TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection
Abstract
:Abstract
Simple Summary
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells, Bacteria Strains, Antibodies and Chemicals
2.3. Mouse Pyelonephritis Model and In Situ M1/M2 Polarization Examination
2.4. Macrophage Differentiation, Polarization and Treatment
2.5. Quantitative RT-PCR
2.6. ELISA and Griess Method
2.7. Flow Cytometry
2.8. Protein Extraction and Western Blot
2.9. Statistical Analysis
3. Results
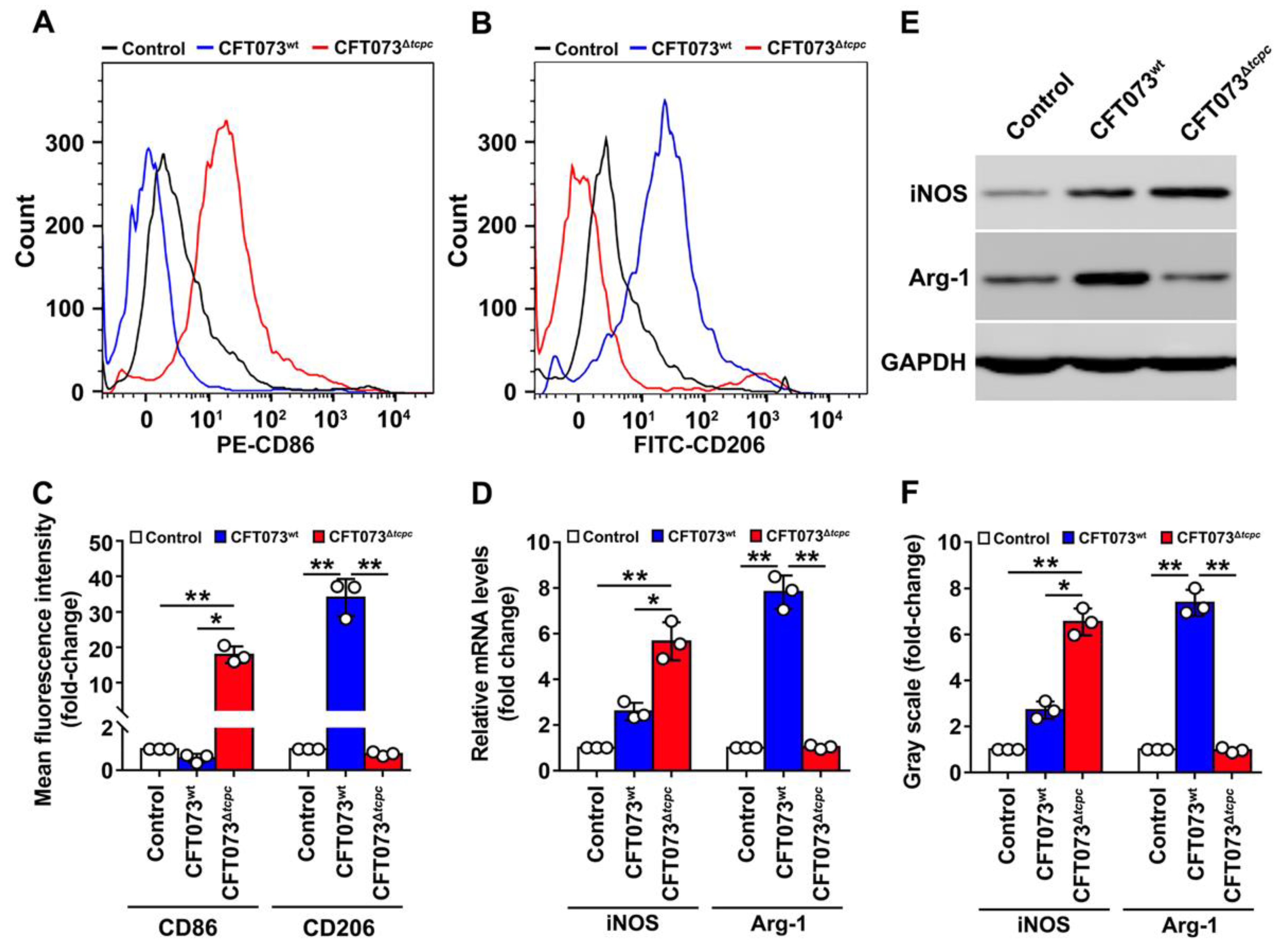
3.1. CFT073wt Infection Inhibits In Vivo M1, but Enhances M2 Macrophage Polarization In Kidneys from Mouse PN Models
3.2. CFT073wt Suppresses M1 While Promoting M2 Macrophage Polarization In Vitro
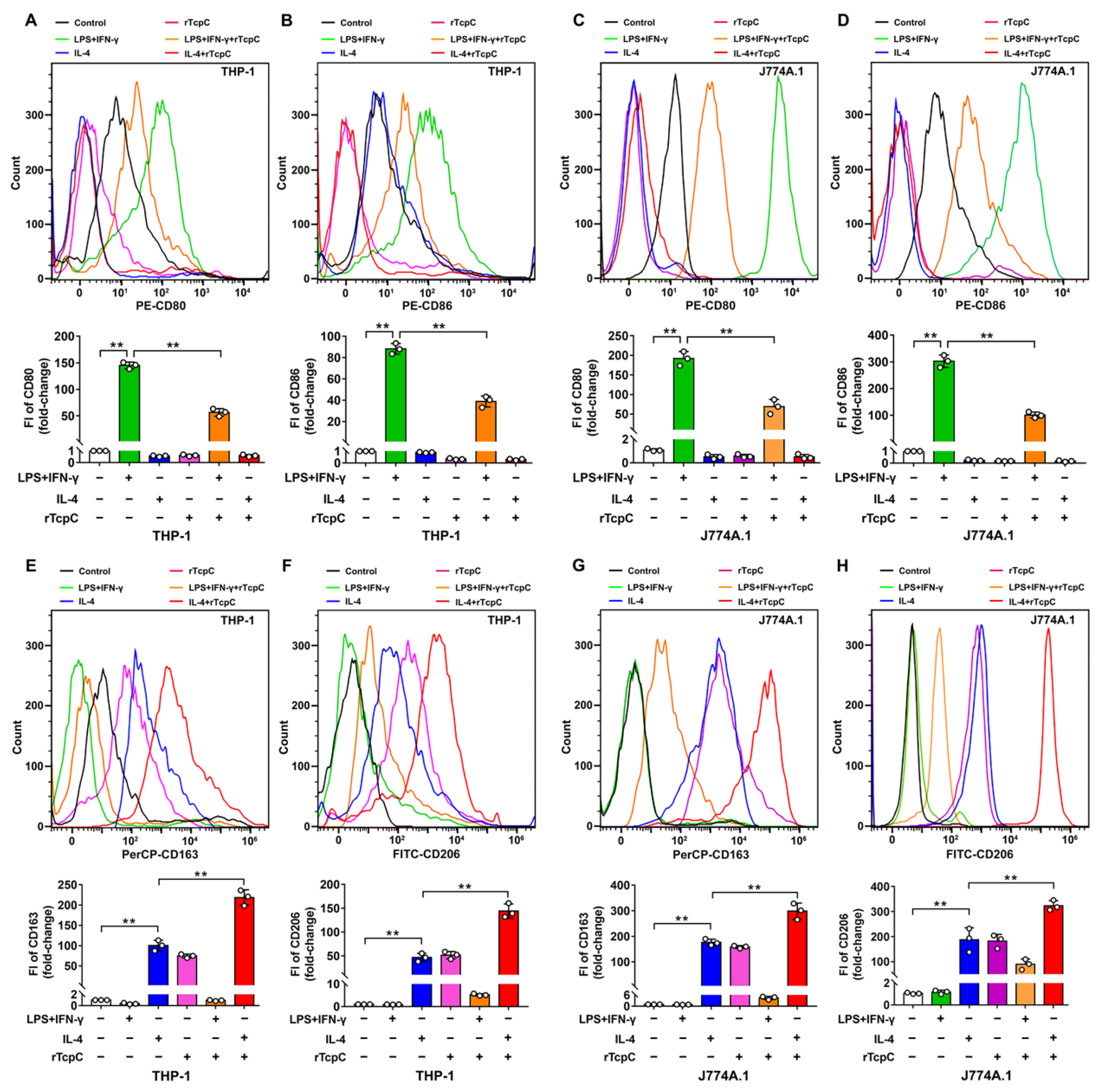
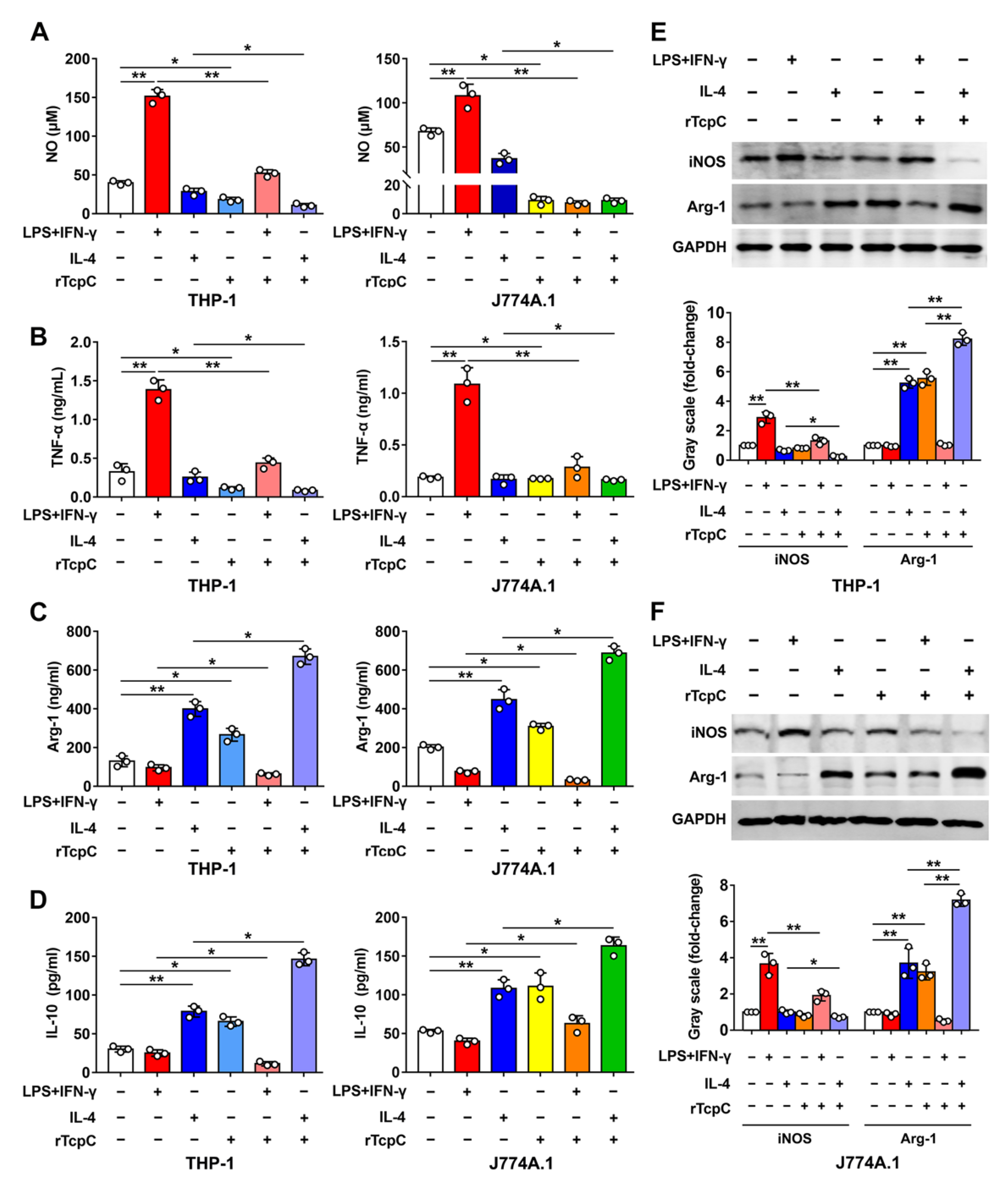
3.3. rTcpC Inhibits M1 but Promotes M2 Polarization in Both Human and Murine Macrophage Cell Lines
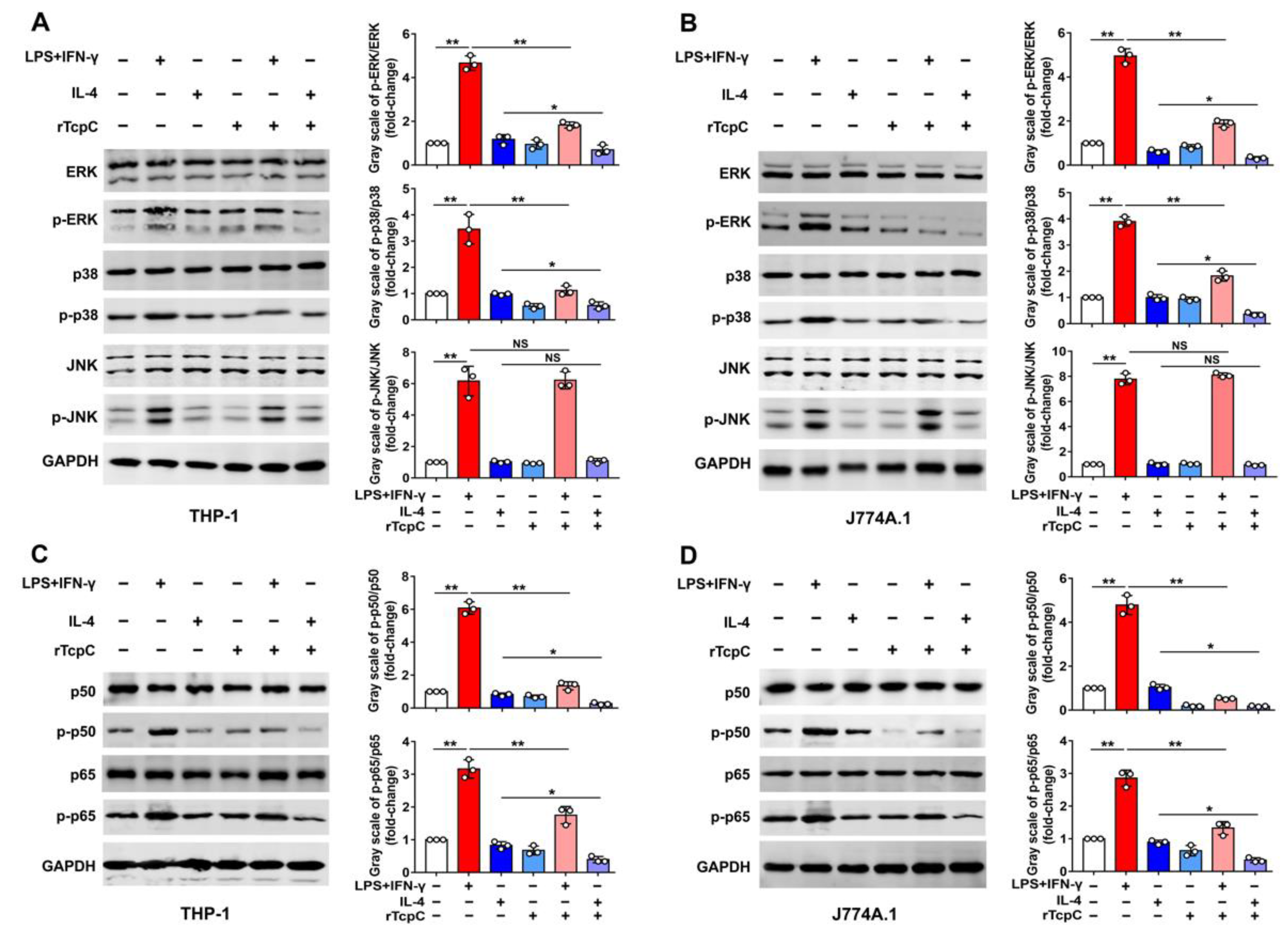
3.4. rTcpC Regulates M1/M2 Macrophage Polarization via Regulating MAPK/NF-κB and Akt/STAT Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Hickling, D. Medical Treatment for Urinary Tract Infections. Urol. Clin. North. Am. 2022, 49, 283–297. [Google Scholar] [CrossRef]
- Gupta, K.; Grigoryan, L.; Trautner, B. Urinary tract infection. Ann. Intern. Med. 2017, 167, ITC49–ITC64. [Google Scholar] [CrossRef]
- Lindblad, A.; Johansson, C.; Persson, K.; Demirel, I. The role of caspase-1, caspase-4 and NLRP3 in regulating the host cell response evoked by uropathogenic Escherichia coli. Sci. Rep. 2022, 12, 2005. [Google Scholar] [CrossRef]
