Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics
Abstract
1. Introduction
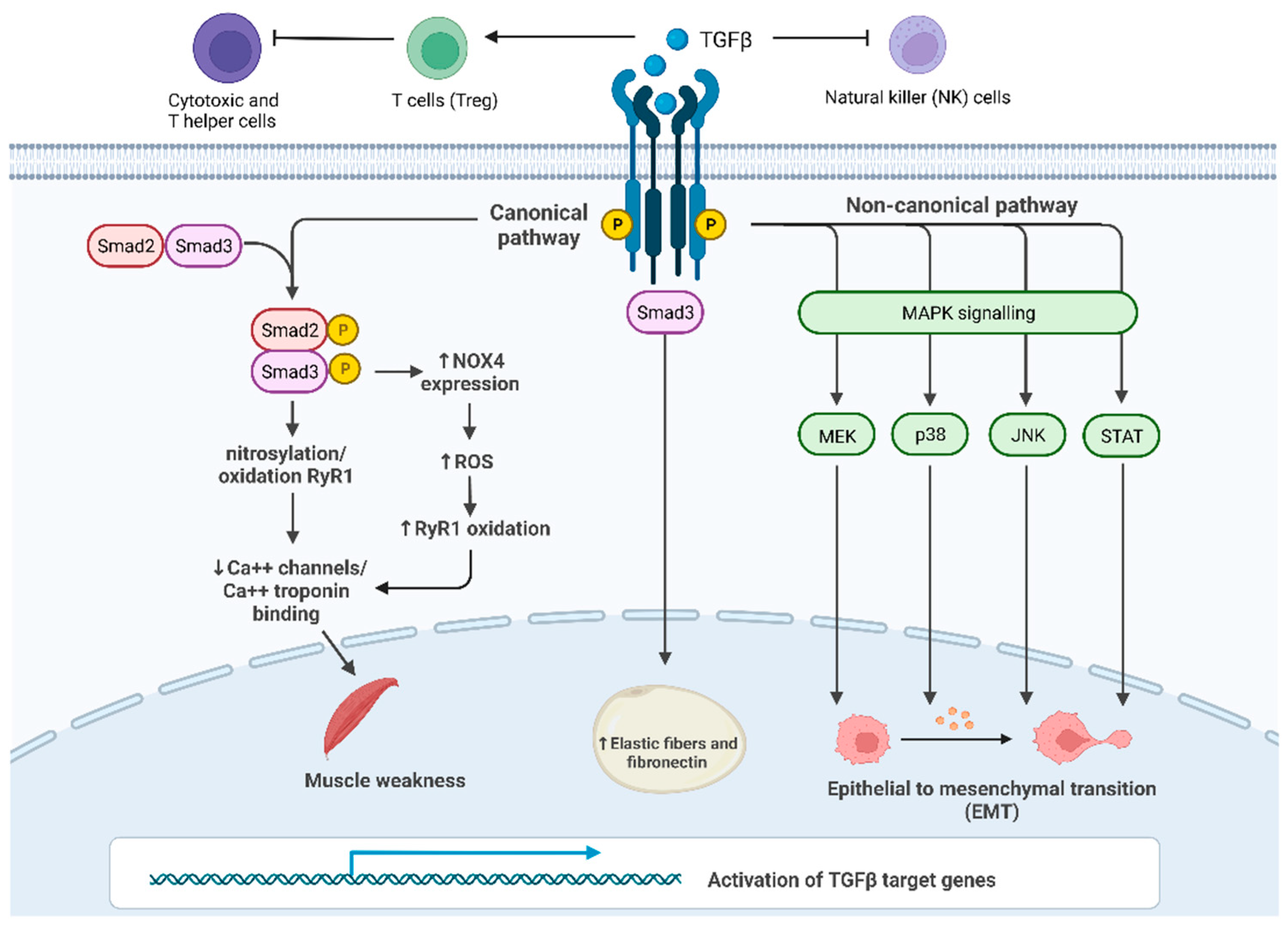
2. TGF-β Signaling Activation
3. TGF-β Effects in the Tumor Microenvironment
4. The Interplay between TGF-β and Cancer Cachexia
5. Other Players Involved in Cachexia Syndrome
5.1. TNFα
5.2. IL-6
5.3. Myostatin and Activin
5.4. Growth Differentiation Factor 15
5.5. Lipocalin 2
5.6. Insulin-Like Peptide 3
6. Role of microRNA in Cachexia
7. Potential Strategies to Prevent Cachexia
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tisdale, M.J. Molecular Pathways Leading to Cancer Cachexia. Physiology 2005, 20, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Tisdale, M.J. The “Cancer Cachectic Factor. ” Supportive Care Cancer 2003, 11, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Holcomb, R.; Muscaritoli, M.; von Haehling, S.; Haverkamp, W.; Jatoi, A.; Morley, J.E.; Strasser, F.; Landmesser, U.; Coats, A.J.S.; et al. Orphan Disease Status of Cancer Cachexia in the USA and in the European Union: A Systematic Review. J. Cachexia Sarcopenia Muscle 2019, 10, 22–34. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Bekaii-Saab, T.; Doyle, L.A.; Shrestha, S.; Ghosh, S.; Baracos, V.E.; Sawyer, M.B. Skeletal Muscle Anabolism Is a Side Effect of Therapy with the MEK Inhibitor: Selumetinib in Patients with Cholangiocarcinoma. Br. J. Cancer 2012, 106, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of Muscle Mass as a Strategy to Reduce the Toxic Effects of Cancer Chemotherapy on Body Composition. Curr. Opin. Support Palliat Care 2018, 12, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine Patterns in Cancer Patients: A Review of the Correlation between Interleukin 6 and Prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Landskron, G.; de La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.W.; Lin, S.; Brown, D.A.; Salis, A.; Breit, S.N. Anorexia-Cachexia and Obesity Treatment May Be Two Sides of the Same Coin: Role of the TGF-b Superfamily Cytokine MIC-1/GDF15. Int. J. Obes 2016, 40, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Walton, K.L.; Hagg, A.; Colgan, T.D.; Johnson, K.; Qian, H.; Gregorevic, P.; Harrison, C.A. Specific Targeting of TGF-β Family Ligands Demonstrates Distinct Roles in the Regulation of Muscle Mass in Health and Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E5266–E5275. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming Growth Factor-Beta Induces Skeletal Muscle Atrophy and Fibrosis through the Induction of Atrogin-1 and Scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef]
- Greco, S.H.; Tomkötter, L.; Vahle, A.K.; Rokosh, R.; Avanzi, A.; Mahmood, S.K.; Deutsch, M.; Alothman, S.; Alqunaibit, D.; Ochi, A.; et al. TGF-β Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia. PLoS ONE 2015, 10, e0132786. [Google Scholar] [CrossRef]
- Farkas, J.; von Haehling, S.; Kalantar-Zadeh, K.; Morley, J.E.; Anker, S.D.; Lainscak, M. Cachexia as a Major Public Health Problem: Frequent, Costly, and Deadly. J. Cachexia Sarcopenia Muscle 2013, 4, 173–178. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Fabbro, E.D.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Chen, W.J.; Wahl, S.M. TGF-Beta: The Missing Link in CD4+CD25+ Regulatory T Cell-Mediated Immunosuppression. Cytokine Growth Factor Rev. 2003, 14, 85–89. [Google Scholar] [CrossRef]
