Aldosterone Increases Vascular Permeability in Rat Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Vascular Permeability Measurement
2.4. Histological and Immunohistochemical (IHC) Analysis
2.5. mRNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (rt-qPCR)
2.6. Blood Morphology
2.7. Serum and Skin ALDO Concentrations
2.8. Statistical Analysis
3. Results
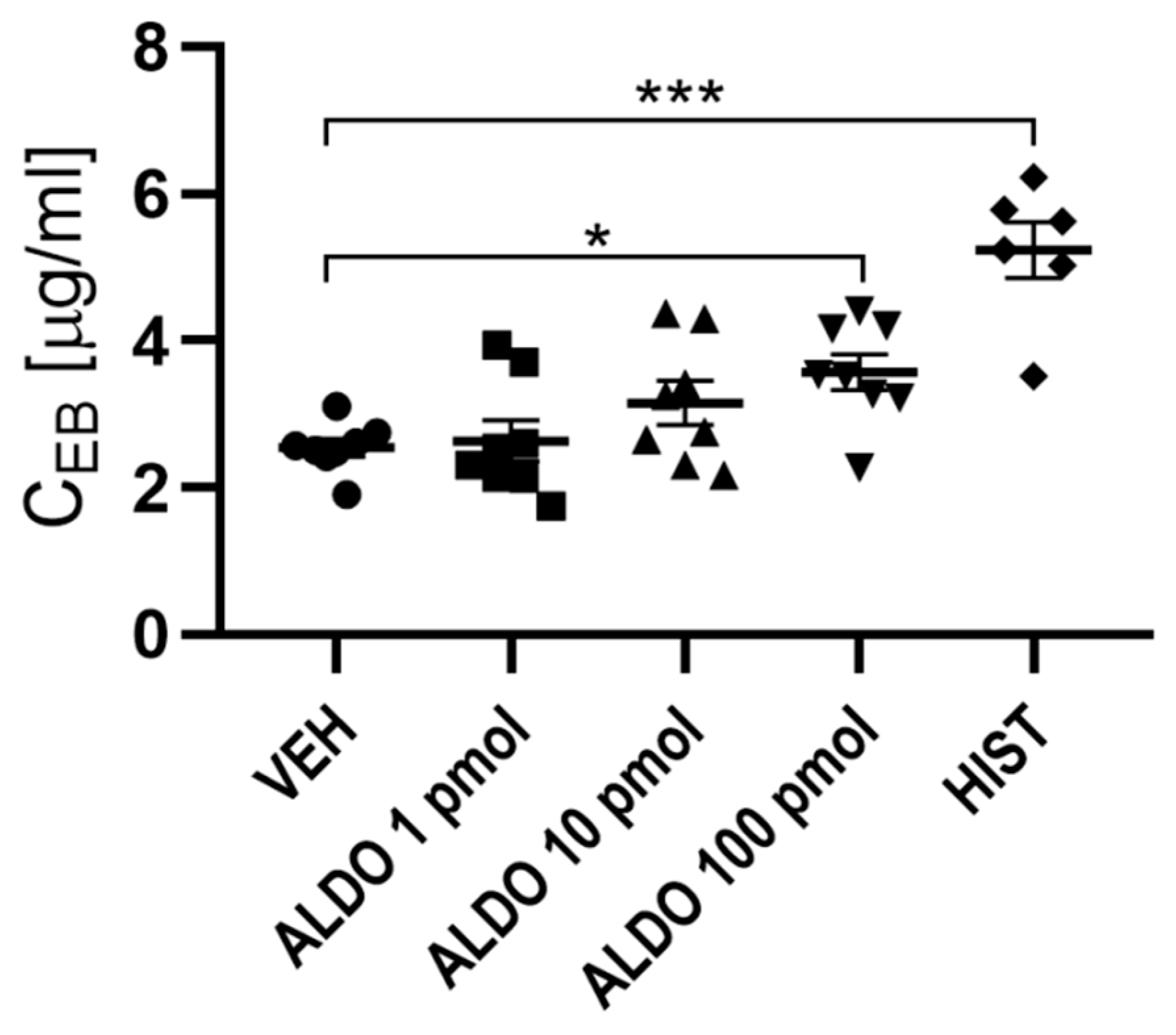
3.1. ALDO Increased Vascular Permeability
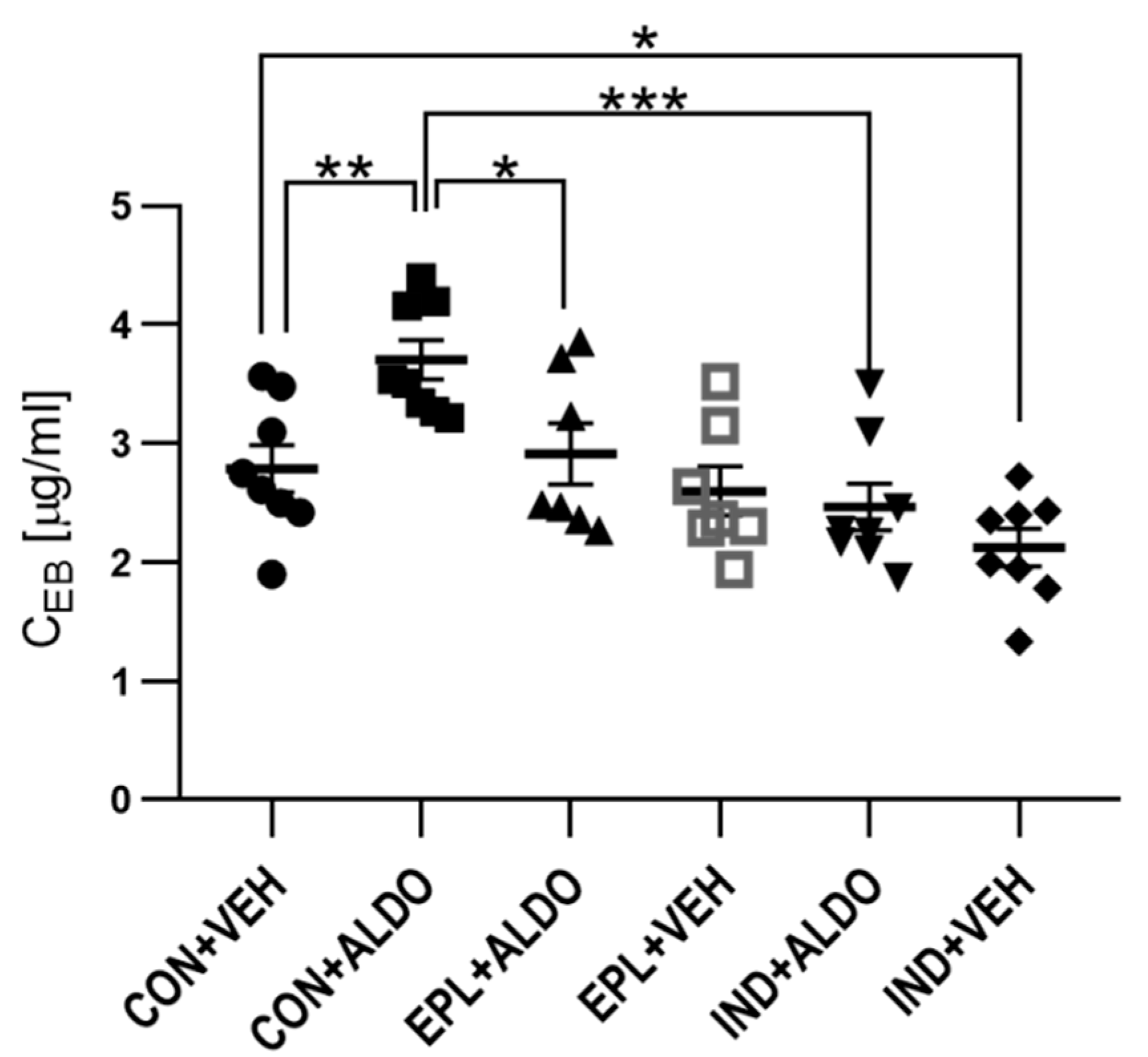
3.1.1. Acute EPL and IND Administration Diminished the ALDO-Increased Vascular Permeability
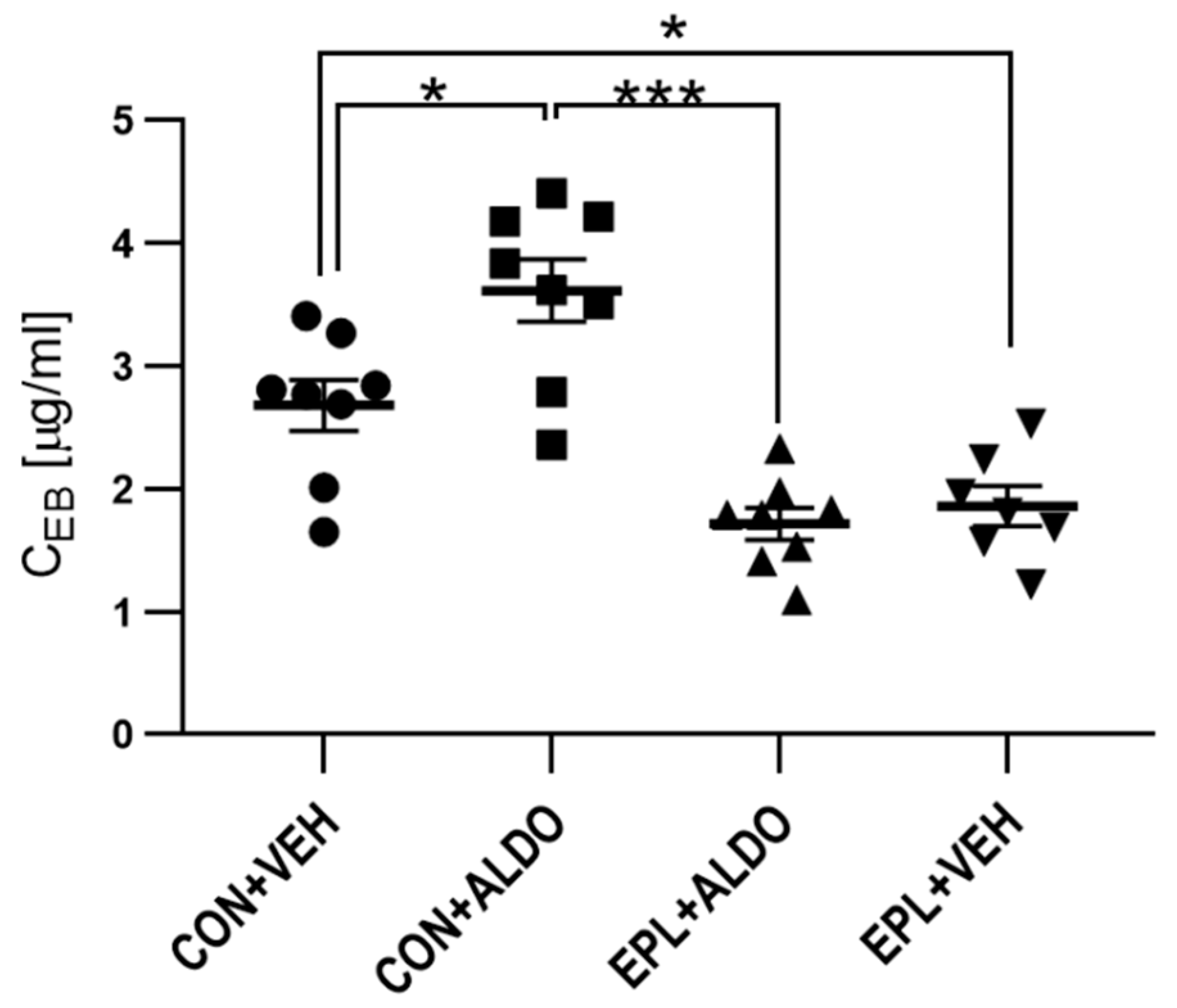
3.1.2. Chronic EPL Administration Reduced Basal Vascular Permeability
3.2. Histological and Morphometric Analysis
3.3. Blood Morphology
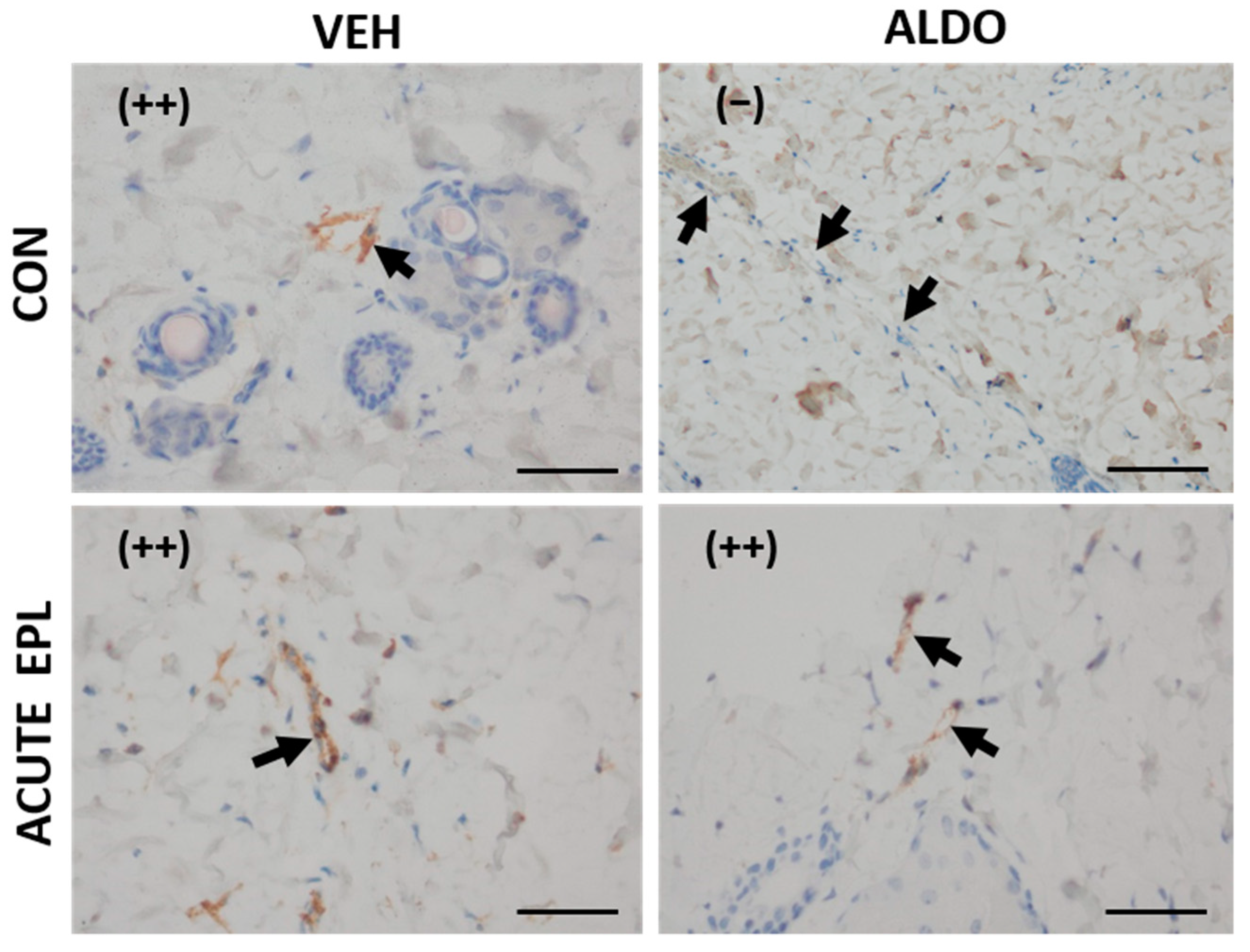
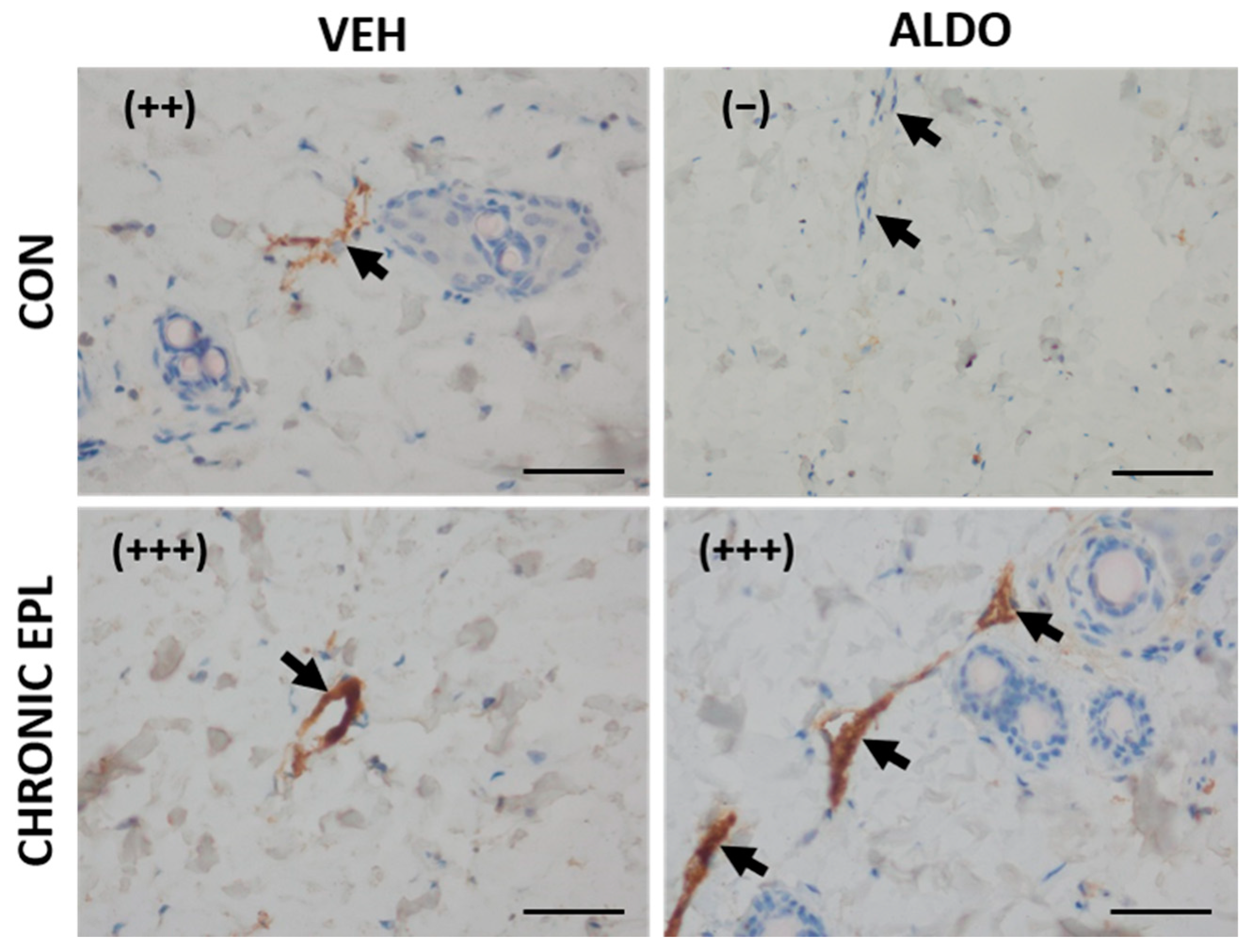
3.4. IHC Staining for vWF, VEGF, MR, HSD11β2, and ZO-1
3.4.1. Acute ALDO Administration Activated Endothelial vWF Exocytosis
3.4.2. Chronic EPL Administration Led to vWF Accumulation in Blood Vessels and VEGF Staining Reduction
3.5. mRNA Level of vWF, VEGF, MR, HSD11β2, and ZO-1
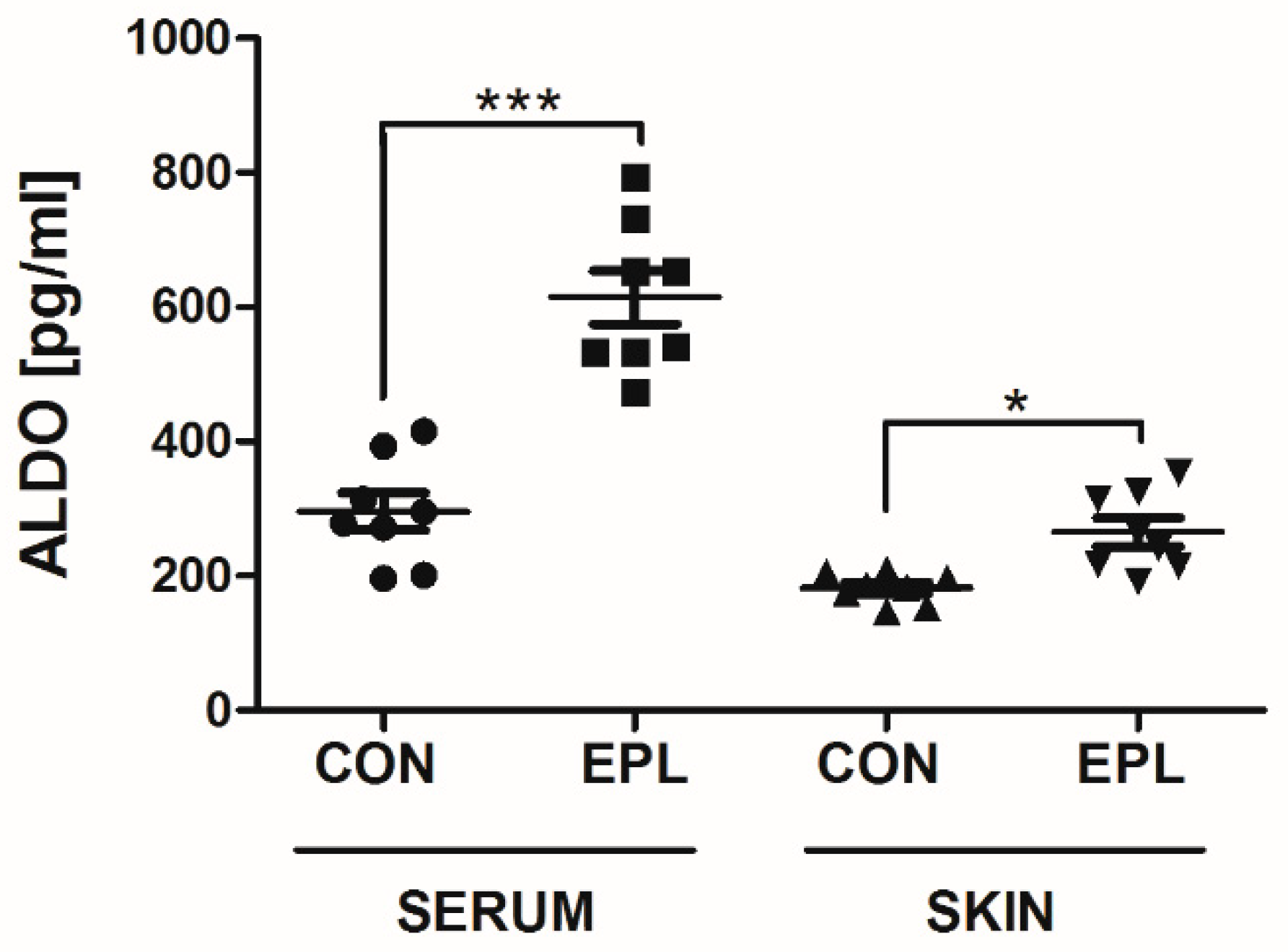
3.6. EPL Increased Serum and Skin ALDO Concentrations
4. Discussion
4.1. Used Model
4.2. Acute ALDO Effects on Vascular Permeability
4.3. Chronic EPL Effect on Vascular Permeability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenouch, S.; Lombes, M.; Delahaye, F.; Eugene, E.; Bonvalet, J.P.; Farman, N. Human skin as target for aldosterone: Coexpression of mineralocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab. 1994, 79, 1334–1341. [Google Scholar] [PubMed]
- Steckelings, U.M.; Czarnelzki, B.M. The renin-angiotensin-system in the skin. Evidence for its presence and possible functional implications. Exp. Dermatol. 1995, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y. Vascular synthesis of aldosterone: Role in hypertension. Mol. Cell. Endocrinol. 2004, 217, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Przylipiak, A.; Chabielska, E. The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J. Physiol. Pharmacol. 