles of the Cerebrospinal Fluid System. Dev. Cell 2020, 52, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.C.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef]
- Laufer, M.; Vanegas, H. The optic tectum of a perciform teleost II. Fine structure. J. Comp. Neurol. 1974, 154, 61–95. [Google Scholar] [CrossRef]
- Mugnaini, E.; Walberg, F. Ultrastructure of Neuroglia. Ergeb. Anat. Entwicklungsgesch. 1964, 37, 194–236. [Google Scholar]
- Kriebel, R.M. The caudal neurosecretory system of Poecilia sphenops (Poeciliidae). J. Morphol. 1980, 165, 157–165. [Google Scholar] [CrossRef]
- Ibañez-Tallon, I.; Pagenstecher, A.; Fliegauf, M.; Olbrich, H.; Kispert, A.; Ketelsen, U.P.; North, A.; Heintz, N.; Omran, H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymanl flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 2004, 13, 2133–2141. [Google Scholar] [CrossRef]
- Rocamonde, B.; Herranz-Pérez, V.; Garcia-Verdugo, J.M.; Huillard, E. ID4 Is Required for Normal Ependymal Cell Development. Front. Neurosci. 2021, 15, 668243. [Google Scholar] [CrossRef]
- Marcus, R.C.; Delaney, C.L.; Easter, S.S. Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio). Vis. Neurosci. 1999, 16, 417–424. [Google Scholar] [CrossRef]
- Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 2012, 520, 3471–3491. [Google Scholar] [CrossRef]
- Teles, M.C.; Sîrbulescu, R.F.; Wellbrock, U.M.; Oliveira, R.F.; Zupanc, G.K.H. Adult neurogenesis in the brain of the Mozambique tilapia, Oreochromis mossambicus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012, 198, 427–449. [Google Scholar] [CrossRef]
- Zupanc, G.K.H.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef]
- Zupanc, G.K.H.; Sîrbulescu, R.F. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur. J. Neurosci. 2011, 34, 917–929. [Google Scholar] [CrossRef]
- Radaelli, G.; Rowlerson, A.; Mascarello, F.; Patruno, M.; Funkenstein, B. Myostatin precursor is present in several tissues in teleost fish: A comparative immunolocalization study. Cell Tissue Res. 2003, 311, 239–250. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, Y.P.; Zhao, Y.; Deng, S.L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 85712. [Google Scholar] [CrossRef]
- Saraswathy, V.M.; Zhou, L.; Burris, B.; Dogra, D.; Reischauer, S.; Mokalled, M.H. Myostatin is a negative regulator of adult neurogenesis in zebrafish. bioRxiv 2021. [Google Scholar] [CrossRef]
- Augustin, H.; McGourty, K.; Steinert, J.R.; Cochemé, H.M.; Adcott, J.; Cabecinha, M.; Vincent, A.; Halff, E.F.; Kittler, J.T.; Boucrot, E.; et al. Myostatin-like proteins regulate synaptic function and neuronal morphology. Developement 2017, 144, 2445–2455. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Capillo, G.; Laurà, R.; Abbate, F.; Levanti, M.; Guerrera, M.C.; Ciriaco, E.; Germanà, A. Neuromast hair cells retain the capacity of regeneration during heavy metal exposure. Ann. Anat. 2018, 218, 183–189. [Google Scholar] [CrossRef]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V.; et al. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef]
- Spokony, R.F.; Aoki, Y.; Saint-Germain, N.; Magner-Fink, E.; Saint-Jeannet, J.P. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development 2002, 129, 421–432. [Google Scholar] [CrossRef]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef]
- Pritchett, J.; Athwal, V.; Roberts, N.; Hanley, N.A.; Hanley, K.P. Understanding the role of SOX9 in acquired diseases: Lessons from development. Trends Mol. Med. 2011, 17, 166–174. [Google Scholar] [CrossRef]
- Sarkar, A.; Hochedlinger, K. The Sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef]
- Scott, C.E.; Wynn, S.L.; Sesay, A.; Cruz, C.; Cheung, M.; Gaviro, M.V.G.; Booth, S.; Gao, B.; Cheah, K.S.E.; Lovell-Badge, R.; et al. SOX9 induces and maintains neural stem cells. Nat. Neurosci. 2010, 13, 1181–1189. [Google Scholar] [CrossRef]
- Nunn, A.C. The Role of SOX9 in Neural Progenitor Identity. Ph.D. Thesis, University College London, London, UK, 2012; pp. 1–241. [Google Scholar]
- Hoser, M.; Potzner, M.R.; Koch, J.M.C.; Bösl, M.R.; Wegner, M.; Sock, E. Sox12 Deletion in the Mouse Reveals Nonreciprocal Redundancy with the Related Sox4 and Sox11 Transcription Factors. Mol. Cell. Biol. 2008, 28, 4675–4687. [Google Scholar] [CrossRef]
