Pharmacological Inhibition of S100A4 Attenuates Fibroblast Activation and Renal Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Antibodies, and Plasmids
2.2. Animal Experiments
2.3. Histological Examination and Immunohistochemistry
2.4. Cell Culture
2.5. Overexpression and Knockdown Experiments
2.6. Luciferase Reporter Assay
2.7. Protein Immunoprecipitation
2.8. Nuclear and Cytoplasmic Protein Extraction
2.9. Western Blot Analysis
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
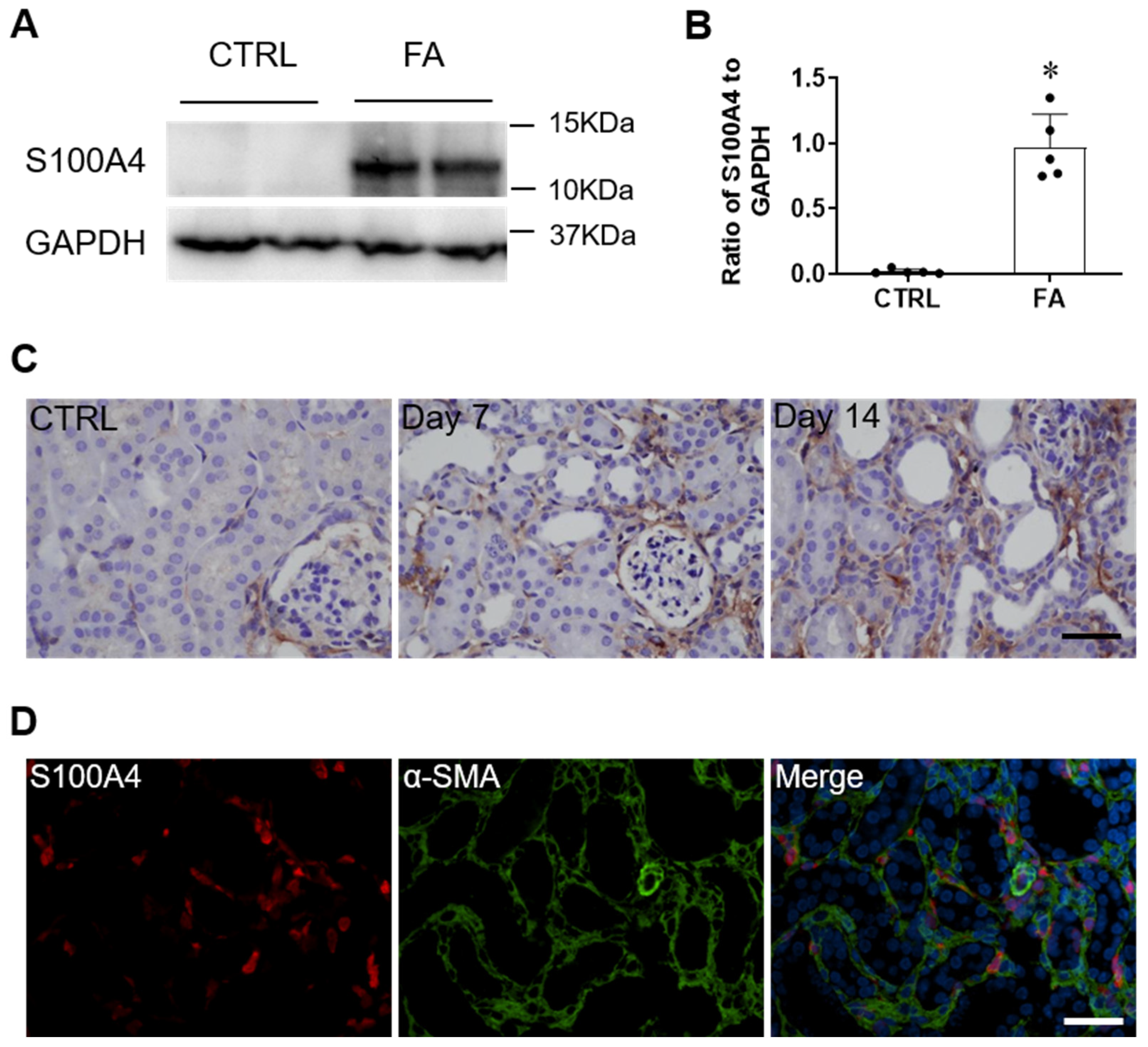
3.1. S100A4 Is Upregulated in the Kidney of Folic Acid Nephropathy
3.2. Pharmacologic Inhibition of S100A4 Reduces Renal Fibrosis in Folic Acid Nephropathy
3.3. S100A4 Interacts with Smad3 under Physiological Conditions
3.4. S100A4 Promotes Smad3 Nuclear Translocation by Maintaining the Smad3/Smad4 Complex
3.5. Knockdown of S100A4 Abrogates TGF-β1 Induced Fibroblast Activation
3.6. Overexpression of S100A4 Promotes TGF-β1-Induced Fibroblast Activation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BUN | serum urea nitrogen |
| CKD | chronic kidney disease |
| COL-1 | collagen type I |
| ECM | extracellular matrix |
| EMT | epithelial mesenchymal transition |
| FBS | fetal bovine serum |
| FA | folic acid |
| FN | Fibronectin |
| NRK-49F | Normal rat kidney fibroblast |
| TGF-β1 | transforming growth factor β1 |
| α-SMA | alpha-smooth muscle actin. |
References
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Kottgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, M.L.; Song, E.; Zhou, Z.; Ma, T.; Wang, J.; Jia, N.; Wang, G.; Nie, S.; Liu, Y.; et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-beta/Smad3 pathway. Sci. Transl. Med. 2018, 10, eaat2039. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Lu, D.W.; Zhao, H.; Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Zhao, Y.Y. Central role of dysregulation of TGF-beta/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Lin, X.; Feng, X.H.; Wang, Y. Smad3 signaling activates bone marrow-derived fibroblasts in renal fibrosis. Lab. Investig. 2014, 94, 545–556. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Fan, J.; Li, X.; Wen, Q.; Luo, N. New strategy for renal fibrosis: Targeting Smad3 proteins for ubiquitination and degradation. Biochem. Pharm. 2016, 116, 200–209. [Google Scholar] [CrossRef]
- Bottinger, E.P.; Bitzer, M. TGF-beta signaling in renal disease. J. Am. Soc. Nephrol. 2002, 13, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumian, N.; Klingelhofer, J.; Grigorian, M. The Multifaceted S100A4 Protein in Cancer and Inflammation. Methods Mol. Biol. 2019, 1929, 339–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, S.; Gu, J.; Tian, T.; Yuan, Q.; Jia, J.; Qin, Z.; Chen, Z. S100A4 promotes colon inflammation and colitis-associated colon tumorigenesis. Oncoimmunology 2018, 7, e1461301. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Nesland, J.M.; Fodstad, O.; Maelandsmo, G.M. Expression of S100A4, E-cadherin, alpha- and beta-catenin in breast cancer biopsies. Br. J. Cancer 2002, 87, 1281–1286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stewart, R.L.; Carpenter, B.L.; West, D.S.; Knifley, T.; Liu, L.; Wang, C.; Weiss, H.L.; Gal, T.S.; Durbin, E.B.; Arnold, S.M.; et al. S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide. Oncotarget 2016, 7, 34630–34642. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Pan, H.; Du, Q.; Liang, J. Clinicopathological significance of S100A4 expression in human hepatocellular carcinoma. J. Int. Med. Res. 2013, 41, 457–462. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Zhang, M.; Li, Y.; Zhang, S. S100A4 in cancer progression and metastasis: A systematic review. Oncotarget 2017, 8, 73219–73239. [Google Scholar] [CrossRef]
- Li, Z.H.; Bresnick, A.R. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006, 66, 5173–5180. [Google Scholar] [CrossRef]
- Orre, L.M.; Panizza, E.; Kaminskyy, V.O.; Vernet, E.; Graslund, T.; Zhivotovsky, B.; Lehtio, J. S100A4 interacts with p53 in the nucleus and promotes p53 degradation. Oncogene 2013, 32, 5531–5540. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, S.; Qin, Z. Extracellular S100A4 as a key player in fibrotic diseases. J. Cell Mol. Med. 2020, 24, 5973–5983. [Google Scholar] [CrossRef]
- Tamaki, Y.; Iwanaga, Y.; Niizuma, S.; Kawashima, T.; Kato, T.; Inuzuka, Y.; Horie, T.; Morooka, H.; Takase, T.; Akahashi, Y.; et al. Metastasis-associated protein, S100A4 mediates cardiac fibrosis potentially through the modulation of p53 in cardiac fibroblasts. J. Mol. Cell Cardiol. 2013, 57, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, Z.; Yan, J.; Wang, Y.; Hu, Z.; Mitch, W.E.; Wang, Y. The IL-4 receptor alpha has a critical role in bone marrow-derived fibroblast activation and renal fibrosis. Kidney Int. 2017, 92, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; An, C.; Tran, M.; Du, H.; Wang, P.; Zhou, D.; Wang, Y. Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis. Front. Immunol. 2021, 12, 735014. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Folic acid-induced animal model of kidney disease. Anim. Model. Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Entman, M.L.; Wang, Y. Critical Role of CXCL16 in Hypertensive Kidney Injury and Fibrosis. Hypertension 2013, 62, 1129–1137. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, X.; Yan, J.; Entman, M.L.; Wang, Y. CXCR6 Plays a Critical Role in Angiotensin II-Induced Renal Injury and Fibrosis. Arter. Thromb. Vasc. Biol. 2014, 34, 1422–1428. [Google Scholar] [CrossRef]