2019, 70, 325–336. [Google Scholar]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Bleich, M.; Schmid, W.; Cole, T.J.; Peters, J.; Watanabe, H.; Kriz, W.; Warth, R.; Greger, R.; Schütz, G. Mineralocorticoid receptor knockout mice: Pathophysiology of Na + metabolism. Proc. Natl. Acad. Sci. USA 1998, 95, 9424–9429. [Google Scholar] [CrossRef] [PubMed]
- Marie, Y.S.; Toulon, A.; Paus, R.; Maubec, E.; Cherfa, A.; Grossin, M.; Descamps, V.; Clemessy, M.; Gasc, J.M.; Peuchmaur, M.; et al. Targeted Skin Overexpression of the Mineralocorticoid Receptor in Mice Causes Epidermal Atrophy, Premature Skin Barrier Formation, Eye Abnormalities, and Alopecia. Am. J. Pathol. 2007, 171, 846–860. [Google Scholar] [CrossRef]
- Pérez, P. The mineralocorticoid receptor in skin disease. J. Cereb. Blood Flow Metab. 2021, 179, 3178–3189. [Google Scholar] [CrossRef]
- Boix, J.; Bigas, J.; Sevilla, L.M.; Iacobone, M.; Citton, M.; Torresan, F.; Caroccia, B.; Rossi, G.P.; Pérez, P. Primary aldosteronism patients show skin alterations and abnormal activation of glucocorticoid receptor in keratinocytes. Sci. Rep. 2017, 7, 15806. [Google Scholar] [CrossRef]
- Concistrè, A.; Petramala, L.; Bonvicini, M.; Gigante, A.; Collalti, G.; Pellicano, C.; Olmati, F.; Iannucci, G.; Soldini, M.; Rosato, E.; et al. Comparisons of skin microvascular changes in patients with primary aldosteronism and essential hypertension. Hypertens. Res. 2020, 43, 1222–1230. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability-the essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, S.; Egawa, G.; Kabashima, K. Regulation of blood vascular permeability in the skin. Inflamm. Regen. 2017, 37, 11. [Google Scholar] [CrossRef]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Ruhs, S.; Nolze, A.; Hübschmann, R.; Grossmann, C. 30 Years of the mineralocorticoid receptor: Nongenomic effects via the mineralocorticoid receptor. J. Endocrinol. 2017, 234, T107–T124. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, I.Z.; Mendelsohn, M.E. Angiotensin II and Aldosterone Regulate Gene Transcription Via Functional Mineralocortocoid Receptors in Human Coronary Artery Smooth Muscle Cells. Circ. Res. 2005, 96, 643–650. [Google Scholar] [CrossRef]