- Colak, S.; ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Neil, J.R.; Schiemann, W.P. Transforming Growth Factor-β and the Hallmarks of Cancer. Cell Signal. 2011, 23, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Dardare, J.; Witz, A.; Merlin, J.L.; Gilson, P.; Harlé, A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 3534. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, X.; Zhang, F.; Clemens, T.L.; Wan, M.; Cao, X. TGF-Beta Type II Receptor Phosphorylates PTH Receptor to Integrate Bone Remodelling Signalling. Nat. Cell Biol. 2010, 12, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Muthusamy, B.P.; Budi, E.H.; Katsuno, Y.; Lee, M.K.; Smith, S.M.; Mirza, A.M.; Akhurst, R.J.; Derynck, R. ShcA Protects against Epithelial–Mesenchymal Transition through Compartmentalized Inhibition of TGF-β-Induced Smad Activation. PLOS Biol. 2015, 13, e1002325. [Google Scholar] [CrossRef]
- Papageorgis, P.; Lambert, A.W.; Ozturk, S.; Gao, F.; Pan, H.; Manne, U.; Alekseyev, Y.O.; Thiagalingam, A.; Abdolmaleky, H.M.; Lenburg, M.; et al. Smad Signaling Is Required to Maintain Epigenetic Silencing during Breast Cancer Progression. Cancer Res. 2010, 70, 968–978. [Google Scholar] [CrossRef]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal Modulators of TGF-β in Cancer. J. Clin. Med. 2017, 6, 7. [Google Scholar] [CrossRef]
- Norton, J.; Foster, D.; Chinta, M.; Titan, A.; Longaker, M. Pancreatic Cancer Associated Fibroblasts (CAF): Under-Explored Target for Pancreatic Cancer Treatment. Cancers 2020, 12, 1347. [Google Scholar] [CrossRef]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in Regulation of the Tumor Microenvironment and Drug Delivery (Review). Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin Inhibition Enhances Drug Delivery and Potentiates Chemotherapy by Decompressing Tumour Blood Vessels. Nat. Commun. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Ding, W.; Rountree, C.B. Epigenetic Regulation of Cancer Stem Cell Marker CD133 by Transforming Growth Factor-Beta. Hepatology 2010, 51, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.M.; Jing, Y.Y.; Yu, G.F.; Kou, X.R.; Ye, F.; Gao, L.; Li, R.; Zhao, Q.D.; Yang, Y.; Lu, Z.H.; et al. Tumor-Associated Macrophages Promote Cancer Stem Cell-like Properties via Transforming Growth Factor-Beta1-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Cancer Lett. 2014, 352, 160–168. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Karnoub, A.E. Mesenchymal Stem Cells in Tumor Development: Emerging Roles and Concepts. Cell Adh. Migr. 2012, 6, 220–230. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ Signalling Pathway in Disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Gong, D.; Shi, W.; Yi, S.J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFβ Signaling Plays a Critical Role in Promoting Alternative Macrophage Activation. BMC Immunol. 2012, 13. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Nana, A.W.; Yang, P.M.; Lin, H.Y. Overview of Transforming Growth Factor β Superfamily Involvement in Glioblastoma Initiation and Progression. Asian Pac. J. Cancer Prev. 2015, 16, 6813–6823. [Google Scholar] [CrossRef][Green Version]
- Neuzillet, C.; de Gramont, A.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. Perspectives of TGF-β Inhibition in Pancreatic and Hepatocellular Carcinomas. Oncotarget 2014, 5, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J. Behavior of Immune Players in the Tumor Microenvironment. Curr. Opin. Oncol. 2009, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, S.; Zeng, J. TGF-β Signaling: A Complex Role in Tumorigenesis (Review). Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A Multigenic Program Mediating Breast Cancer Metastasis to Bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Sánchez-Elsner, T.; Botella, L.M.; Velasco, B.; Corbí, A.; Attisano, L.; Bernabéu, C. Synergistic Cooperation between Hypoxia and Transforming Growth Factor-Beta Pathways on Human Vascular Endothelial Growth Factor Gene Expression. J. Biol. Chem. 2001, 276, 38527–38535. [Google Scholar] [CrossRef]
