Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells
Abstract
:1. Introduction
2. Connecting AKT/AKT Effectors and LncRNAs to Cell Functions
2.1. Connecting AKT and LncRNAs to Cell Functions
2.1.1. Apoptosis by AKT-Regulating LncRNAs
2.1.2. Autophagy by AKT-Regulating LncRNAs
2.1.3. ER Stress by AKT-Regulating LncRNAs
2.1.4. DNA Damage Response by AKT-Regulating LncRNAs
2.1.5. Senescence by AKT-Regulating LncRNAs
2.1.6. Migration by AKT-Regulating LncRNAs
2.1.7. Potential Future Directions
2.2. Connecting AKT1/AKT2/AKT3 and Database-LncRNAs to Cell Functions
2.2.1. AKT1-, AKT2-, and AKT3-Targeting Database LncRNAs and Cell Functions
- (1)
- AKT1-Targeting ENST00113 and Cell Functions
- (2)
- AKT1-Targeting MALAT1 and Cell Functions
- (3)
- AKT1-Targeting GAS5 and Cell Functions
- (4)
- AKT1-Targeting CDKN2B-AS1 and Cell Functions
- (5)
- AKT1-Targeting HULC and Cell Functions
- (6)
- AKT1-Targeting LUCAT1 and Cell Functions
- (7)
- AKT1-Targeting RP11-708H21.4, AFAP1-AS1, LINC00462, and Cell Functions
- (8)
- AKT1-Targeting LOXL1-AS1, FOXD2-AS1, AB073614, and Cell Functions
- (9)
- AKT1-Targeting H19 and Cell Functions
- (10)
- AKT1-Targeting SPRY4-IT1, LINC00312, and Cell Functions
- (11)
- AKT2-Targeting LncRNA-p3134 and Cell Functions
- (12)
- AKT3-Targeting FEZF1-AS1 and Cell Functions
2.2.2. Potential Future Directions
2.3. Connecting AKT Effectors and LncRNAs to Cell Functions
2.3.1. AKT Effector (FOXO)-Regulating LncRNAs and Cell Functions
- (1)
- Apoptosis by FOXO-Regulating LncRNAs
- (2)
- Ferroptosis by FOXO-Regulating LncRNAs
- (3)
- Migration by FOXO-Regulating LncRNAs
2.3.2. AKT Effector (c-Myc)-Regulating LncRNAs and Cell Functions
- (1)
- Apoptosis by c-Myc-Regulating LncRNAs
- (2)
- Autophagy by c-Myc-Regulating LncRNAs
- (3)
- ER Stress by c-Myc-Regulating LncRNAs
- (4)
- Ferroptosis by c-Myc-Regulating LncRNAs
- (5)
- Necroptosis by c-Myc-Regulating LncRNAs
- (6)
- DNA Damage Response by c-Myc-Regulating LncRNAs
- (7)
- Senescence by c-Myc-Regulating LncRNAs
- (8)
- Migration by c-Myc-Regulating LncRNAs
2.3.3. AKT Effector (mTORC1)-Regulating LncRNAs and Cell Functions
- (1)
- Apoptosis by mTORC1-Regulating LncRNAs
- (2)
- Autophagy by mTORC1-Regulating LncRNAs
- (3)
- Senescence by mTORC1-Regulating LncRNAs
- (4)
- Migration by mTORC1-Regulating LncRNAs
2.3.4. AKT Effector (SREBP1)-Regulating LncRNAs and Cell Functions
- (1)
- Apoptosis by SREBP1-Regulating LncRNAs
- (2)
- Autophagy by SREBP1-Regulating LncRNAs
2.3.5. AKT Effector (HIF)-Regulating LncRNAs and Cell Functions
- (1)
- Apoptosis by HIF-Regulating LncRNAs
- (2)
- Autophagy by HIF-Regulating LncRNAs
- (3)
- Ferroptosis by HIF-Regulating LncRNAs
- (4)
- DNA Damage Response by HIF-Regulating LncRNAs
- (5)
- Migration by HIF-Regulating LncRNAs
2.3.6. Potential Future Directions
2.4. Connecting AKT Effectors and Database LncRNAs to Cell Functions
2.4.1. AKT Effector (c-Myc)-Targeting LncRNAs and Cell Functions
- (1)
- c-Myc-Targeting PVT1 and Cell Functions
- (2)
- c-Myc-Targeting HOTTIP and Cell Functions
- (3)
- c-Myc-Targeting CRNDE and Cell Functions
- (4)
- c-Myc-Targeting CCAT2 and HNF1A-AS1 and Cell Functions
- (5)
- c-Myc-Targeting PCAT1 and Cell Functions
- (6)
- c-Myc-Targeting SNHG1 and LncRNA-BCAT1 and Cell Functions
- (7)
- c-Myc-Targeting NEAT1 and Cell Functions
- (8)
- c-Myc-Targeting CERNA2 and PCAT6 and Cell Functions
- (9)
- c-Myc-Targeting TUG1 and Cell Functions