- Fejes-Tóth, G.; Náray-Fejes-Tóth, A. Early aldosterone-regulated genes in cardiomyocytes: Clues to cardiac remodeling? Endocrinology 2007, 148, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rivero, J.; Cachofeiro, V.; Lahera, V.; Aras-Lopez, R.; Marquez-Rodas, I.; Salaices, M.; Xavier, F.; Ferrer, M.; Balfagón, G. Participation of Prostacyclin in Endothelial Dysfunction Induced by Aldosterone in Normotensive and Hypertensive Rats. Hypertension 2005, 46, 107–112. [Google Scholar] [CrossRef]
- Rocha, R.; Martin-Berger, C.L.; Yang, P.; Scherrer, R.; Delyani, J.; McMahon, B. Selective Aldosterone Blockade Prevents Angiotensin II/Salt-Induced Vascular Inflammation in the Rat Heart. Endocrinology 2002, 143, 4828–4836. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Q.; Zhou, J.; Liu, H.-H.; Hua, F.; Yang, H.-Z.; Hu, Z.-W. The mineralocorticoid receptor-p38MAPK-NFκB or ERK-Sp1 signal pathways mediate aldosterone-stimulated inflammatory and profibrotic responses in rat vascular smooth muscle cells. Acta Pharmacol. Sin. 2012, 33, 873–878. [Google Scholar] [CrossRef]
- Kirsch, T.; Beese, M.; Wyss, K.; Klinge, U.; Haller, H.; Haubitz, M.; Fiebeler, A. Aldosterone Modulates Endothelial Permeability and Endothelial Nitric Oxide Synthase Activity by Rearrangement of the Actin Cytoskeleton. Hypertension 2013, 61, 501–508. [Google Scholar] [CrossRef]
- Canonica, J.; Mehanna, C.; Bonnard, B.; Jonet, L.; Gelize, E.; Jais, J.-P.; Jaisser, F.; Zhao, M.; Behar-Cohen, F. Effect of acute and chronic aldosterone exposure on the retinal pigment epithelium-choroid complex in rodents. Exp. Eye Res. 2019, 187, 107747. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, A.; Kratschmar, D.V. Tissue-specific modulation of mineralocorticoid receptor function by 11β-hydroxysteroid dehydrogenases: An overview. Mol. Cell Endocrinol. 