- Cirl, C.; Wieser, A.; Yadav, M.; Duerr, S.; Schubert, S.; Fischer, H.; Stappert, D.; Wantia, N.; Rodriguez, N.; Wagner, H.; et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 2008, 14, 399–406. [Google Scholar] [CrossRef]
- Waldhuber, A.; Puthia, M.; Wieser, A.; Cirl, C.; Dürr, S.; Neumann-Pfeifer, S.; Albrecht, S.; Römmler, F.; Müller, T.; Zheng, Y.; et al. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J. Clin. Investig. 2016, 126, 2425–2436. [Google Scholar] [CrossRef]
- Fang, J.-Q.; Ou, Q.; Pan, J.; Fang, J.; Zhang, D.-Y.; Qiu, M.-Q.; Li, Y.-Q.; Wang, X.-H.; Yang, X.-Y.; Chi, Z.; et al. TcpC inhibits toll-like receptor signaling pathway by serving as an E3 ubiquitin ligase that promotes degradation of myeloid differentiation factor 88. PLoS Pathog. 2021, 17, e1009481. [Google Scholar] [CrossRef]
- Ou, Q.; Fang, J.-Q.; Zhang, Z.-S.; Chi, Z.; Fang, J.; Xu, D.-Y.; Lu, K.-Z.; Qian, M.-Q.; Zhang, D.-Y.; Guo, J.-P.; et al. TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4. Nat. Commun. 2021, 12, 3481. [Google Scholar] [CrossRef]
- Murray, P.J. On macrophage diversity and inflammatory metabolic timers. Nat. Rev. Immunol. 2020, 20, 89–90. [Google Scholar] [CrossRef] [PubMed]
- To, S.; Chavula, T.; Pedroza, M.; Smith, J.; Agarwal, S.K. Cadherin-11 Regulates Macrophage Development and Function. Front. Immunol. 2022, 13, 795337. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Jin, X.; Liao, Q.; Chen, Z.; Peng, H.; Zhou, Y. CD38: A Significant Regulator of Macrophage Function. Front. Oncol. 2022, 12, 775649. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D. NF-kappaB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Pflug, K.M.; Sitcheran, R. Targeting NF-kappaB-inducing kinase (NIK) in immunity, inflammation, and cancer. Int. J. Mol. Sci. 2020, 21, 8470. [Google Scholar] [CrossRef]