- Ebadi, M.; Mazurak, V.C. Potential Biomarkers of Fat Loss as a Feature of Cancer Cachexia. Mediat. Inflamm. 2015, 2015, 820934. [Google Scholar] [CrossRef]
- Batista, M.L.; Henriques, F.S.; Neves, R.X.; Olivan, M.R.; Matos-Neto, E.M.; Alcântara, P.S.M.; Maximiano, L.F.; Otoch, J.P.; Alves, M.J.; Seelaender, M. Cachexia-Associated Adipose Tissue Morphological Rearrangement in Gastrointestinal Cancer Patients. J. Cachexia Sarcopenia Muscle 2016, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Figuerêdo, R.G.; Azevedo, F.F.; Cavallaro, D.A.; Neto, N.I.P.; Lima, J.D.C.; Matos-Neto, E.; Radloff, K.; Riccardi, D.M.; Camargo, R.G.; et al. Adipose Tissue Fibrosis in Human Cancer Cachexia: The Role of TGFβ Pathway. BMC Cancer 2017, 17, 1–2. [Google Scholar] [CrossRef]
- David, C.J.; Huang, Y.H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massagué, J. TGF-β Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming Growth Factor Beta1 Release by Human Adipose Tissue Is Enhanced in Obesity. Metabolism 2005, 54, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.D.C.C.; Simoes, E.; de Castro, G.; Morais, M.R.P.T.; de Matos-Neto, E.M.; Alves, M.J.; Pinto, N.I.; Figueredo, R.G.; Zorn, T.M.T.; Felipe-Silva, A.S.; et al. Tumour-Derived Transforming Growth Factor-β Signalling Contributes to Fibrosis in Patients with Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Ábrigo, J.; Campos, F.; Simon, F.; Riedel, C.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. TGF-β Requires the Activation of Canonical and Non-Canonical Signalling Pathways to Induce Skeletal Muscle Atrophy. Biol. Chem. 2018, 399, 253–264. [Google Scholar] [CrossRef]
- Cheruku, H.R.; Mohamedali, A.; Cantor, D.I.; Tan, S.H.; Nice, E.C.; Baker, M.S. Transforming Growth Factor-β, MAPK and Wnt Signaling Interactions in Colorectal Cancer. EuPA Open Proteom. 2015, 8, 104–115. [Google Scholar] [CrossRef]
- Hix, L.M.; Karavitis, J.; Khan, M.W.; Shi, Y.H.; Khazaie, K.; Zhang, M. Tumor STAT1 Transcription Factor Activity Enhances Breast Tumor Growth and Immune Suppression Mediated by Myeloid-Derived Suppressor Cells. J. Biol. Chem. 2013, 288, 11676. [Google Scholar] [CrossRef] [PubMed]
- Meissl, K.; Macho-Maschler, S.; Müller, M.; Strobl, B. The Good and the Bad Faces of STAT1 in Solid Tumours. Cytokine 2017, 89, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Neto, N.I.P.; Murari, A.S.D.P.; Oyama, L.M.; Otoch, J.P.; Alcântara, P.S.M.; Tokeshi, F.; Figuerêdo, R.G.; Alves, M.J.; Lima, J.D.C.C.; Matos-Neto, E.M.; et al. Peritumoural Adipose Tissue Pro-inflammatory Cytokines Are Associated with Tumoural Growth Factors in Cancer Cachexia Patients. J. Cachexia Sarcopenia Muscle 2018, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Ondrias, K.; Marks, A.R. Coupled Gating between Individual Skeletal Muscle Ca2+ Release Channels (Ryanodine Receptors). Science 1998, 281, 818–821. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β Mediates Muscle Weakness Associated with Bone Metastases in Mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef]
- Oliff, A.; Defeo-Jones, D.; Boyer, M.; Martinez, D.; Kiefer, D.; Vuocolo, G.; Wolfe, A.; Socher, S.H. Tumors Secreting Human TNF/Cachectin Induce Cachexia in Mice. Cell 1987, 50, 555–563. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Mechanisms of TNF-Alpha-Induced Insulin Resistance. Exp. Clin. Endocrinol. Diabetes 1999, 107, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.Y.; Baldwin, J. NF-KappaB-Induced Loss of MyoD Messenger RNA: Possible Role in Muscle Decay and Cachexia. Science 2000, 289, 2363–2365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Chen, Y.; John, J.; Moylan, J.; Jin, B.; Mann, D.L.; Reid, M.B. TNF-Alpha Acts via P38 MAPK to Stimulate Expression of the Ubiquitin Ligase Atrogin1/MAFbx in Skeletal Muscle. FASEB J. 2005, 19, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Llovera, M.; García-Martínez, C.; López-Soriano, J.; Carbó, N.; Agell, N.; López-Soriano, F.J.; Argiles, J.M. Role of TNF Receptor 1 in Protein Turnover during Cancer Cachexia Using Gene Knockout Mice. Mol. Cell Endocrinol. 1998, 142, 183–189. [Google Scholar] [CrossRef]
