Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
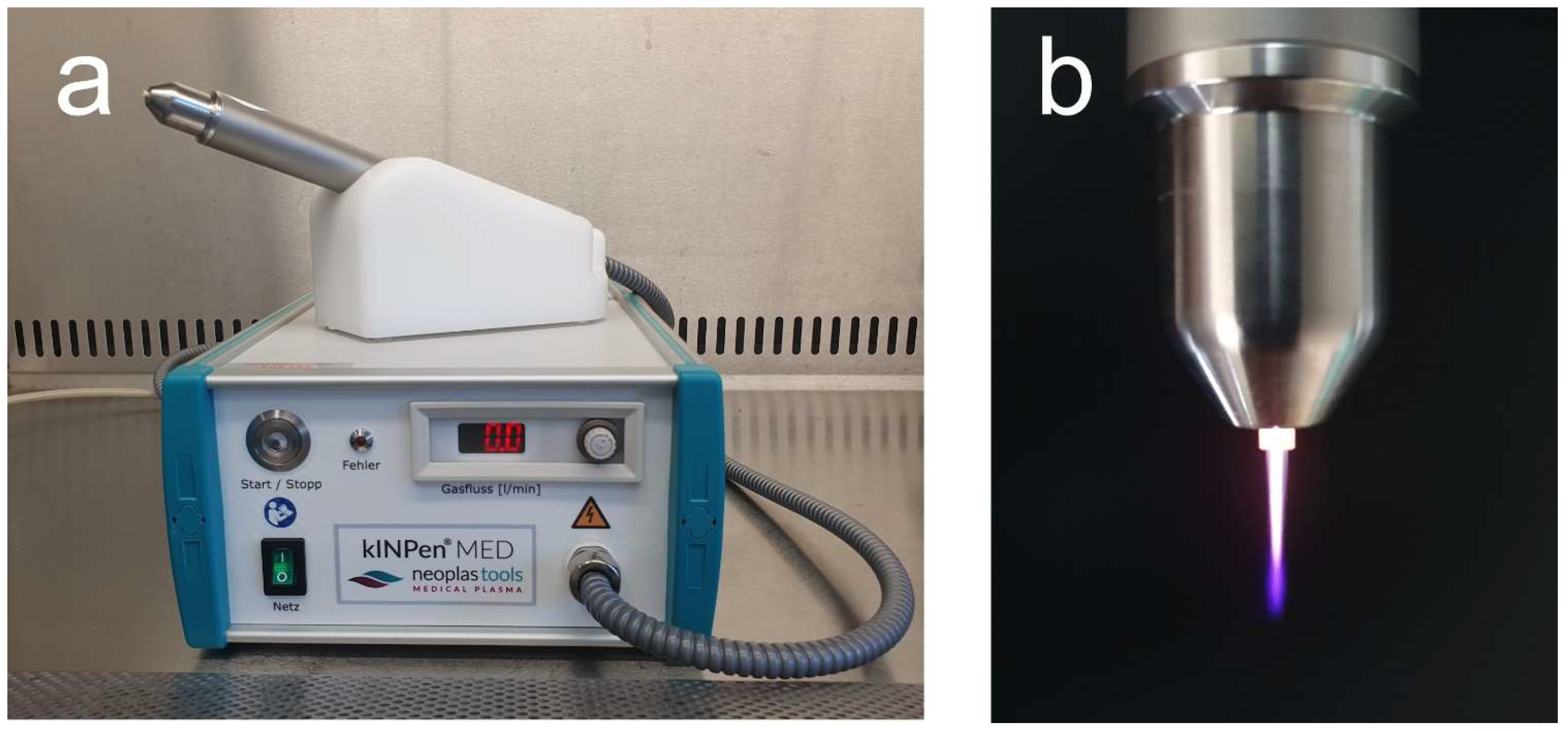
2.2. NIPP Application
2.3. Immunofluorescence
2.4. Analysis of mRNA Levels
2.5. Analysis of Protein Levels
2.6. Statistical Analysis
3. Results
3.1. Impact of NIPP on Cell Viability and Cytoskeleton Morphology
3.2. Effects of NIPP on Regulation of COX2
3.3. Effects of NIPP on Regulation of TNF
3.4. Effects of NIPP on Regulation of CCL2
3.5. Effects of NIPP on Regulation of IL1B
3.6. Effects of NIPP on Regulation of IL6
3.7. Effects of NIPP on Regulation of IL8
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the Human Immune Cell Network at the Gingival Barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Winning, T.A.; Townsend, G.C. Oral Mucosal Embryology and Histology. Clin. Dermatol. 2000, 18, 499–511. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound Healing-A Literature Review. An. Bras. Derm. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hübner, G.; Brauchle, M.; Smola, H.; Madlener, M.; Fässler, R.; Werner, S. Differential Regulation of Pro-Inflammatory Cytokines during Wound Healing in Normal and Glucocorticoid-Treated Mice. Cytokine 1996, 8, 548–556. [Google Scholar] [CrossRef]
- McFarland-Mancini, M.M.; Funk, H.M.; Paluch, A.M.; Zhou, M.; Giridhar, P.V.; Mercer, C.A.; Kozma, S.C.; Drew, A.F. Differences in Wound Healing in Mice with Deficiency of IL-6 versus IL-6 Receptor. J. Immunol. 2010, 184, 7219–7228. [Google Scholar] [CrossRef]
- Takamiya, M.; Fujita, S.; Saigusa, K.; Aoki, Y. Simultaneous Detection of Eight Cytokines in Human Dermal Wounds with a Multiplex Bead-Based Immunoassay for Wound Age Estimation. Int. J. Leg. Med. 2008, 122, 143–148. [Google Scholar] [CrossRef]
- Low, Q.E.H.; Drugea, I.A.; Duffner, L.A.; Quinn, D.G.; Cook, D.N.; Rollins, B.J.; Kovacs, E.J.; DiPietro, L.A. Wound Healing in MIP-1α−/− and MCP-1−/− Mice. Am. J. Pathol. 2001, 159, 457–463. [Google Scholar] [CrossRef]
- .Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound Healing Involves Induction of Cyclooxygenase-2 Expression in Rat Skin. Lab. Investig. 2002, 82, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Furuta, M.; Kimura, A.; Taruya, A.; Yamamoto, H.; Shimada, E.; Akiyama, M.; Mukaida, N.; et al. CCL2-Mediated Reversal of Impaired Skin Wound Healing in Diabetic Mice by Normalization of Neovascularization and Collagen Accumulation. J. Investig. Dermatol. 2019, 139, 2517–2527.e5. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of Interleukin-8 (IL-8) and IL-8 Receptors on the Migration of Human Keratinocytes, the Role of PLC-γ and Potential Clinical Implications. Exp. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, D.; Höijer, P.; Engstrand, T.; Rubin, K.; Gerdin, B.; Ivarsson, M. Keratinocytes Inhibit Expression of Connective Tissue Growth Factor in Fibroblasts in Vitro by an Interleukin-1alpha-Dependent Mechanism. J. Investig. Derm. 2002, 119, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Garcia, M.S.; Isseroff, R.R. Wound Re-Epithelialization: Modulating Keratinocyte Migration in Wound Healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Mast, B.A.; Schultz, G.S. Interactions of Cytokines, Growth Factors, and Proteases in Acute and Chronic Wounds. Wound Repair Regen. 1996, 4, 411–420. [Google Scholar] [CrossRef]