- (10)
- c-Myc-Targeting LINC-ROR and FILNC1 and Cell Functions
2.4.2. AKT Effector (mTOR)-Targeting LncRNAs and Cell Functions
- (1)
- mTOR-Targeting HOTAIR and Cell Functions
- (2)
- mTOR-Targeting UCA1 and Cell Functions
2.4.3. AKT Effector (S6K1/2)-Targeting LncRNAs and Cell Functions
2.4.4. AKT Effector (SREBP1)-Targeting LncRNAs and Cell Functions
2.4.5. AKT Effector (HIF)-Targeting LncRNAs and Cell Functions
- (1)
- HIF1A-Targeting CPS1-IT1 and Cell Functions
- (2)
- HIF1A-Targeting MIR31HG and Cell Functions
- (3)
- HIF1A-Targeting MEG3 and Cell Functions
2.4.6. Relationship between AKT- and AKT Effector-Targeting LncRNAs
2.4.7. Potential Future Directions
3. Connecting AKT/AKT Effectors and CircRNAs to Cell Functions
3.1. Connecting AKT and CircRNAs to Cell Functions
3.1.1. Apoptosis by AKT-Regulating CircRNAs
3.1.2. Autophagy by AKT-Regulating CircRNAs
3.1.3. ER Stress by AKT-Regulating CircRNAs
3.1.4. Senescence by AKT-Regulating CircRNAs
3.1.5. Migration by AKT-Regulating CircRNAs
3.1.6. Potential Future Directions
3.2. Connecting AKT1/AKT2/AKT3 and Database CircRNAs to Cell Functions
3.3. Connecting AKT Effectors and CircRNAs to Cell Functions
3.3.1. Apoptosis and Migration by AKT Effector (c-Myc)-Regulating CircRNAs
3.3.2. Apoptosis, Autophagy, and Migration by AKT Effector (mTORC1)-Regulating CircRNAs
3.3.3. Apoptosis and Migration by AKT Effector (HIF)-Regulating CircRNAs
3.3.4. Potential Future Directions
3.4. Connecting AKT Effectors and Database-CircRNAs to Cell Functions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Tang, J.Y.; Cheng, Y.B.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Liu, W.; Chang, H.W. Oxidative stress and AKT-associated angiogenesis in a zebrafish model and its potential application for withanolides. Cells 2022, 11, 961. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Cheng, Y.B.; Tang, J.Y.; Hou, M.F.; Yen, C.Y.; Chang, H.W. Impacts of oxidative stress and PI3K/AKT/mTOR on metabolism and the future direction of investigating fucoidan-modulated metabolism. Antioxidants 2022, 11, 911. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, E.G.; Yoo, N.J.; Lee, S.H. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. Br. J. Cancer 2008, 98, 1533–1535. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Mundi, P.S.; Sachdev, J.; McCourt, C.; Kalinsky, K. AKT in cancer: New molecular insights and advances in drug development. Br. J. Clin. Pharm. 2016, 82, 943–956. [Google Scholar] [CrossRef]
- Yi, K.H.; Lauring, J. Recurrent AKT mutations in human cancers: Functional consequences and effects on drug sensitivity. Oncotarget 2016, 7, 4241–4251. [Google Scholar] [CrossRef]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Yang, G.; Lu, X.; Yuan, L. LncRNA: A link between RNA and cancer. Biochim. Biophys. Acta 2014, 1839, 1097–1109. [Google Scholar] [CrossRef]
- Peng, W.X.; Koirala, P.; Mo, Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circ RNA s. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Lee, Y.C.; Wang, W.Y.; Lin, H.H.; Huang, Y.R.; Lin, Y.C.; Hsiao, K.Y. The functional roles and regulation of circular RNAs during cellular stresses. Non-Coding RNA 2022, 8, 38. [Google Scholar] [CrossRef]
- Quan, J.; Pan, X.; Zhao, L.; Li, Z.; Dai, K.; Yan, F.; Liu, S.; Ma, H.; Lai, Y. LncRNA as a diagnostic and prognostic biomarker in bladder cancer: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 6415–6424. [Google Scholar] [CrossRef]