- Rydén, M.; Agustsson, T.; Laurencikiene, J.; Britton, T.; Sjölin, E.; Isaksson, B.; Permert, J.; Arner, P. Lipolysis--Not Inflammation, Cell Death, or Lipogenesis--Is Involved in Adipose Tissue Loss in Cancer Cachexia. Cancer 2008, 113, 1695–1704. [Google Scholar] [CrossRef]
- Maltoni, M.; Fabbri, L.; Nanni, O.; Scarpi, E.; Pezzi, L.; Flamini, E.; Riccobon, A.; Derni, S.; Pallotti, G.; Amadori, D. Serum Levels of Tumour Necrosis Factor Alpha and Other Cytokines Do Not Correlate with Weight Loss and Anorexia in Cancer Patients. Support. Care Cancer 1997, 5, 130–135. [Google Scholar] [CrossRef]
- Jatoi, A.; Ritter, H.L.; Dueck, A.; Nguyen, P.L.; Nikcevich, D.A.; Luyun, R.F.; Mattar, B.I.; Loprinzi, C.L. A Placebo-Controlled, Double-Blind Trial of Infliximab for Cancer-Associated Weight Loss in Elderly and/or Poor Performance Non-Small Cell Lung Cancer Patients (N01C9). Lung Cancer 2010, 68, 234–239. [Google Scholar] [CrossRef]
- Scott, H.R.; McMillan, D.C.; Crilly, A.; McArdle, C.S.; Milroy, R. The Relationship between Weight Loss and Interleukin 6 in Non-Small-Cell Lung Cancer. Br. J. Cancer 1996, 73, 1560. [Google Scholar] [CrossRef]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 Is the Major Regulator of Acute Phase Protein Synthesis in Adult Human Hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.O.; Davis, J.M.; Muga, S.J.; Carson, J.A. Interleukin-6 and Cachexia in ApcMin/+ Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R393–R401. [Google Scholar] [CrossRef]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 Transcription Factors Control Muscle Mass in Adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef] [PubMed]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.J. Induction of Cachexia in Mice by Systemically Administered Myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 Axis Is a Critical Driver of Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-Dependent Suppression of Appetite by Bone-Derived Lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef]
- Black, K.; Garrett, I.R.; Mundy, G.R. Chinese Hamster Ovarian Cells Transfected with the Murine Interleukin-6 Gene Cause Hypercalcemia as Well as Cachexia, Leukocytosis and Thrombocytosis in Tumor-Bearing Nude Mice. Endocrinology 1991, 128, 2657–2659. [Google Scholar] [CrossRef]
- White, J.P.; Baynes, J.W.; Welle, S.L.; Kostek, M.C.; Matesic, L.E.; Sato, S.; Carson, J.A. The Regulation of Skeletal Muscle Protein Turnover during the Progression of Cancer Cachexia in the ApcMin/+ Mouse. PLoS ONE 2011, 6, e24650. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S.; Koniaris, L.G.; Zimmers, T.A. STAT3 Activation in Skeletal Muscle Links Muscle Wasting and the Acute Phase Response in Cancer Cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Fearon, K.C.H.; Maingay, J.P.; Ross, J.A. Down-Regulation of the Acute-Phase Response in Patients with Pancreatic Cancer Cachexia Receiving Oral Eicosapentaenoic Acid Is Mediated via Suppression of Interleukin-6. Clin. Sci. 1997, 92, 215–221. [Google Scholar] [CrossRef]
- Bayliss, T.J.; Smith, J.T.; Schuster, M.; Dragnev, K.H.; Rigas, J.R. A Humanized Anti-IL-6 Antibody (ALD518) in Non-Small Cell Lung Cancer. Expert Opin. Biol. Ther. 2011, 11, 1663–1668. [Google Scholar] [CrossRef]