- Maas-Szabowski, N.; Shimotoyodome, A.; Fusenig, N.E. Keratinocyte Growth Regulation in Fibroblast Cocultures via a Double Paracrine Mechanism. J. Cell Sci. 1999, 112, 1843–1853. [Google Scholar] [CrossRef]
- Maas-Szabowski, N.; Stark, H.J.; Fusenig, N.E. Keratinocyte Growth Regulation in Defined Organotypic Cultures through IL-1-Induced Keratinocyte Growth Factor Expression in Resting Fibroblasts. J. Investig. Derm. 2000, 114, 1075–1084. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The Diverse Contribution of Keratinocytes to Immune Responses in Skin. JCI Insight 2020, 5, 142067. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic Wound Pathogenesis and Current Treatment Strategies: A Unifying Hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [Green Version]
- Posnett, J.; Franks, P.J. The Burden of Chronic Wounds in the UK. Nurs. Times 2008, 104, 44–45. [Google Scholar] [PubMed]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Jahandideh, A.; Amini, M.; Porbagher, H.; Amini, M. Evaluating the Effect of Cold Plasma on the Healing of Gingival Wound. J. Diabetes. Metab. Disord. 2021, 20, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Pekbağrıyanık, T.; Dadas, F.K.; Enhoş, Ş. Effects of Non-Thermal Atmospheric Pressure Plasma on Palatal Wound Healing of Free Gingival Grafts: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 6269–6278. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.L.; Morfill, G.; Schmidt, H.-U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of a Randomized Controlled Trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Haralambiev, L.; Wien, L.; Gelbrich, N.; Lange, J.; Bakir, S.; Kramer, A.; Burchardt, M.; Ekkernkamp, A.; Gümbel, D.; Stope, M.B. Cold Atmospheric Plasma Inhibits the Growth of Osteosarcoma Cells by Inducing Apoptosis, Independent of the Device Used. Oncol. Lett. 2020, 19, 283–290. [Google Scholar] [CrossRef]
- Shimatani, A.; Toyoda, H.; Orita, K.; Hirakawa, Y.; Aoki, K.; Oh, J.-S.; Shirafuji, T.; Nakamura, H. In Vivo Study on the Healing of Bone Defect Treated with Non-Thermal Atmospheric Pressure Gas Discharge Plasma. PLoS ONE 2021, 16, e0255861. [Google Scholar] [CrossRef]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of Efficacy of Non-Thermal Atmospheric Pressure Plasma in Treatment of Periodontitis: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Deschner, J.; Stope, M.B.; Mustea, A.; Kramer, F.-J.; Nokhbehsaim, M. Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 5280. [Google Scholar] [CrossRef]
- Eggers, B.; Stope, M.B.; Marciniak, J.; Götz, W.; Mustea, A.; Deschner, J.; Nokhbehsaim, M.; Kramer, F.-J. Non-Invasive Physical Plasma Generated by a Medical Argon Plasma Device Induces the Expression of Regenerative Factors in Human Gingival Keratinocytes, Fibroblasts, and Tissue Biopsies. Biomedicines 2022, 10, 889. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The Beneficial Effect of Cold Atmospheric Plasma on Parameters of Molecules and Cell Function Involved in Wound Healing in Human Osteoblast-like Cells in Vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of Cold Plasma on Periodontal Wound Healing-an in Vitro Study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Eggers, B.; Marciniak, J.; Memmert, S.; Wagner, G.; Deschner, J.; Kramer, F.