2012, 350, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Horowitz, J.R.; Silver, M.; Tsurumi, Y.; Chen, D.; Sullivan, A.; Isner, J.M. Vascular Endothelial Growth Factor/Vascular Permeability Factor Enhances Vascular Permeability Via Nitric Oxide and Prostacyclin. Circulation 1998, 97, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Jones-Bolin, S. Guidelines for the Care and Use of Laboratory Animals in Biomedical Research. Curr. Protoc. Pharmacol. 2012, 59, A.4B.1–A.4B.9. [Google Scholar] [CrossRef]
- Brash, J.T.; Ruhrberg, C.; Fantin, A. Evaluating Vascular Hyperpermeability-inducing Agents in the Skin with the Miles Assay. J. Vis. Exp. 2018, 136, e57524. [Google Scholar] [CrossRef]
- Hilfenhaus, M. Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch. Toxicol. 1976, 36, 305–316. [Google Scholar] [CrossRef]
- Miles, A.A.; Miles, E.M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J. Physiol. 1952, 118, 228–257. [Google Scholar] [CrossRef]
- Udaka, K.; Takeuchi, Y.; Movat, H.Z. Simple Method for Quantitation of Enhanced Vascular Permeability. Exp. Biol. Med. 1970, 133, 1384–1387. [Google Scholar] [CrossRef]
- Pietrzak, L.; Mogielnicki, A.; Buczko, W. Nicotinamide and its metabolite N-methylnicotinamide increase skin vascular permeability in rats. Clin. Exp. Dermatol. 2009, 34, 380–384. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomar, A.R.; Aguilar, G.M.; Garcia, M.C.; Rojas, C.P.; Mancera, A.V. Production of aldosterone in cardiac tissues of healthy dogs and with dilated myocardiopathy. Veter. World 2017, 10, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.S.; Zhang, L.; Ames, G.B.; Fischer, J.; Zhang, J.; Levin, S. Single-and repeated-dose pharmacokinetics of eplerenone, a selective aldosterone receptor blocker, in rats. Xenobiotica 2003, 33, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Gromotowicz-Poplawska, A.; Stankiewicz, A.; Kramkowski, K.; Gradzka, A.; Wojewodzka-Zelezniakowicz, M.; Dzięcioł, J.; Szemraj, J.; Chabielska, E. The acute prothrombotic effect of aldosterone in rats is partially mediated via angiotensin II receptor type 1. Thromb. Res. 2015, 138, 114–120. [Google Scholar] [CrossRef]