- Kandarian, S.C.; Nosacka, R.L.; Delitto, A.E.; Judge, A.R.; Judge, S.M.; Ganey, J.D.; Moreira, J.D.; Jackman, R.W. Tumour-Derived Leukaemia Inhibitory Factor Is a Major Driver of Cancer Cachexia and Morbidity in C26 Tumour-Bearing Mice. J. Cachexia Sarcopenia Muscle 2018, 9, 1109–1120. [Google Scholar] [CrossRef]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet. 2007, 3, 779–786. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovich, S.; Krag, T.O.B.; Barton, E.R.; Morris, L.D.; Whittemore, L.A.; Ahima, R.S.; Khurana, T.S. Functional Improvement of Dystrophic Muscle by Myostatin Blockade. Nature 2002, 420, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin Reduces Akt/TORC1/P70S6K Signaling, Inhibiting Myoblast Differentiation and Myotube Size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Nissinen, T.A.; Penna, F.; Bonetto, A. Targeting the Activin Receptor Signaling to Counteract the Multi-Systemic Complications of Cancer and Its Treatments. Cells 2021, 10, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Butchbach, M.E.R.; Sahenk, Z.; Wang, H.; Saji, M.; Carathers, M.; Ringel, M.D.; Skipworth, R.J.E.; Fearon, K.C.H.; Hollingsworth, M.A.; et al. Dystrophin Glycoprotein Complex Dysfunction: A Regulatory Link between Muscular Dystrophy and Cancer Cachexia. Cancer Cell 2005, 8, 421–432. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and Myostatin in Human Cancer Cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M. The Regulation and Functions of Activin and Follistatin in Inflammation and Immunity. Vitam. Horm. 2011, 85, 255–297. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Jacobi, C.; Feige, J.N.; Glass, D.J. TAK-1/P38/NNFκB Signaling Inhibits Myoblast Differentiation by Increasing Levels of Activin, A. Skelet. Muscle 2012, 2, 1–14. [Google Scholar] [CrossRef]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Isabel Chiu, M.; Gyuris, J.; Garcia, J.M. Plasma Growth Differentiation Factor 15 Is Associated with Weight Loss and Mortality in Cancer Patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef]
- Zhong, X.; Pons, M.; Poirier, C.; Jiang, Y.; Liu, J.; Sandusky, G.E.; Shahda, S.; Nakeeb, A.; Schmidt, C.M.; House, M.G.; et al. The Systemic Activin Response to Pancreatic Cancer: Implications for Effective Cancer Cachexia Therapy. J. Cachexia Sarcopenia Muscle 2019, 10, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.D.; Ward, C.W. Myostatin/Activin Receptor Ligands in Muscle and the Development Status of Attenuating Drugs. Endocr. Rev. 2022, 43, 329–365. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Geva, R.; Richards, D.; Madhusudan, S.; Lin, B.K.; Wang, H.T.; Walgren, R.A.; Stemmer, S.M. LY2495655, an Antimyostatin Antibody, in Pancreatic Cancer: A Randomized, Phase 2 Trial. J. Cachexia Sarcopenia Muscle 2018, 9, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Cangemi, N.A.; Makker, V.; Cadoo, K.A.; Liu, J.F.; Rasco, D.W.; Navarro, W.H.; Haqq, C.M.; Hyman, D.M. First-in-Human Phase i Study of the Activin a Inhibitor, STM 434, in Patients with Granulosa Cell Ovarian Cancer and Other Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 5458–5465. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Hanna, D.L.; Zhang, W.; Baba, H.; Lenz, H.J. Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clin. Cancer Res. 2016, 22, 3999–4004. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The Metabolic Effects of GDF15 Are Mediated by the Orphan Receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Study of NGM120 in Subjects With Advanced Solid Tumors and Pancreatic Cancer Using Combination Therapy—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04068896 (accessed on 14 March 2022).