-J.; Nokhbehsaim, M. Influences of Cold Atmospheric Plasma on Apoptosis Related Molecules in Osteoblast-like Cells in Vitro. Head Face Med. 2021, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, P.; Chernets, N.; Song, Y.; Dobrynin, D.; Pleshko, N.; Steinbeck, M.J.; Freeman, T.A. Chemical Modification of Extracellular Matrix by Cold Atmospheric Plasma-Generated Reactive Species Affects Chondrogenesis and Bone Formation. J. Tissue Eng. Regen. Med. 2016, 10, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Zhang, X.; Schwarz, E.M.; Young, D.A.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J. Cyclooxygenase-2 Regulates Mesenchymal Cell Differentiation into the Osteoblast Lineage and Is Critically Involved in Bone Repair. J. Clin. Investig. 2002, 109, 1405–1415. [Google Scholar] [CrossRef]
- Fairweather, M.; Heit, Y.I.; Buie, J.; Rosenberg, L.M.; Briggs, A.; Orgill, D.P.; Bertagnolli, M.M. Celecoxib Inhibits Early Cutaneous Wound Healing. J. Surg. Res. 2015, 194, 717–724. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Hikiji, H.; Takato, T.; Shimizu, T.; Ishii, S. The Roles of Prostanoids, Leukotrienes, and Platelet-Activating Factor in Bone Metabolism and Disease. Prog. Lipid. Res. 2008, 47, 107–126. [Google Scholar] [CrossRef]
- Noguchi, K.; Ishikawa, I. The Roles of Cyclooxygenase-2 and Prostaglandin E2 in Periodontal Disease. Periodontol 2000, 43, 85–101. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, K.-N. Effects of a Nonthermal Atmospheric Pressure Plasma Jet on Human Gingival Fibroblasts for Biomedical Application. Biomed. Res. Int. 2016, 2016, 2876916. [Google Scholar] [CrossRef]
- Gümbel, D.; Suchy, B.; Wien, L.; Gelbrich, N.; Napp, M.; Kramer, A.; Ekkernkamp, A.; Daeschlein, G.; Stope, M.B. Comparison of Cold Atmospheric Plasma Devices’ Efficacy on Osteosarcoma and Fibroblastic In Vitro Cell Models. Anticancer. Res. 2017, 37, 5407–5414. [Google Scholar] [PubMed]
- Marino, M.W.; Dunn, A.; Grail, D.; Inglese, M.; Noguchi, Y.; Richards, E.; Jungbluth, A.; Wada, H.; Moore, M.; Williamson, B.; et al. Characterization of Tumor Necrosis Factor-Deficient Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical Role of Tumor Necrosis Factor-α in the Early Process of Wound Healing in Skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. General Principles of Wound Healing. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef]
- Siqueira, M.F.; Li, J.; Chehab, L.; Desta, T.; Chino, T.; Krothpali, N.; Behl, Y.; Alikhani, M.; Yang, J.; Braasch, C.; et al. Impaired Wound Healing in Mouse Models of Diabetes Is Mediated by TNF-Alpha Dysregulation and Associated with Enhanced Activation of Forkhead Box O1 (FOXO1). Diabetologia 2010, 53, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Dong, M.; Li, Y.; Zhou, Q.; Yang, L. The Proinflammatory Cytokines IL-1β and TNF-α Modulate Corneal Epithelial Wound Healing through P16Ink4a Suppressing STAT3 Activity. J. Cell. Physiol. 2020, 235, 10081–10093. [Google Scholar] [CrossRef]
- de Souza, L.B.; Silva, J.I.D.S.; Bagne, L.; Pereira, A.T.; de Oliveira, M.A.; Lopes, B.B.; do Amaral, M.E.C.; de Aro, A.A.; Esquisatto, M.A.M.; Santos, G.M.T.D.; et al. Argon Atmospheric Plasma Treatment Promotes Burn Healing by Stimulating Inflammation and Controlling the Redox State. Inflammation 2020, 43, 2357–2371. [Google Scholar] [CrossRef]