- Gromotowicz-Poplawska, A.; Marcinczyk, N.; Misztal, T.; Golaszewska, A.; Aleksiejczuk, M.; Rusak, T.; Chabielska, E. Rapid effects of aldosterone on platelets, coagulation, and fibrinolysis lead to experimental thrombosis augmentation. Vascul. Pharmacol. 2019, 122–123, 106598. [Google Scholar] [CrossRef]
- Hausner, E. Review and evaluation of pharmacology and toxicology data. Inspra. For the indication of heart failure after myocardial infraction. FDA 2003, 21, 437. [Google Scholar]
- Oberleithner, H.; Riethmüller, C.; Ludwig, T.; Shahin, V.; Stock, C.; Schwab, A.; Hausberg, M.; Kusche, K.; Schillers, H. Differential action of steroid hormones on human endothelium. J. Cell Sci. 2006, 119, 1926–1932. [Google Scholar] [CrossRef]
- Williams, T.J.; Morley, J. Prostaglandins as Potentiators of Increased Vascular Permeability in Inflammation. Nature 1973, 246, 215–217. [Google Scholar] [CrossRef]
- Walczak, C.; Gaignier, F.; Gilet, A.; Zou, F.; Thornton, S.N.; Ropars, A. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 2125–2132. [Google Scholar] [CrossRef]
- Lai, L.; Pen, A.; Hu, Y.; Ma, J.; Chen, J.; Hao, C.-M.; Gu, Y.; Lin, S. Aldosterone upregulates vascular endothelial growth factor expression in mouse cortical collecting duct epithelial cells through classic mineralocorticoid receptor. Life Sci. 2007, 81, 570–576. [Google Scholar] [CrossRef]
- Marumo, T.; Uchimura, H.; Hayashi, M.; Hishikawa, K.; Fujita, T. Aldosterone Impairs Bone Marrow–Derived Progenitor Cell Formation. Hypertension 2006, 48, 490–496. [Google Scholar] [PubMed]
- McCormack, J.J.; da Silva, M.L.; Ferraro, F.; Patella, F.; Cutler, D.F. Weibel-Palade bodies at a glance. J. Cell Sci. 2017, 130, 3611–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.; Chaupin, D.F.; Matsushita, K.; Yamakuchi, M.; Cameron, S.J.; Morrell, C.N.; Lowenstein, C.J. Aldosterone activates endothelial exocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3782–3787. [Google Scholar] [CrossRef]