- A Phase 1 Study of AV-380 in Healthy Subjects—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04815551 (accessed on 14 March 2022).
- First-in-Human Study of the GDF-15 Neutralizing Antibody CTL-002 in Patients With Advanced Cancer (GDFATHER)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04725474 (accessed on 14 March 2022).
- Olson, B.; Zhu, X.; Norgard, M.A.; Diba, P.; Levasseur, P.R.; Buenafe, A.C.; Huisman, C.; Burfeind, K.G.; Michaelis, K.A.; Kong, G.; et al. Chronic Cerebral Lipocalin 2 Exposure Elicits Hippocampal Neuronal Dysfunction and Cognitive Impairment. Brain Behav. Immun. 2021, 97, 102–118. [Google Scholar] [CrossRef]
- Dallmann, R.; Weyermann, P.; Anklin, C.; Boroff, M.; Bray-French, K.; Cardel, B.; Courdier-Fruh, I.; Deppe, H.; Dubach-Powell, J.; Erb, M.; et al. The Orally Active Melanocortin-4 Receptor Antagonist BL-6020/979: A Promising Candidate for the Treatment of Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 163. [Google Scholar] [CrossRef]
- Yeo, G.S.H.; Chao, D.H.M.; Siegert, A.M.; Koerperich, Z.M.; Ericson, M.D.; Simonds, S.E.; Larson, C.M.; Luquet, S.; Clarke, I.; Sharma, S.; et al. The Melanocortin Pathway and Energy Homeostasis: From Discovery to Obesity Therapy. Mol. Metab. 2021, 48. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Lopez, M.; Agoulnik, A.I. Diverse Functions of Insulin-like 3 Peptide. J. Endocrinol. 2020, 247, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Perilli, L.; Gianesello, L.; Taglialavoro, G.; Foresta, C. Profiling Insulin like Factor 3 (INSL3) Signaling in Human Osteoblasts. PLoS ONE 2011, 6, e29733. [Google Scholar] [CrossRef]
- Yeom, E.; Shin, H.; Yoo, W.; Jun, E.; Kim, S.; Hong, S.H.; Kwon, D.W.; Ryu, T.H.; Suh, J.M.; Kim, S.C.; et al. Tumour-Derived Dilp8/INSL3 Induces Cancer Anorexia by Regulating Feeding Neuropeptides via Lgr3/8 in the Brain. Nat. Cell Biol. 2021, 23, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Song, W.; Droujinine, I.A.; Hu, Y.; Asara, J.M.; Perrimon, N. Systemic Organ Wasting Induced by Localized Expression of the Secreted Insulin/IGF Antagonist ImpL2. Dev. Cell 2015, 33, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef]
- Pramanik, D.; Campbell, N.R.; Karikari, C.; Chivukula, R.; Kent, O.A.; Mendell, J.T.; Maitra, A. Restitution of Tumor Suppressor MicroRNAs Using a Systemic Nanovector Inhibits Pancreatic Cancer Growth in Mice. Mol. Cancer Ther. 2011, 10, 1470–1480. [Google Scholar] [CrossRef]
- Stahlhut, C.; Slack, F.J. Combinatorial Action of MicroRNAs Let-7 and MiR-34 Effectively Synergizes with Erlotinib to Suppress Non-Small Cell Lung Cancer Cell Proliferation. Cell Cycle 2015, 14, 2171–2180. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of MicroRNA-Target Recognition. PLoS Biol. 2005, 3, 0404–0418. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206: The Skeletal Muscle-Specific MyomiR. Biochim. Biophys Acta 2008, 1779, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNA-206 Promotes Skeletal Muscle Regeneration and Delays Progression of Duchenne Muscular Dystrophy in Mice. J. Clin. Investig. 2012, 122, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-Beta Regulates MiR-206 and MiR-29 to Control Myogenic Differentiation through Regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Y.; Yang, G.; Chen, X.; Zhang, Y.; Cao, G.; Wang, J.; Sun, Y.; Zhang, P.; Fan, M.; et al. Transforming Growth Factor-Beta-Regulated MiR-24 Promotes Skeletal Muscle Differentiation. Nucleic Acids Res. 2008, 36, 2690–2699. [Google Scholar] [CrossRef]
- Sartori, R.; Hagg, A.; Zampieri, S.; Armani, A.; Winbanks, C.E.; Viana, L.R.; Haidar, M.; Watt, K.I.; Qian, H.; Pezzini, C.; et al. Perturbed BMP Signaling and Denervation Promote Muscle Wasting in Cancer Cachexia. Sci. Transl. Med. 2021, 13, eaay9592. [Google Scholar] [CrossRef]
- Re Cecconi, A.D.; Barone, M.; Gaspari, S.; Tortarolo, M.; Bendotti, C.; Porcu, L.; Terribile, G.; Piccirillo, R. The P97-Nploc4 ATPase Complex Plays a Role in Muscle Atrophy during Cancer and Amyotrophic Lateral Sclerosis. J. Cachexia Sarcopenia Muscle 2022, 13, 225–2241. [Google Scholar] [CrossRef]