- Haralambiev, L.; Wien, L.; Gelbrich, N.; Kramer, A.; Mustea, A.; Burchardt, M.; Ekkernkamp, A.; Stope, M.B.; Gümbel, D. Effects of Cold Atmospheric Plasma on the Expression of Chemokines, Growth Factors, TNF Superfamily Members, Interleukins, and Cytokines in Human Osteosarcoma Cells. Anticancer. Res. 2019, 39, 151–157. [Google Scholar] [CrossRef]
- Bekeschus, S.; Ressel, V.; Freund, E.; Gelbrich, N.; Mustea, A.; Stope, M.B. Gas Plasma-Treated Prostate Cancer Cells Augment Myeloid Cell Activity and Cytotoxicity. Antioxidants 2020, 9, 323. [Google Scholar] [CrossRef]
- Tian, Q.; Stepaniants, S.B.; Mao, M.; Weng, L.; Feetham, M.C.; Doyle, M.J.; Yi, E.C.; Dai, H.; Thorsson, V.; Eng, J.; et al. Integrated Genomic and Proteomic Analyses of Gene Expression in Mammalian Cells. Mol. Cell. Proteom. 2004, 3, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Chiba, T.; Kurimoto, R.; Asahara, H. Post-Transcriptional Regulation of Inflammation by RNA-Binding Proteins via Cis-Elements of MRNAs. J. Biochem. 2019, 166, 375–382. [Google Scholar] [CrossRef]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef] [PubMed]
- Elmanfi, S.; Zhou, J.; Sintim, H.O.; Könönen, E.; Gürsoy, M.; Gürsoy, U.K. Regulation of Gingival Epithelial Cytokine Response by Bacterial Cyclic Dinucleotides. J. Oral Microbiol. 2018, 11, 1538927. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold Atmospheric Plasma (CAP) Activates Angiogenesis-Related Molecules in Skin Keratinocytes, Fibroblasts and Endothelial Cells and Improves Wound Angiogenesis in an Autocrine and Paracrine Mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- March, C.J.; Mosley, B.; Larsen, A.; Cerretti, D.P.; Braedt, G.; Price, V.; Gillis, S.; Henney, C.S.; Kronheim, S.R.; Grabstein, K. Cloning, Sequence and Expression of Two Distinct Human Interleukin-1 Complementary DNAs. Nature 1985, 315, 641–647. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 Is Required for Tumor Invasiveness and Angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Al-Roujayee, A.S. Naringenin Improves the Healing Process of Thermally-Induced Skin Damage in Rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking Interleukin-1β Induces a Healing-Associated Wound Macrophage Phenotype and Improves Healing in Type 2 Diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikh Hosseini, M.; Fatollah, S.; Mirpour, S.; Ghoranneviss, M.; Larijani, B.; Mohajeri-Tehrani, M.R.; Khorramizadeh, M.R. Beneficial Effects of Cold Atmospheric Plasma on Inflammatory Phase of Diabetic Foot Ulcers; a Randomized Clinical Trial. J. Diabetes. Metab. Disord. 2020, 19, 895–905. [Google Scholar] [CrossRef]
- Weissenbach, M.; Clahsen, T.; Weber, C.; Spitzer, D.; Wirth, D.; Vestweber, D.; Heinrich, P.C.; Schaper, F. Interleukin-6 Is a Direct Mediator of T Cell Migration. Eur. J. Immunol. 2004, 34, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Cross, A.L.; Edwards, S.W.; Moots, R.J. Effects of IL-6 and IL-6 Blockade on Neutrophil Function in Vitro and in Vivo. Rheumatology 2014, 53, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, D.; Liang, D.; Zhang, W.; Shi, Q.; Cao, Y. Evaluation of Modified Cold-Atmospheric Pressure Plasma (MCAP) for the Treatment of Peri-Implantitis in Beagles. Oral Dis. 2022, 28, 495–502. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential Involvement of Interleukin-8 (IL-8) in Acute Inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Bhartiya, P.; Masur, K.; Shome, D.; Kaushik, N.; Nguyen, L.N.; Kaushik, N.K.; Choi, E.H. Influence of Redox Stress on Crosstalk between Fibroblasts and Keratinocytes. Biology 2021, 10, 1338. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kramer, A.; Schmidt, A. Gas Plasma-Augmented Wound Healing in Animal Models and Veterinary Medicine. Molecules 2021, 26, 5682. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Redd, M.J.; Cooper, L.; Wood, W.; Stramer, B.; Martin, P. Wound Healing and Inflammation: Embryos Reveal the Way to Perfect Repair. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, M.W.J.; O’Kane, S. Scar-Free Healing: From Embryonic Mechanisms to Adult Therapeutic Intervention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Teplyakova, O.; Vinnik, Y.; Drobushevskaya, A.; Malinovskaya, N.; Kirichenko, A.; Ponedelnik, D. Ozone Improved the Wound Healing in Type 2 Diabetics via Down-Regulation of IL- 8, 10 and Induction of FGFR Expression. Acta. Biomed. 2022, 93, e2022060. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.R.; Cavalcanti, B.N.; Marques, M.M. Effect of Laser Phototherapy on the Release of TNF-Alpha and MMP-1 by Endodontic Sealer-Stimulated Macrophages. Photomed. Laser. Surg. 2009, 27, 37–42. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for Medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Eggers, B.; Wagenheim, A.-M.; Jung, S.; Kleinheinz, J.; Nokhbehsaim, M.; Kramer, F.-J.; Sielker, S. Effect of Cold Atmospheric Plasma (CAP) on Osteogenic Differentiation Potential of Human Osteoblasts. Int. J. Mol. Sci. 2022, 23, 2503. [Google Scholar] [CrossRef]
- Crespi, R.; Capparé, P.; Crespi, G.; Lo Giudice, G.; Gastaldi, G.; Gherlone, E. Immediate Implant Placement in Sockets with Asymptomatic Apical Periodontitis. Clin. Implant. Dent. Relat. Res. 2017, 19, 20–27. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An Innovative Therapeutic Option for the Treatment of Skeletal Sarcomas: Elimination of Osteo- and Ewing’s Sarcoma Cells Using Physical Gas Plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Ye, G.; Zhang, Q.; Wang, J.; Zhang, J.; Fang, J. In Vitro Studies of the Antimicrobial Effect of Non-Thermal Plasma-Activated Water as a Novel Mouthwash. Eur. J. Oral Sci. 2017, 125, 463–470. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggers, B.; Stope, M.B.; Marciniak, J.; Mustea, A.; Deschner, J.; Nokhbehsaim, M.; Kramer, F.-J. Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing. Cells 2022, 11, 2740. https://doi.org/10.3390/cells11172740
Eggers B, Stope MB, Marciniak J, Mustea A, Deschner J, Nokhbehsaim M, Kramer F-J. Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing. Cells. 2022; 11(17):2740. https://doi.org/10.3390/cells11172740
Chicago/Turabian StyleEggers, Benedikt, Matthias Bernhard Stope, Jana Marciniak, Alexander Mustea, James Deschner, Marjan Nokhbehsaim, and Franz-Josef Kramer. 2022. "Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing" Cells 11, no. 17: 2740. https://doi.org/10.3390/cells11172740
APA StyleEggers, B., Stope, M. B., Marciniak, J., Mustea, A., Deschner, J., Nokhbehsaim, M., & Kramer, F.-J. (2022). Modulation of Inflammatory Responses by a Non-Invasive Physical Plasma Jet during Gingival Wound Healing. Cells, 11(17), 2740. https://doi.org/10.3390/cells11172740





