Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Virus
2.3. Viral Load Measurement
2.4. Measurement of Inflammatory Mediators
2.5. Plasma Levels of AnxA1
2.6. Flow Cytometry
2.7. Histology and Immunohistochemistry
2.8. Ac2–26 Treatments
2.9. Statistical Analysis
3. Results
3.1. CHIKV Infection Does Not Alter AnxA1 Plasma Levels but Increases Its Expression at the Site of Virus Inoculation
3.2. Mice Lacking AnxA1 or the Fpr2/3 Receptor Exhibit Exacerbated Inflammation and Greater Tissue Damage at Site of Infection
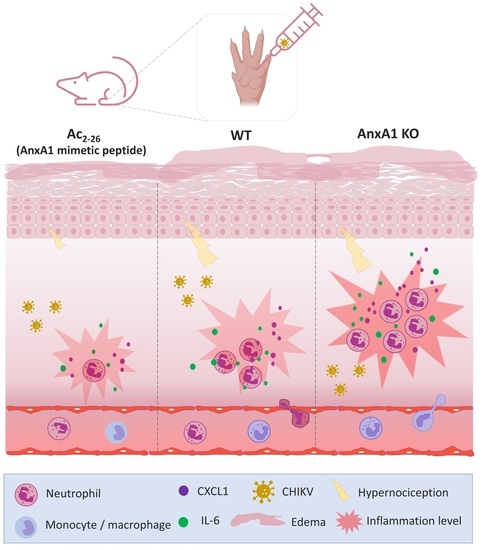
3.3. Treatment with Exogenous AnxA1 (Ac2–26 Peptide) Reduces Inflammation and Mechanical Hypernociception Induced by CHIKV
4. Discussion
- (i)
- Immunostaining of AnxA1 in the footpad was increased at 7 days after CHIKV infection;
- (ii)
- AnxA1 deficiency was associated with increased production of inflammatory mediators, enhanced accumulation of neutrophils, exacerbated tissue damage, with minor effects on viral clearance;
- (iii)
- in the absence of the FPR2 receptor, there was an increase in the production of MPO in the footpad, elevated histopathological damage, and prolonged hypernociception with minimal impact on viral clearance;
- (iv)
- prophylactic treatment with Ac2–26 decreased the production of inflammatory mediators in the footpad, reduced neutrophil accumulation in the infection site and resulted in a decrease in paw edema and hypernociception, without interfering with the viral titers of treated mice.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Her, Z.; Malleret, B.; Chan, M.; Ong, E.K.S.; Wong, S.-C.; Kwek, D.J.C.; Tolou, H.; Lin, R.T.; Tambyah, P.A.; Rénia, L.; et al. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J. Immunol. 2010, 184, 5903–5913. Available online: https://pubmed.ncbi.nlm.nih.gov/20404274/ (accessed on 11 June 2022). [CrossRef] [PubMed]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; de Franca, R.F.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. Available online: https://pubmed.ncbi.nlm.nih.gov/35632709/ (accessed on 11 June 2022). [CrossRef] [PubMed]
- Ng, L.F.P.; Chow, A.; Sun, Y.-J.; Kwek, D.J.C.; Lim, P.-L.; Dimatatac, F.; Ng, L.-C.; Ooi, E.E.; Choo, K.-H.; Her, Z.; et al. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009, 4, e4261. Available online: https://pubmed.ncbi.nlm.nih.gov/19156204/ (accessed on 9 June 2022). [CrossRef] [PubMed]
- Chow, A.; Her, Z.; Ong, E.K.S.; Chen, J.M.; Dimatatac, F.; Kwek, D.J.C.; Barkham, T.; Yang, H.; Rénia, L.; Leo, Y.S.; et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011, 203, 149–157. Available online: https://pubmed.ncbi.nlm.nih.gov/21288813/ (accessed on 9 June 2022). [CrossRef]
- Stoermer, K.A.; Burrack, A.; Oko, L.; Montgomery, S.A.; Borst, L.; Gill, R.G.; Morrison, T.E. Genetic Ablation of Arginase 1 in Macrophages and Neutrophils Enhances Clearance of an Arthritogenic Alphavirus. J. Immunol. 2012, 189, 4047–4059. Available online: https://pubmed.ncbi.nlm.nih.gov/22972923/ (accessed on 3 June 2022). [CrossRef]