- Bergsland, M.; Werme, M.; Malewicz, M.; Perlmann, T.; Muhr, J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006, 20, 3475–3486. [Google Scholar] [CrossRef]
- Reiprich, S.; Cantone, M.; Weider, M.; Baroti, T.; Wittstatt, J.; Schmitt, C.; Küspert, M.; Vera, J.; Wegner, M. Transcription factor Sox10 regulates oligodendroglial Sox9 levels via microRNAs. Glia 2017, 65, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 2011, 25, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.; Zhang, C.L.; Lie, D.C. Transcription-factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018879. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Drakulic, D.; Lazic, A.; Ninkovic, D.S.; Schwirtlich, M.; Mojsin, M. SOX Transcription Factors as Important Regulators of Neuronal and Glial Differentiation During Nervous System Development and Adult Neurogenesis. Front. Mol. Neurosci. 2021, 14, 51. [Google Scholar] [CrossRef]
- Güven, A.; Kalebic, N.; Long, K.R.; Florio, M.; Vaid, S.; Brandl, H.; Stenzel, D.; Huttner, W.B. Extracellular matrix-inducing Sox9 promotes both basal progenitor proliferation and gliogenesis in developing neocortex. Elife 2020, 9, e49808. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Zhu, L.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J. zhong Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef]
- Maina, J.N.; Icardo, J.M.; Zaccone, G.; Aragona, M.; Lauriano, E.R.; Alesci, A.; Albano, M.; Guerrera, M.C.; Germana, A.; Fernandes, J.M.O.; et al. Immunohistochemical and ultrastructural study of the immune cell system and epithelial surfaces of the respiratory organs in the bimodally-breathing African sharptooth catfish (Clarias gariepinus Burchell, 1822). Anat. Rec. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zaccone, G.; Cupello, C.; Capillo, G.; Kuciel, M.; Nascimento, A.L.R.; Gopesh, A.; Germanà, G.P.; Spanò, N.; Guerrera, M.C.; Aragona, M.; et al. Expression of Acetylcholine- and G protein coupled Muscarinic receptor in the Neuroepithelial cells (NECs) of the obligated air-breathing fish, Arapaima gigas (Arapaimatidae: Teleostei). Zoology 2020, 139, 125755. [Google Scholar] [CrossRef]
- Netea, M.G.; Simon, A.; Van De Veerdonk, F.; Kullberg, B.J.; Van Der Meer, J.W.M.; Joosten, L.A.B. IL-1β processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- O’Léime, C.S.; Cryan, J.F.; Nolan, Y.M. Nuclear deterrents: Intrinsic regulators of IL-1β-induced effects on hippocampal neurogenesis. Brain. Behav. Immun. 2017, 66, 394–412. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauriano, E.R.; Capillo, G.; Kuciel, M. Air- breathing in fish: Air- breathing organs and control of respiration: Nerves and neurotransmitters in the air-breathing organs and the skin. Acta Histochem. 2018, 120, 630–641. [Google Scholar] [CrossRef]
- Huang, F.P.; Wang, Z.Q.; Wu, D.C.; Schielke, G.P.; Sun, Y.; Yang, G.Y. Early NFκB activation is inhibited during focal cerebral ischemia in interleukin-1β-converting enzyme deficient mice. J. Neurosci. Res. 2003, 73, 698–707. [Google Scholar] [CrossRef]
- Loddick, S.A.; Rothwell, N.J. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab. 1996, 16, 932–940. [Google Scholar] [CrossRef]
- Zaccone, G.; Fudge, D.S.; Winegard, T.M.; Capillo, G.; Kuciel, M.; Funakoshi, K.; Lauriano, E.R. Confocal imaging and phylogenetic considerations of the subcutaneous neurons in the Atlantic hagfish Myxine glutinosa. Acta Zool. 2015, 96, 209–217. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, M.J.; Han, J.S. Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol. Brain 2018, 11, 39. [Google Scholar] [CrossRef]
- Yao, Z.; Delorme-Axford, E.; Backues, S.K.; Klionsky, D.J. Atg41/Icy2 regulates autophagosome formation. Autophagy 2015, 11, 2288–2299. [Google Scholar] [CrossRef]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef]
- Lv, X.; Jiang, H.; Li, B.; Liang, Q.; Wang, S.; Zhao, Q.; Jiao, J. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci. Rep. 2014, 4, 6010. [Google Scholar] [CrossRef]