- Liu, G.; Yin, G.-S.; Tang, J.-Y.; Ma, D.-J.; Ru, J.; Huang, X.-H. Endothelial dysfunction in patients with primary aldosteronism: A biomarker of target organ damage. J. Hum. Hypertens. 2014, 28, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Glucocorticoid and mineralocorticoid receptors: Biology and clinical relevance. Annu. Rev. Med. 1997, 48, 231–240. [Google Scholar] [PubMed]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Hammami, M.M.; Siiteri, P.K. Regulation of 11 beta-hydroxysteroid dehydrogenase activity in human skin fibroblasts: Enzymatic modulation of glucocorticoid action. J. Clin. Endocrinol. Metab. 1991, 73, 326–334. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11β-Hydroxysteroid Dehydrogenase Type 1: A Tissue-Specific Regulator of Glucocorticoid Response. Endocr Rev. 2004, 25, 831–866. [Google Scholar]
- Schipper, L.; Spee, B.; Rothuizen, J.; Nijnanten, F.W.V.; Fink-Gremmels, J. Characterisation of 11 beta-hydroxysteroid dehydrogenases in feline kidney and liver. Biochim. Biophys. Acta 2004, 1688, 68–77. [Google Scholar] [CrossRef]
- Brem, A.S.; Bina, R.B.; King, T.C.; Morris, D.J. Localization of 2 11beta-OH steroid dehydrogenase isoforms in aortic endothelial cells. Hypertension 1998, 31, 459–462. [Google Scholar] [CrossRef]
- Zhao, M.; Célérier, I.; Bousquet, E.; Jeanny, J.-C.; Jonet, L.; Savoldelli, M.; Offret, O.; Curan, A.; Farman, N.; Jaisser, F.; et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J. Clin. Investig. 2012, 122, 2672–2679. [Google Scholar]
- Eatman, D.; Layas, M.F.; Bayorh, M.A. Eplerenone Suppresses Salt-Induced Vascular Endothelial Growth Factor Expression in the Kidney. Kidney Blood Press. Res. 2010, 33, 167–173. [Google Scholar]
- Lother, A.; Deng, L.; Huck, M.; Fürst, D.; Kowalski, J.; Esser, J.S.; Moser, M.; Bode, C.; Hein, L. Endothelial cell mineralocorticoid receptors oppose VEGF-induced gene expression and angiogenesis. J. Endocrinol. 2019, 240, 15–26. [Google Scholar] [PubMed]
- Ukimura, A.; Iimori, A. Pleiotropic Effects of Eplerenone on Murine Coxsackievirus B3 Myocarditis. J. Card. Fail. 2010, 16, S157. [Google Scholar]
- Zakrzeska, A.; Gromotowicz-Popławska, A.; Szemraj, J.; Zsoka, P.; Kisiel, W.; Purta, T.; Kasacka, I.; Chabielska, E. Eplerenone reduces arterial thrombosis in diabetic rats. J. Renin-Angiotensin Aldosterone Syst. 2015, 16, 1085–1094. [Google Scholar]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar]