- Lum, F.M.; Ng, L.F.P. Cellular and molecular mechanisms of chikungunya pathogenesis. Antivir. Res. 2015, 120, 165–174. Available online: https://pubmed.ncbi.nlm.nih.gov/26092642/ (accessed on 9 June 2022). [CrossRef]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.; Figueiredo, L.T.M.; Fonseca, B.A.L.D.; Franca, R.F.O.; Cunha, F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2020, 10, 3108. Available online: https://pubmed.ncbi.nlm.nih.gov/32082301/ (accessed on 11 July 2022). [CrossRef]
- Muralidharan, A.; Reid, S.P. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. Available online: https://pubmed.ncbi.nlm.nih.gov/34073501/ (accessed on 9 June 2022). [CrossRef]
- Zaid, A.; Gérardin, P.; Taylor, A.; Mostafavi, H.; Malvy, D.; Mahalingam, S. Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018, 70, 484–495. Available online: https://pubmed.ncbi.nlm.nih.gov/29287308/ (accessed on 11 July 2022). [CrossRef]
- Chang, A.Y.; Encinales, L.; Porras, A.; Pacheco, N.; Reid, S.P.; Martins, K.A.O.; Pacheco, S.; Bravo, E.; Navarno, M.; Rico Mendoza, A.; et al. Frequency of Chronic Joint Pain Following Chikungunya Virus Infection: A Colombian Cohort Study. Arthritis Rheumatol. 2018, 70, 578–584. Available online: https://pubmed.ncbi.nlm.nih.gov/29266783/ (accessed on 3 July 2022). [CrossRef] [Green Version]
- Edington, F.; Varjão, D.; Melo, P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Jt. Bone Spine 2018, 85, 669–678. Available online: https://pubmed.ncbi.nlm.nih.gov/30053609/ (accessed on 27 July 2022). [CrossRef] [PubMed]
- Kam, Y.W.; Ong, E.K.S.; Rénia, L.; Tong, J.C.; Ng, L.F.P. Immuno-biology of Chikungunya and implications for disease intervention. Microbes Infect. 2009, 11, 1186–1196. Available online: https://pubmed.ncbi.nlm.nih.gov/19737625/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Suhrbier, A. Rheumatic manifestations of chikungunya: Emerging concepts and interventions. Nat. Rev. Rheumatol. 2019, 15, 597–611. Available online: https://www.nature.com/articles/s41584-019-0276-9 (accessed on 21 July 2022). [CrossRef] [PubMed]
- Amaral, J.K.; Taylor, P.C.; Martins Teixeira, M.; Morrison, T.E.T.; Schoen, R.T. The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses 2019, 11, 289. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6466451/ (accessed on 11 June 2022). [CrossRef]
- Srivastava, P.; Kumar, A.; Hasan, A.; Mehta, D.; Kumar, R.; Sharma, C.; Sunil, S. Disease Resolution in Chikungunya—What Decides the Outcome? Front. Immunol. 2020, 11, 695. Available online: https://pubmed.ncbi.nlm.nih.gov/32411133/ (accessed on 2 August 2022). [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. Available online: https://pubmed.ncbi.nlm.nih.gov/27199985/ (accessed on 18 July 2022). [CrossRef]
- Machado, L.C.; de Morais-Sobral, M.C.; de Campos, T.L.; Pereira, M.R.; de Albuquerque, M.D.F.P.M.; Gilbert, C.; Franca, R.F.O.; Wallau, G.L. Genome sequencing reveals coinfection by multiple chikungunya virus genotypes in a recent outbreak in Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007332. Available online: https://pubmed.ncbi.nlm.nih.gov/31095561/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Garcia, C.C.; Guabiraba, R.; Soriani, F.M.; Teixeira, M.M.T. The development of anti-inflammatory drugs for infectious diseases. Discov. Med. 2010, 10, 479–488. Available online: https://pubmed.ncbi.nlm.nih.gov/21189219/ (accessed on 18 July 2022).