- Huang, Q.K.; Qiao, H.Y.; Fu, M.H.; Li, G.; Li, W.B.; Chen, Z.; Wei, J.; Liang, B.S. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats Through Regulation of Satellite Cell Differentiation via TGF-Β1, Smad3, and HDAC4 Signaling. Med. Sci. Monit. 2016, 22, 1161–1170. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, L.; Jiang, P.; Lu, L.; Chen, X.; Lan, H.; Guttridge, D.C.; Sun, H.; Wang, H. Loss of MiR-29 in Myoblasts Contributes to Dystrophic Muscle Pathogenesis. Mol. Ther. 2012, 20, 1222–1233. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Wang, B.B.; Wu, W.J.; Wei, J.; Li, P.; Huang, R. MiR-22 Regulates C2C12 Myoblast Proliferation and Differentiation by Targeting TGFBR1. Eur. J. Cell Biol. 2018, 97, 257–268. [Google Scholar] [CrossRef]
- Khanna, N.; Ge, Y.; Chen, J. MicroRNA-146b Promotes Myogenic Differentiation and Modulates Multiple Gene Targets in Muscle Cells. PLoS ONE 2014, 9, e100657. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- van de Worp, W.R.P.H.; Schols, A.M.W.J.; Dingemans, A.M.C.; Op den Kamp, C.M.H.; Degens, J.H.R.J.; Kelders, M.C.J.M.; Coort, S.; Woodruff, H.C.; Kratassiouk, G.; Harel-Bellan, A.; et al. Identification of MicroRNAs in Skeletal Muscle Associated with Lung Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Liu, M.; Zhang, Y.; Zhou, Z.; Luo, W.; Fung, K.M.; Xu, C.; Bronze, M.S.; Houchen, C.W.; et al. Circular RNA ANAPC7 Inhibits Tumor Growth and Muscle Wasting via PHLPP2-AKT-TGF-β Signaling Axis in Pancreatic Cancer. Gastroenterology 2022, 162, 2004–2017. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Plester, C.E.; Richardson, R.A.; Fearon, K.C.H. Changes in Nutritional Status Associated with Unresectable Pancreatic Cancer. Br. J. Cancer 1997, 75, 106–109. [Google Scholar] [CrossRef]
- Li, Y.; Schwartz, R.J.; Waddell, I.D.; Holloway, B.R.; Reid, M.B. Skeletal Muscle Myocytes Undergo Protein Loss and Reactive Oxygen-Mediated NF-KappaB Activation in Response to Tumor Necrosis Factor Alpha. FASEB J. 1998, 12, 871–880. [Google Scholar] [CrossRef]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.W.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKbeta/NF-KappaB Activation Causes Severe Muscle Wasting in Mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef]
- Narsale, A.A.; Carson, J.A. Role of Interleukin-6 in Cachexia: Therapeutic Implications. Curr. Opin. Support. Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular Therapeutic Strategies Targeting Pancreatic Cancer Induced Cachexia. World J. Gastrointest. Surg. 2018, 10, 95. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J. Catabolic Proinflammatory Cytokines. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 245–251. [Google Scholar] [CrossRef]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A Placebo-Controlled Double Blind Trial of Etanercept for the Cancer Anorexia/Weight Loss Syndrome: Results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Friberg, G.; Singh, D.A.; Locker, G.; Nattam, S.; Kozloff, M.; Taber, D.A.; Karrison, T.; Dachman, A.; Stadler, W.M.; et al. Phase II Trial of Bevacizumab plus Gemcitabine in Patients with Advanced Pancreatic Cancer. J. Clin. Oncol. 2005, 23, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Hui, D.; Bruera, E.; Janku, F.; Naing, A.; Falchook, G.S.; Piha-Paul, S.; Wheler, J.J.; Fu, S.; Tsimberidou, A.M.; et al. MABp1, a First-in-Class True Human Antibody Targeting Interleukin-1α in Refractory Cancers: An Open-Label, Phase 1 Dose-Escalation and Expansion Study. Lancet Oncol 2014, 15, 656–666. [Google Scholar] [CrossRef]
- Fisher, G. A Phase III Study of Xilonix in Refractory Colorectal Cancer Patients with Weight Loss. J. Clin. Oncol. 2015, 33, 685. [Google Scholar] [CrossRef]
- Schuster, M.; Rigas, J.R.; Orlov, S.V.; Milovanovic, B.; Prabhash, K.; Smith, J.T.; ALD518 study group. A Humanized Anti-IL-6 Antibody, Treats Anemia in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): Results of a Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Oncol. 2010, 28 (Suppl. 15), 7631. [Google Scholar] [CrossRef]