- Shidlovskyi, V.O.; Shidlovskyi, O.V.; Tovkai, O.A.; Sheremet, M.I.; Maksymyuk, V.V.; Tarabanchuk, V.V.; Ivanovych, S.M.; Heryak, M.S.; Andreychyn, M.S.; Hanberher, I.I.; et al. Topical Diagnosis and Determination of the Primary Hyperaldosteronism Variant. J. Med. Life 2019, 12, 322–328. [Google Scholar]
- Udelsman, R.; Holbrook, N.J. Endocrine and molecular responses to surgical stress. Curr. Probl. Surg. 1994, 31, 662–720. [Google Scholar]
- Gromotowicz, A.; Szemraj, J.; Stankiewicz, A.; Zakrzeska, A.; Mantur, M.; Jaroszewicz, E.; Rogowski, F.; Chabielska, E. Study of the mechanisms of aldosterone prothrombotic effect in rats. J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 430–439. [Google Scholar]
- Funder, J.W. Aldosterone and Mineralocorticoid Receptors—Physiology and Pathophysiology. Int. J. Mol. Sci. 2017, 18, 1032. [Google Scholar] [CrossRef]
- Turan, G.A.; Eskicioglu, F.; Sivrikoz, O.N.; Cengiz, H.; Adakan, S.; Gur, E.B.; Tatar, S.; Sahin, N.; Yilmaz, O. Myo-inositol is a promising treatment for the prevention of ovarian hyperstimulation syndrome (OHSS): An animal study. Arch. Gynecol. Obstet. 2015, 292, 1163–1171. [Google Scholar]
- Ratajczak-Wielgomas, K.; Kassolik, K.; Grzegrzolka, J.; Halski, T.; Piotrowska, A.; Mieszala, K.; Wilk, I.; Podhorska-Okolow, M.; Dziegiel, P.; Andrzejewski, W. Effects of massage on the expression of proangiogenic markers in rat skin. Folia Histochem. Cytobiol. 2018, 1, 83–91. [Google Scholar]
- Veron, D.; Reidy, K.J.; Bertuccio, C.; Teichman, J.; Villegas, G.; Jimenez, J.; Shen, W.; Kopp, J.B.; Thomas, D.B.; Tufro, A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010, 77, 989–999. [Google Scholar] [PubMed] [Green Version]
- Veron, D.; Bertuccio, C.A.; Marlier, A.; Reidy, K.; Garcia, A.M.; Jimenez, J.; Velazquez, H.; Kashgarian, M.; Moeckel, G.W.; Tufro, A. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 2011, 54, 1227–1241. [Google Scholar] [PubMed]