- Vago, J.P.; Tavares, L.P.; Riccardi, C.; Teixeira, M.M.; Sousa, L.P. Exploiting the pro-resolving actions of glucocorticoid-induced proteins Annexin A1 and GILZ in infectious diseases. Biomed. Pharmacother. 2021, 133, 111033. Available online: https://pubmed.ncbi.nlm.nih.gov/33378946/ (accessed on 18 July 2022). [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 2019, 40, 212–227. Available online: https://pubmed.ncbi.nlm.nih.gov/30772190/ (accessed on 18 July 2022). [CrossRef]
- Flower, R.J.; Blackwell, G.J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature 1979, 278, 456–459. Available online: https://pubmed.ncbi.nlm.nih.gov/450050/ (accessed on 13 July 2022). [CrossRef] [PubMed]
- Perucci, L.O.; Sugimoto, M.A.; Gomes, K.B.; Dusse, L.M.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and specialized proresolving lipid mediators: Promoting resolution as a therapeutic strategy in human inflammatory diseases. Expert Opin. Ther. Targets 2017, 21, 879–896. Available online: https://pubmed.ncbi.nlm.nih.gov/28786708/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. Available online: https://pubmed.ncbi.nlm.nih.gov/26885535/ (accessed on 21 July 2022). [CrossRef]
- Ernst, J.D.; Hoye, E.; Blackwood, R.A.; Jaye, D. Purification and characterization of an abundant cytosolic protein from human neutrophils that promotes Ca2(+)-dependent aggregation of isolated specific granules. J. Clin. Investig. 1990, 85, 1065–1071. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC296536/ (accessed on 16 July 2022). [CrossRef] [PubMed]
- Perretti, M.; Flower, R.J. Measurement of lipocortin 1 levels in murine peripheral blood leukocytes by flow cytometry: Modulation by glucocorticoids and inflammation. Br. J. Pharmacol. 1996, 118, 605–610. Available online: https://pubmed.ncbi.nlm.nih.gov/8762084/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Perretti, M.; Christian, H.; Wheller, S.K.; Aiello, I.; Mugridge, K.G.; Morris, J.F.; Flower, R.J.; Goulding, N.J. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 2000, 24, 163–174. Available online: https://pubmed.ncbi.nlm.nih.gov/10772777/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Filep, J.G.; Sekheri, M.; El Kebir, D. Targeting formyl peptide receptors to facilitate the resolution of inflammation. Eur. J. Pharmacol. 2018, 833, 339–348. Available online: https://pubmed.ncbi.nlm.nih.gov/29935171/ (accessed on 22 July 2022). [CrossRef]
- Senchenkova, E.Y.; Ansari, J.; Becker, F.; Vital, S.A.; Al-Yafeai, Z.; Sparkenbaugh, E.M.; Pawlinski, R.; Stokes, K.Y.; Carroll, J.L.; Dragoi, A.M.; et al. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation 2019, 140, 319–335. Available online: https://pubmed.ncbi.nlm.nih.gov/31154815/ (accessed on 11 July 2022). [CrossRef]
- Machado, M.G.; Tavares, L.P.; Souza, G.V.S.; Queiroz-Junior, C.M.; Ascenção, F.R.; Lopes, M.E.; Garcia, C.C.; Menezes, G.B.; Perretti, M.; Russo, R.C.; et al. The Annexin A1/FPR2 pathway controls the inflammatory response and bacterial dissemination in experimental pneumococcal pneumonia. FASEB J. 2020, 34, 2749–2764. Available online: https://pubmed.ncbi.nlm.nih.gov/31908042/ (accessed on 11 July 2022). [CrossRef]