- Chen, G.; Lin, S.C.; Chen, J.; He, L.; Dong, F.; Xu, J.; Han, S.; Du, J.; Entman, M.L.; Wang, Y. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1876–1886. [Google Scholar] [CrossRef]
- Zhou, J.; An, C.; Jin, X.; Hu, Z.; Safirstein, R.L.; Wang, Y. TAK1 deficiency attenuates cisplatin-induced acute kidney injury. Am. J. Physiol. Renal. Physiol. 2020, 318, F209–F215. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Yang, J.; Mitch, W.E.; Wang, Y. JAK3/STAT6 Stimulates Bone Marrow-Derived Fibroblast Activation in Renal Fibrosis. J. Am. Soc. Nephrol. 2015, 26, 3060–3071. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Hu, Z.; Entman, M.L.; Mitch, W.E.; Wang, Y. AMP-activated protein kinase/myocardin-related transcription factor-A signaling regulates fibroblast activation and renal fibrosis. Kidney Int. 2018, 93, 81–94. [Google Scholar] [CrossRef]
- Yang, J.; Lin, S.C.; Chen, G.; He, L.; Hu, Z.; Chan, L.; Trial, J.; Entman, M.L.; Wang, Y. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J. Am. Soc. Nephrol. 2013, 24, 1644–1659. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Gilbertsen, A.; Herrera, J.; Racila, E.; Smith, K.; Peterson, M.; Griffin, T.; Benyumov, A.; Yang, L.; Bitterman, P.B.; et al. Calcium-binding protein S100A4 confers mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis. J. Clin. Investig. 2017, 127, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Iwano, M.; Yamaguchi, Y.; Harada, K.; Nakatani, K.; Akai, Y.; Nishino, T.; Shiiki, H.; Kanauchi, M.; Saito, Y.; et al. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 2005, 68, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Tomcik, M.; Palumbo-Zerr, K.; Zerr, P.; Avouac, J.; Dees, C.; Sumova, B.; Distler, A.; Beyer, C.; Cerezo, L.A.; Becvar, R.; et al. S100A4 amplifies TGF-beta-induced fibroblast activation in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, 1748–1755. [Google Scholar] [CrossRef]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, J.; Dai, C.; Liu, X.; Wang, J.; Gao, Z.; Guo, H.; Wang, R.; Lu, S.; et al. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J. Hepatol. 2015, 62, 156–164. [Google Scholar] [CrossRef]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Kobelt, D.; Lemm, M.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J. Natl. Cancer Inst. 2011, 103, 1018–1036. [Google Scholar] [CrossRef]
- Chang, X.; Zhen, X.; Liu, J.; Ren, X.; Hu, Z.; Zhou, Z.; Zhu, F.; Ding, K.; Nie, J. The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression. Kidney Int. 2017, 92, 612–624. [Google Scholar] [CrossRef]
- Morin, F.; Kavian, N.; Nicco, C.; Cerles, O.; Chereau, C.; Batteux, F. Niclosamide Prevents Systemic Sclerosis in a Reactive Oxygen Species-Induced Mouse Model. J. Immunol. 2016, 197, 3018–3028. [Google Scholar] [CrossRef]