- Ruan, W.; Ji, X.; Qin, Y.; Zhang, X.; Wan, X.; Zhu, C.; Lv, C.; Hu, C.; Zhou, J.; Lu, L.; et al. Harmine alleviated sepsis-induced cardiac dysfunction by modulating macrophage polarization via the STAT/MAPK/NF-κB pathway. Front. Cell Dev. Biol. 2021, 9, 792257. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Koh, Y.C.; Yang, G.; Lai, C.S.; Weerawatanakorn, M.; Pan, M.H. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int. J. Mol. Sci. 2018, 19, 2208. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fang, J.; Zhang, C.; Pan, J.; Jin, Q.; Yang, Y.; Wang, L.; Wang, B.; Zhang, D.; Pan, J. TcpC secreting uropathogenic E. coli promoted kidney cells to secrete MIP-2 via p38 MAPK pathway. Mol. Med. Rep. 2017, 16, 3528–3534. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-κB and MAPK pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Zhang, J.; Zhu, J.; Xi, Q.; Wang, H.; Zhang, Z.; Cheng, Y.; Yang, G.; Liu, H.; et al. Loss of fragile site-associated tumor suppressor promotes antitumor immunity via macrophage polarization. Nat. Commun. 2021, 12, 4300. [Google Scholar] [CrossRef]
- Fang, J.Q.; Imran, M.; Hu, W.L.; Ojcius, D.M.; Li, Y.; Ge, Y.M.; Li, K.X.; Lin, X.A.; Yan, J. vWA proteins of Leptospira interrogans induce hemorrhage in leptospirosis by competitive inhibition of vWF/GPIb-mediated platelet aggregation. EBioMedicine 2018, 37, 428–441. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Kishore, A.; Petrek, M. Roles of Macrophage Polarization and Macrophage-Derived miRNAs in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Chen, C.Y.; Yang, S.C.; Alalaiwe, A.; Lin, C.H.; Fang, J.Y. 2,4-Dimethoxy-6-Methylbenzene-1,3-diol, a benzenoid from antrodia cinnamomea, mitigates psoriasiform inflammation by suppressing MAPK/NF-κB phosphorylation and GDAP1L1/Drp1 translocation. Front. Immunol. 2021, 12, 664425. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-X.; Jiang, F.-J.; Liu, G.; Wang, Y.-Y.; Gao, Z.-Q.; Jin, S.-H.; Nie, Y.-J.; Chen, D.; Chen, J.-L.; Pang, Q.-F. Dehydrocostus Lactone Attenuates Methicillin-Resistant Staphylococcus aureus-Induced Inflammation and Acute Lung Injury via Modulating Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 9754. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, T.; Li, L.; Sun, L.; Hou, Y.; Hu, X.; Zhang, L.; Tian, H.; Zhao, Q.; Peng, J.; et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat. Commun. 2014, 5, 4696. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhang, Z.; Tang, Z.; Zhang, J.; Luo, Y.; Deng, W.; Dong, Z.; Zhao, Y.; Na, N. TSC1 Affects the Process of Renal Ischemia-Reperfusion Injury by Controlling Macrophage Polarization. Front. Immunol. 2021, 12, 637335. [Google Scholar] [CrossRef]
- Yuan, Q.; Gu, J.; Zhang, J.; Liu, S.; Wang, Q.; Tian, T.; Chen, Z.; Zhang, J. MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization. Cell Rep. 2021, 34, 108724. [Google Scholar] [CrossRef]
- Ambite, I.; Butler, D.; Wan, M.L.Y.; Rosenblad, T.; Tran, T.H.; Chao, S.M.; Svanborg, C. Molecular determinants of disease severity in urinary tract infection. Nat. Rev. Urol. 2021, 18, 468–486. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- Hemberger, J.; Ittensohn, J.; Griffiths, H.; Keller, M.; Costina, V.; Albrecht, S.; Miethke, T. The promoter of the immune-modulating gene TIR-containing protein C of the uropathogenic Escherichia coli strain CFT073 reacts to the pathogen’s environment. Int. J. Mol. Sci. 2022, 23, 1148. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Zhang, J.; Fischer, H.; Huang, W.; Lutay, N.; Cirl, C.; Lum, J.; Miethke, T.; Svanborg, C. Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Escherichia coli Virulence. PLoS Pathog. 2010, 6, e1001120. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef]
- Li, L.; Wei, K.; Ding, Y.; Ahati, P.; Xu, H.; Fang, H.; Wang, H. M2a macrophage-secreted CHI3L1 promotes extracellular matrix metabolic imbalances via activation of IL-13Ralpha2/MAPK pathway in rat intervertebral disc degeneration. Front. Immunol. 2021, 12, 666361. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Wang, S.; Zhang, P.; Wang, Q.; Xiao, J.; Zhang, C.; Zheng, X.; Xu, X.; Xue, S.; et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019, 27, 1176–1189.e5. [Google Scholar] [CrossRef]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freij, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 2013, 4, e00264-13. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pawar, S.; Dutta, O.; Wang, K.; Rivera, A.; Xue, C. Macrophage Mediated Immunomodulation During Cryptococcus Pulmonary Infection. Front. Cell. Infect. Microbiol. 2022, 12, 859049. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Cao, W.; Zhou, L.; Fan, X.; Liu, Q.; Liu, F.; Gai, X.; Chang, C.; Xiong, J.; Rao, Y.; et al. Infection of Mycobacterium tuberculosis promotes both M1/M2 polarization and MMP production in cigarette smoke-exposed macrophages. Front. Immunol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Lauzon-Joset, J.F.; Scott, N.M.; Mincham, K.T.; Stumbles, P.A.; Holt, P.G.; Strickland, D.H. Pregnancy induces a steady-state shift in alveolar macrophage M1/M2 phenotype that is associated with a heightened severity of influenza virus infection: Mechanistic insight using mouse models. J. Infect. Dis. 2019, 219, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Zhang, K.; Zhang, Z.; Duan, F.; Guo, L.; Luo, W.; Mok, B.W.Y.; Thakur, A.; Ke, X.; Motallebnejad, P.; et al. Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nat. Commun. 2022, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kim, H.J.; Na, H.; Nam, M.W.; Kim, J.Y.; Kim, J.S.; Koo, S.H.; Lee, M.O. RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis. Cell Rep. 2017, 20, 124–135. [Google Scholar] [CrossRef]
- Gou, Y.; Yang, D.; Tian, T.; Zhu, X.; Zhang, R.; Ren, J.; Tu, D.; Luo, Y.; Miao, Y.; Zhao, H.; et al. The Transcription of ZIP9 Is Associated With the Macrophage Polarization and the Pathogenesis of Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 725595. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Ahn, Y.R.; Lee, E.-B.; Yu, M.J.; Lee, J.R. Expression of a RhoA-Specific Guanine Nucleotide Exchange Factor, p190RhoGEF, in Mouse Macrophages Negatively Affects M1 Polarization and Inflammatory Responses. Front. Immunol. 2022, 13, 782475. [Google Scholar] [CrossRef]