- Zibert, J.R.; Wallbrecht, K.; Schön, M.; Mir, L.M.; Jacobsen, G.K.; Trochon-Josph, V.; Bouquet, C.; Villadsen, L.S.; Cadossi, R.; Skov, L.; et al. Halting angiogenesis by non-viral somatic gene therapy alleviates psoriasis and murine psoriasiform skin lesions. J. Clin. Investig. 2011, 121, 410–421. [Google Scholar] [PubMed] [Green Version]


| Group | vWF/Actb | VEGF/Actb | MR/Actb | HSD11β2/Actb | ZO-1/Actb | |
|---|---|---|---|---|---|---|
| CON | VEH | 18.04 ± 0.69 | 19.47 ± 1.13 | 22.00 ± 3.93 | 18.91 ± 1.07 | 19.09 ± 1.07 |
| ALDO | 19.16 ± 0.95 | 20.60 ± 0.95 | 17.60 ± 1.34 | 20.30 ± 1.13 | 21.17 ± 1.11 | |
| Acute EPL | VEH | 17.75 ± 1.33 | 21.22 ± 0.84 | 22.47 ± 5.34 | 20.29 ± 0.95 | 19.18 ± 1.45 |
| ALDO | 13.92 ± 2.97 | 19.64 ± 0.76 | 22.48 ± 4.83 | 18.94 ± 0.51 | 18.40 ± 1.16 | |
| CON | VEH | 20.57 ± 0.12 | 14.81 ± 1.44 | 18.03 ± 0.67 | 19.35 ± 0.16 | 17.96 ± 0.52 |
| ALDO | 20.67 ± 0.24 | 17.07 ± 0.51 | 19.25 ± 0.61 | 19.51 ± 0.07 | 19.27 ± 0.46 | |
| Chronic EPL | VEH | 20.42 ± 0.13 | 17.12 ± 1.25 | 17.99 ± 0.56 | 19.41 ± 0.12 | 18.56 ± 0.39 |
| ALDO | 20.10 ± 0.27 | 16.90 ± 0.70 | 18.39 ± 0.61 | 19.46 ± 0.15 | 17.81 ± 0.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Stelmaszewska, J.; Dzieciol, J.; Chabielska, E. Aldosterone Increases Vascular Permeability in Rat Skin. Cells 2022, 11, 2707. https://doi.org/10.3390/cells11172707
Aleksiejczuk M, Gromotowicz-Poplawska A, Marcinczyk N, Stelmaszewska J, Dzieciol J, Chabielska E. Aldosterone Increases Vascular Permeability in Rat Skin. Cells. 2022; 11(17):2707. https://doi.org/10.3390/cells11172707
Chicago/Turabian StyleAleksiejczuk, Michal, Anna Gromotowicz-Poplawska, Natalia Marcinczyk, Joanna Stelmaszewska, Janusz Dzieciol, and Ewa Chabielska. 2022. "Aldosterone Increases Vascular Permeability in Rat Skin" Cells 11, no. 17: 2707. https://doi.org/10.3390/cells11172707
APA StyleAleksiejczuk, M., Gromotowicz-Poplawska, A., Marcinczyk, N., Stelmaszewska, J., Dzieciol, J., & Chabielska, E. (2022). Aldosterone Increases Vascular Permeability in Rat Skin. Cells, 11(17), 2707. https://doi.org/10.3390/cells11172707