- Boyapally, R.; Pulivendala, G.; Bale, S.; Godugu, C. Niclosamide alleviates pulmonary fibrosis in vitro and in vivo by attenuation of epithelial-to-mesenchymal transition, matrix proteins & Wnt/beta-catenin signaling: A drug repurposing study. Life Sci. 2019, 220, 8–20. [Google Scholar] [CrossRef]
- Matsuura, I.; Lai, C.Y.; Chiang, K.N. Functional interaction between Smad3 and S100A4 (metastatin-1) for TGF-beta-mediated cancer cell invasiveness. Biochem. J. 2010, 426, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shi, J.; Xu, Z.; Yao, X.; Mou, T.; Yu, J.; Liu, H.; Li, G. S100A4-MYH9 Axis Promote Migration and Invasion of Gastric Cancer Cells by Inducing TGF-beta-Mediated Epithelial-Mesenchymal Transition. J. Cancer 2018, 9, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, J.; Luo, Y.; Liao, Q.; Niu, Y.; Zhang, F.; Shao, Z.; Ding, Y.; Zhao, L. LIM and SH3 protein 1 induces TGFbeta-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin. Cancer Res. 2014, 20, 5835–5847. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.F.; Docherty, N.G.; Burke, J.P.; O’Connell, P.R. S100A4 expression is increased in stricture fibroblasts from patients with fibrostenosing Crohn’s disease and promotes intestinal fibroblast migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G457–G466. [Google Scholar] [CrossRef] [PubMed]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Huang, X.R.; Chung, A.C.; Qin, W.; Shao, X.; Igarashi, P.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J. Am. Soc. Nephrol. JASN 2010, 21, 1477–1487. [Google Scholar] [CrossRef]
- Loeffler, I.; Liebisch, M.; Allert, S.; Kunisch, E.; Kinne, R.W.; Wolf, G. FSP1-specific SMAD2 knockout in renal tubular, endothelial, and interstitial cells reduces fibrosis and epithelial-to-mesenchymal transition in murine STZ-induced diabetic nephropathy. Cell Tissue Res. 2018, 372, 115–133. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Jiao, B.; Tran, M.; Wang, Y. Pharmacological Inhibition of S100A4 Attenuates Fibroblast Activation and Renal Fibrosis. Cells 2022, 11, 2762. https://doi.org/10.3390/cells11172762
Wen J, Jiao B, Tran M, Wang Y. Pharmacological Inhibition of S100A4 Attenuates Fibroblast Activation and Renal Fibrosis. Cells. 2022; 11(17):2762. https://doi.org/10.3390/cells11172762
Chicago/Turabian StyleWen, Jia, Baihai Jiao, Melanie Tran, and Yanlin Wang. 2022. "Pharmacological Inhibition of S100A4 Attenuates Fibroblast Activation and Renal Fibrosis" Cells 11, no. 17: 2762. https://doi.org/10.3390/cells11172762
APA StyleWen, J., Jiao, B., Tran, M., & Wang, Y. (2022). Pharmacological Inhibition of S100A4 Attenuates Fibroblast Activation and Renal Fibrosis. Cells, 11(17), 2762. https://doi.org/10.3390/cells11172762