- Casares-Crespo, L.; Calatayud-Baselga, I.; García-Corzo, L.; Mira, H. On the role of Basal autophagy in adult neural stem cells and neurogenesis. Front. Cell. Neurosci. 2018, 12, 339. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Z. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Sun, Q.; Ji, X.; Zhu, L.; Cong, Z.; Zhou, Y.; Liu, H.; Zhou, M. Nrf2 is required to maintain the self-renewal of glioma stem cells. BMC Cancer 2013, 13, 380. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 Signaling Regulates Oxidative Stress Tolerance and Lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity. Stem Cells 2014, 32, 1904–1916. [Google Scholar] [CrossRef]
- Jang, J.; Wang, Y.; Kim, H.S.; Lalli, M.A.; Kosik, K.S. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells 2014, 32, 2616–2625. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef]
- La Rosa, P.; Russo, M.; D’Amico, J.; Petrillo, S.; Aquilano, K.; Lettieri-Barbato, D.; Turchi, R.; Bertini, E.S.; Piemonte, F. Nrf2 Induction Re-establishes a Proper Neuronal Differentiation Program in Friedreich’s Ataxia Neural Stem Cells. Front. Cell. Neurosci. 2019, 13, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, M.; Suarez, I.; Bodega, G.; Fernandez, B. Glial fibrillary acidic protein and vimentin immunohistochemistry in the posterior rhombencephalon of the Iberian barb (Barbus comiza). Neurosci. Lett. 1992, 134, 203–206. [Google Scholar] [CrossRef]
- Onteniente, B.; Kimura, H.; Maeda, T. Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J. Comp. Neurol. 1983, 215, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Kamphuis, W.; Middeldorp, J.; Kooijman, L.; Sluijs, J.A.; Kooi, E.J.; Moeton, M.; Freriks, M.; Mizee, M.R.; Hol, E.M. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 492–510. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K.W. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Robinson, H.A.; Pozzo-Miller, L. The role of MeCP2 in learning and memory. Learn. Mem. 2019, 26, 343–350. [Google Scholar] [CrossRef]
- Garcia, A.D.R.; Doan, N.B.; Imura, T.; Bush, T.G.; Sofroniew, M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004, 7, 1233–1241. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New insights into neuron-glia communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef]
- Menuet, A.; Pellegrini, E.; Brion, F.; Gueguen, M.M.; Anglade, I.; Pakdel, F.; Kah, O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005, 485, 304–320. [Google Scholar] [CrossRef]
- Zupanc, G.K.H. Neurogenesis, cell death and regeneration in the adult gymnotiform brain. J. Exp. Biol. 1999, 202, 1435–1446. [Google Scholar] [CrossRef]
- Ma, P.M. Tanycytes in the sunfish brain: NADPH-diaphorase histochemistry and regional distribution. J. Comp. Neurol. 1993, 336, 77–95. [Google Scholar] [CrossRef]
- Clint, S.C.; Zupanc, G.K.H. Neuronal regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: Guidance of migrating young cells by radial glia. Dev. Brain Res. 2001, 130, 15–23. [Google Scholar] [CrossRef]
- Nelson, C.M.; Lennon, V.A.; Lee, H.; Krug, R.G.; Kamalova, A.; Madigan, N.N.; Clark, K.J.; Windebank, A.J.; Henley, J.R. Glucocorticoids Target Ependymal Glia and Inhibit Repair of the Injured Spinal Cord. Front. Cell Dev. Biol. 2019, 7, 56. [Google Scholar] [CrossRef]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Santamaria-Kisiel, L.; Rintala-Dempsey, A.C.; Shaw, G.S. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006, 396, 201–214. [Google Scholar] [CrossRef]
- Germanà, A.; Marino, F.; Guerrera, M.C.; Campo, S.; De Girolamo, P.; Montalbano, G.; Germanà, G.P.; Ochoa-Erena, E.J.; Ciriaco, E.; Vega, J.A. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 2008, 71, 248–255. [Google Scholar] [CrossRef]
- Sorci, G.; Agneletti, A.L.; Donato, R. Effects of S100A1 and S100B on microtubule stability. An in vitro study using triton-cytoskeletons from astrocyte and myoblast cell lines. Neuroscience 2000, 99, 773–783. [Google Scholar] [CrossRef]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Sayed, R.K.A.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells 2022, 11, 2659. https://doi.org/10.3390/cells11172659
Mokhtar DM, Sayed RKA, Zaccone G, Albano M, Hussein MT. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells. 2022; 11(17):2659. https://doi.org/10.3390/cells11172659
Chicago/Turabian StyleMokhtar, Doaa M., Ramy K. A. Sayed, Giacomo Zaccone, Marco Albano, and Manal T. Hussein. 2022. "Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies" Cells 11, no. 17: 2659. https://doi.org/10.3390/cells11172659