- Galvão, I.; Melo, E.M.; de Oliveira, V.L.S.; Vago, J.P.; Queiroz-Junior, C.; de Gaetano, M.; Brennan, E.; Gahan, K.; Guiry, P.J.; Godson, C.; et al. Therapeutic potential of the FPR2/ALX agonist AT-01-KG in the resolution of articular inflammation. Pharmacol. Res. 2021, 165, 105445. Available online: https://pubmed.ncbi.nlm.nih.gov/33493655/ (accessed on 18 July 2022). [CrossRef]
- das Dores Pereira, R.; Rabelo, R.A.N.; Leite, P.G.; Cramer, A.; Botelho, A.F.M.; Cruz, J.S.; Régis, W.C.B.; Perretti, M.; Teixeira, M.M.; Machado, F.S. Role of formyl peptide receptor 2 (FPR2) in modulating immune response and heart inflammation in an experimental model of acute and chronic Chagas disease. Cell. Immunol. 2021, 369, 104427. Available online: https://pubmed.ncbi.nlm.nih.gov/34482259/ (accessed on 18 July 2022). [CrossRef]
- Costa, V.V.; Sugimoto, M.A.; Hubner, J.; Bonilha, C.S.; Queiroz-Junior, C.M.; Gonçalves-Pereira, M.H.; Chen, J.; Gobbetti, T.; Rodrigues, G.O.L.; Bambirra, J.L.; et al. Targeting the Annexin A1-FPR2/ALX pathway for host-directed therapy in dengue disease. Elife 2022, 11, e73853. Available online: https://pubmed.ncbi.nlm.nih.gov/35293862/ (accessed on 18 July 2022). [CrossRef]
- Vago, J.P.; Nogueira, C.R.C.; Tavares, L.P.; Soriani, F.M.; Lopes, F.; Russo, R.C.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukoc. Biol. 2012, 92, 249–258. Available online: http://doi.wiley.com/10.1189/jlb.0112008 (accessed on 10 July 2022). [CrossRef] [PubMed]
- Puttamallesh, V.N.; Sreenivasamurthy, S.K.; Singh, P.K.; Harsha, H.C.; Ganjiwale, A.; Broor, S.; Pandey, A.; Narayana, J.; Prasad, T.S. Proteomic profiling of serum samples from chikungunya-infected patients provides insights into host response. Clin. Proteomics. 2013, 10, 1–11. Available online: https://pubmed.ncbi.nlm.nih.gov/24124767/ (accessed on 10 July 2022). [CrossRef] [PubMed]
- Molás, R.B.; Ribeiro, M.R.; dos Santos, M.J.C.R.; Borbely, A.U.; Oliani, D.V.; Oliani, A.H.; Nadkarni, S.; Nogueira, M.L.; Moreli, J.B.; Oliani, S.M. The involvement of annexin A1 in human placental response to maternal Zika virus infection. Antivir. Res. 2020, 179, 104809. [Google Scholar] [CrossRef]
- Hannon, R.; Croxtall, J.D.; Getting, S.J.; Roviezzo, F.; Yona, S.; Paul-Clark, M.J.; Gavins, F.N.; Perretti, M.; Morris, J.F.; Buckingham, J.C.; et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003, 17, 253–255. Available online: https://pubmed.ncbi.nlm.nih.gov/12475898/ (accessed on 15 July 2022). [CrossRef]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010, 184, 2611–2619. Available online: http://www.jimmunol.org/cgi/content/full/184/5/2611 (accessed on 27 July 2022). [CrossRef] [PubMed]
- Costa, V.V.; Fagundes, C.T.; Valadã, D.F.; Cisalpino, D.; Carolina Dias, A.F.; tia Silveira, K.D.; Kangussu, L.M.; Avila, T.V.; Bonfim, M.R.Q.; Bonaventura, D.; et al. A Model of DENV-3 Infection That Recapitulates Severe Disease and Highlights the Importance of IFN-c in Host Resistance to Infection. PLoS Negl. Trop. Dis. 2012, 6, e1663. Available online: https://pubmed.ncbi.nlm.nih.gov/22666512/ (accessed on 22 July 2022). [CrossRef]