- Kurtzeborn, K.; Kwon, H.N.; Kuure, S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int. J. Mol. Sci. 2019, 20, 1779. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Gaojian, T.; Dingfei, Q.; Linwei, L.; Xiaowei, W.; Zheng, Z.; Wei, L.; Tong, Z.; Benxiang, N.; Yanning, Q.; Wei, Z.; et al. Parthenolide promotes the repair of spinal cord injury by modulating M1/M2 polarization via the NF-kappaB and STAT 1/3 signaling pathway. Cell Death Discov. 2020, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wang, Y.; Dou, C.; Liu, F.; Guan, G.; Wei, K.; Yang, J.; Yang, M.; Tan, J.; Zeng, W.; et al. Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. J. Cell. Physiol. 2019, 234, 8788–8796. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK signaling: Regulation and functions based on complex protein-protein partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, B.; Campos, A.D.; Webster, W.K.; Gopinathan, A.; Hur, L.; Darnay, B.G. The RING Domain and First Zinc Finger of TRAF6 Coordinate Signaling by Interleukin-1, Lipopolysaccharide, and RANKL. J. Biol. Chem. 2008, 283, 24871–24880. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Ou, Q.; Wu, B.; Li, S.; Wu, M.; Qiu, J.; Cen, N.; Hu, K.; Che, Y.; Ma, Y.; et al. TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection. Cells 2022, 11, 2674. https://doi.org/10.3390/cells11172674
Fang J, Ou Q, Wu B, Li S, Wu M, Qiu J, Cen N, Hu K, Che Y, Ma Y, et al. TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection. Cells. 2022; 11(17):2674. https://doi.org/10.3390/cells11172674
Chicago/Turabian StyleFang, Jiaqi, Qian Ou, Boheng Wu, Sisi Li, Mian Wu, Jialing Qiu, Nuo Cen, Kaixin Hu, Yangfei Che, Yuan Ma, and et al. 2022. "TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection" Cells 11, no. 17: 2674. https://doi.org/10.3390/cells11172674