- Rigas, J.R.; Schuster, M.; Orlov, S.V.; Milovanovic, B.; Prabhash, K.; Smith, J.T.; ALD518 study group. Efect of ALD518, a Humanized Anti-IL-6 Antibody, on Lean Body Mass Loss and Symptoms in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): Results of a Phase II Randomized, Double-Blind Safety and Efficacy Trial. J. Clin. Oncol. 2010, 28 (Suppl. 15), 7622. [Google Scholar] [CrossRef]
- Padrão, A.I.; Oliveira, P.; Vitorino, R.; Colaço, B.; Pires, M.J.; Márquez, M.; Castellanos, E.; Neuparth, M.J.; Teixeira, C.; Costa, C.; et al. Bladder Cancer-Induced Skeletal Muscle Wasting: Disclosing the Role of Mitochondria Plasticity. Int. J. Biochem. Cell Biol. 2013, 45, 1399–1409. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moosavi, F.; Martini, M.; Giovannetti, E.; Firuzi, O. Prospects of Targeting PI3K/AKT/MTOR Pathway in Pancreatic Cancer. Crit Rev. Oncol. Hematol. 2022, 176, 103749. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-I Stimulates Muscle Growth by Suppressing Protein Breakdown and Expression of Atrophy-Related Ubiquitin Ligases, Atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef]
- Barton-Davis, E.R.; Shoturma, D.I.; Musaro, A.; Rosenthal, N.; Lee Sweeney, H. Viral Mediated Expression of Insulin-like Growth Factor I Blocks the Aging-Related Loss of Skeletal Muscle Function. Proc. Natl. Acad. Sci. USA 1998, 95, 15603–15607. [Google Scholar] [CrossRef]
- Young, S.C.J.; Underwood, L.E.; Celniker, A.; Clemmons, D.R. Effects of Recombinant Insulin-like Growth Factor-I (IGF-I) and Growth Hormone on Serum IGF-Binding Proteins in Calorically Restricted Adults. J. Clin. Endocrinol. Metab. 1992, 75, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and Exercise against Muscle Wasting during Cancer Cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef]
- Avan, A.; Avan, A.; le Large, T.Y.S.; Mambrini, A.; Funel, N.; Maftouh, M.; Ghayour-Mobarhan, M.; Cantore, M.; Boggi, U.; Peters, G.J.; et al. AKT1 and SELP Polymorphisms Predict the Risk of Developing Cachexia in Pancreatic Cancer Patients. PLoS ONE 2014, 9, e108057. [Google Scholar] [CrossRef]

| Mediator | Source | Effects | References |
|---|---|---|---|
| TNFα | Immune cells, adipocytes | Proinflammatory, muscle atrophy, lipid mobilization from adipocyte stores, insulin resistance | [52,62,64] |
| IL-6 | Activated macrophages | Proinflammatory, weight loss, muscle atrophy, lipid mobilization | [69,70,71] |
| Myostatin and activin | Skeletal muscle cells | Muscle atrophy | [72,73] |
| GDF15 | Tumor cells | Muscle atrophy, weight loss | [74] |
| LCN2 | Bone marrow-derived neutrophils | Anorexia, muscle atrophy, lipid mobilization | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balsano, R.; Kruize, Z.; Lunardi, M.; Comandatore, A.; Barone, M.; Cavazzoni, A.; Re Cecconi, A.D.; Morelli, L.; Wilmink, H.; Tiseo, M.; et al. Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics. Cells 2022, 11, 2671. https://doi.org/10.3390/cells11172671
Balsano R, Kruize Z, Lunardi M, Comandatore A, Barone M, Cavazzoni A, Re Cecconi AD, Morelli L, Wilmink H, Tiseo M, et al. Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics. Cells. 2022; 11(17):2671. https://doi.org/10.3390/cells11172671
Chicago/Turabian StyleBalsano, Rita, Zita Kruize, Martina Lunardi, Annalisa Comandatore, Mara Barone, Andrea Cavazzoni, Andrea David Re Cecconi, Luca Morelli, Hanneke Wilmink, Marcello Tiseo, and et al. 2022. "Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics" Cells 11, no. 17: 2671. https://doi.org/10.3390/cells11172671
APA StyleBalsano, R., Kruize, Z., Lunardi, M., Comandatore, A., Barone, M., Cavazzoni, A., Re Cecconi, A. D., Morelli, L., Wilmink, H., Tiseo, M., Garajovà, I., van Zuylen, L., Giovannetti, E., & Piccirillo, R. (2022). Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics. Cells, 11(17), 2671. https://doi.org/10.3390/cells11172671