- Bonilha, C.S.; Benson, R.A.; Scales, H.E.; Brewer, J.M.; Garside, P. Junctional adhesion molecule-A on dendritic cells regulates Th1 differentiation. Immunol. Lett. 2021, 235, 32–40. Available online: https://pubmed.ncbi.nlm.nih.gov/34000305/ (accessed on 15 July 2022). [CrossRef]
- Chirathaworn, C.; Chansaenroj, J.; Pongsuchart, P.; Poovorawan, Y. IL-18: A suggested target for immunomodulation in chikungunya virus infection. Arch. Virol. 2021, 166, 219–223. Available online: https://link.springer.com/article/10.1007/s00705-020-04849-3 (accessed on 18 July 2022). [CrossRef]
- Reece, M.D.; Taylor, R.R.; Song, C.; Gavegnano, C. Targeting Macrophage Dysregulation for Viral Infections: Novel Targets for Immunomodulators. Front. Immunol. 2021, 12, 4552. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8591232/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Galvão, I.; Vago, J.P.; Barroso, L.C.; Tavares, L.P.; Queiroz-Junior, C.M.; Costa, V.V.; Carneiro, F.S.; Ferreira, T.P.; Silva, P.M.; Amaral, F.A.; et al. Annexin A1 promotes timely resolution of inflammation in murine gout. Eur. J. Immunol. 2017, 47, 585–596. Available online: https://pubmed.ncbi.nlm.nih.gov/27995621/ (accessed on 18 July 2022). [CrossRef] [PubMed]
- Galvão, I.; de Carvalho, R.V.H.; Vago, J.P.; Silva, A.L.N.; Carvalho, T.G.; Antunes, M.M.; Ribeiro, F.M.; Menezes, G.B.; Zamboni, D.S.; Sousa, L.P.; et al. The role of annexin A1 in the modulation of the NLRP3 inflammasome. Immunology 2020, 160, 78–89. Available online: https://pubmed.ncbi.nlm.nih.gov/32107769/ (accessed on 27 July 2022). [CrossRef] [PubMed]
- Getting, S.J.; Flower, R.J.; Perretti, M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997, 120, 1075–1082. Available online: https://pubmed.ncbi.nlm.nih.gov/9134220/ (accessed on 18 July 2022). [CrossRef]
- Yang, Y.H.; Morand, E.F.; Getting, S.J.; Paul-Clark, M.; Liu, D.L.; Yona, S.; Hannon, R.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheum. 2004, 50, 976–984. Available online: https://pubmed.ncbi.nlm.nih.gov/15022342/ (accessed on 2 August 2022). [CrossRef]
- Arora, S.; Lim, W.; Bist, P.; Perumalsamy, R.; Lukman, H.M.; Li, F.; Welker, L.B.; Yan, B.; Sethi, G.; Tambyah, P.A.; et al. Influenza A virus enhances its propagation through the modulation of Annexin-A1 dependent endosomal trafficking and apoptosis. Cell Death Differ. 2016, 23, 1243–1256. Available online: https://www.nature.com/articles/cdd201619 (accessed on 2 August 2022). [CrossRef]
- Cunha, T.M.; Verri, W.A.; Schivo, I.R.; Napimoga, M.H.; Parada, C.A.; Poole, S.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 2008, 83, 824–832. Available online: https://pubmed.ncbi.nlm.nih.gov/18203872/ (accessed on 2 August 2022). [CrossRef]
- Liu, X.; Poo, Y.-S.; Alves, J.C.; Almeida, R.P.; Mostafavi, H.; Tang, P.C.H.; Bucala, R.; Teixeira, M.M.; Taylor, A.; Zaid, A.; et al. Interleukin-17 Contributes to Chikungunya Virus-Induced Disease. MBio 2022, 13, e00289-22. Available online: https://pubmed.ncbi.nlm.nih.gov/35254128/ (accessed on 2 August 2022). [CrossRef]
- Gonçalves, W.A.; Rezende, B.M.; de Oliveira, M.P.E.; Ribeiro, L.S.; Fattori, V.; da Silva, W.N.; Prazeres, P.H.D.M.; Queiroz-Junior, C.M.; Santana, K.T.D.O.; Costa, W.C.; et al. Sensory Ganglia-Specific TNF Expression Is Associated With Persistent Nociception After Resolution of Inflammation. Front. Immunol. 2019, 10, 3120. Available online: https://pubmed.ncbi.nlm.nih.gov/32038637/ (accessed on 2 August 2022). [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. Available online: https://pubmed.ncbi.nlm.nih.gov/20519386/ (accessed on 2 August 2022). [CrossRef]
- Morrison, T.E.; Oko, L.; Montgomery, S.A.; Whitmore, A.C.; Lotstein, A.R.; Gunn, B.M.; Elmore, S.A.; Heise, M.T. A Mouse Model of Chikungunya Virus–Induced Musculoskeletal Inflammatory Disease: Evidence of Arthritis, Tenosynovitis, Myositis, and Persistence. Am. J. Pathol. 2011, 178, 32. Available online: https://pubmed.ncbi.nlm.nih.gov/21224040/ (accessed on 2 August 2022). [CrossRef] [PubMed]
- Pei, L.; Zhang, J.; Zhao, F.; Su, T.; Wei, H.; Tian, J.; Li, M.; Shi, J. Annexin 1 exerts anti-nociceptive effects after peripheral inflammatory pain through formyl-peptide-receptor-like 1 in rat dorsal root ganglion. Br. J. Anaesth. 2011, 107, 948–958. Available online: https://pubmed.ncbi.nlm.nih.gov/21990306/ (accessed on 2 August 2022). [CrossRef] [PubMed]
- Chatterjee, B.E.; Yona, S.; Rosignoli, G.; Young, R.E.; Nourshargh, S.; Flower, R.J.; Perretti, M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J. Leukoc. Biol. 2005, 78, 639–646. Available online: https://pubmed.ncbi.nlm.nih.gov/16000391/ (accessed on 2 August 2022). [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, S.; de Melo Costa, V.R.; Santos, F.M.; de Sousa, C.D.F.; Moreira, T.P.; Gonçalves, M.R.; Félix, F.B.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; Nogueira, M.L.; et al. Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells 2022, 11, 2717. https://doi.org/10.3390/cells11172717
de Araújo S, de Melo Costa VR, Santos FM, de Sousa CDF, Moreira TP, Gonçalves MR, Félix FB, Queiroz-Junior CM, Campolina-Silva GH, Nogueira ML, et al. Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells. 2022; 11(17):2717. https://doi.org/10.3390/cells11172717
Chicago/Turabian Stylede Araújo, Simone, Victor R. de Melo Costa, Franciele M. Santos, Carla D. Ferreira de Sousa, Thaiane P. Moreira, Matheus R. Gonçalves, Franciel B. Félix, Celso M. Queiroz-Junior, Gabriel H. Campolina-Silva, Maurício Lacerda Nogueira, and et al. 2022. "Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection" Cells 11, no. 17: 2717. https://doi.org/10.3390/cells11172717
APA Stylede Araújo, S., de Melo Costa, V. R., Santos, F. M., de Sousa, C. D. F., Moreira, T. P., Gonçalves, M. R., Félix, F. B., Queiroz-Junior, C. M., Campolina-Silva, G. H., Nogueira, M. L., Sugimoto, M. A., Bonilha, C. S., Perretti, M., Souza, D. G., Costa, V. V., & Teixeira, M. M. (2022). Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells, 11(17), 2717. https://doi.org/10.3390/cells11172717