- Guglas, K.; Bogaczynska, M.; Kolenda, T.; Rys, M.; Teresiak, A.; Blizniak, R.; Lasinska, I.; Mackiewicz, J.; Lamperska, K. lncRNA in HNSCC: Challenges and potential. Contemp. Oncol. 2017, 21, 259–266. [Google Scholar] [CrossRef]
- Chang, Y.S.; Lee, Y.T.; Yen, J.C.; Chang, Y.C.; Lin, L.L.; Chan, W.L.; Chang, W.C.; Lin, S.Y.; Chang, J.G. Long noncoding RNA NTT context-dependently regulates MYB by interacting with activated complex in hepatocellular carcinoma cells. Front. Oncol. 2021, 11, 592045. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.P.; Zeng, X.; Zheng, Y.; Guo, Q.H.; Ji, R.; Zhou, Y.N. Circular RNA is a popular molecule in tumors of the digestive system (Review). Int. J. Oncol. 2020, 57, 21–42. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Moafian, Z.; Maghrouni, A.; Soltani, A.; Hashemy, S.I. Cross-talk between non-coding RNAs and PI3K/AKT/mTOR pathway in colorectal cancer. Mol. Biol. Rep. 2021, 48, 4797–4811. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Baghery Saghchy Khorasani, A.; Pourbagheri-Sigaroodi, A.; Shahrokh, S.; Zali, M.R.; Bashash, D. The PI3K/Akt/mTOR axis in colorectal cancer: Oncogenic alterations, non-coding RNAs, therapeutic opportunities, and the emerging role of nanoparticles. J. Cell. Physiol. 2022, 237, 1720–1752. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing miRNA-lncRNA interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar] [CrossRef]
- Sakshi, S.; Jayasuriya, R.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Role of circRNA-miRNA-mRNA interaction network in diabetes and its associated complications. Mol. Ther. Nucleic Acids 2021, 26, 1291–1302. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Koseoglu, S.; Lu, Z.; Kumar, C.; Kirschmeier, P.; Zou, J. AKT1, AKT2 and AKT3-dependent cell survival is cell line-specific and knockdown of all three isoforms selectively induces apoptosis in 20 human tumor cell lines. Cancer Biol. Ther. 2007, 6, 755–762. [Google Scholar] [CrossRef]
- Brand, Y.; Levano, S.; Radojevic, V.; Naldi, A.M.; Setz, C.; Ryan, A.F.; Pak, K.; Hemmings, B.A.; Bodmer, D. All Akt isoforms (Akt1, Akt2, Akt3) are involved in normal hearing, but only Akt2 and Akt3 are involved in auditory hair cell survival in the mammalian inner ear. PLoS ONE 2015, 10, e0121599. [Google Scholar] [CrossRef]
- Du, Z.; Yang, D.; Zhang, Y.; Xuan, X.; Li, H.; Hu, L.; Ruan, C.; Li, L.; Chen, A.; Deng, L.; et al. AKT2 deficiency impairs formation of the BCR signalosome. Cell Commun. Signal. 2020, 18, 56. [Google Scholar] [CrossRef]
- Cohen, M.M., Jr. The AKT genes and their roles in various disorders. Am. J. Med. Genet. Part A 2013, 161A, 2931–2937. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., 3rd; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Tschopp, O.; Hemmings-Mieszczak, M.; Feng, J.; Brodbeck, D.; Perentes, E.; Hemmings, B.A. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 2003, 278, 32124–32131. [Google Scholar] [CrossRef]
- Yong, H.; Wu, G.; Chen, J.; Liu, X.; Bai, Y.; Tang, N.; Liu, L.; Wei, J. lncRNA MALAT1 accelerates skeletal muscle cell apoptosis and inflammatory response in sepsis by decreasing BRCA1 expression by recruiting EZH2. Mol. Ther. Nucleic Acids 2020, 19, 97–108. [Google Scholar] [CrossRef]
- Qu, F.; Cao, P. Long noncoding RNA SOX2OT contributes to gastric cancer progression by sponging miR-194-5p from AKT2. Exp. Cell Res. 2018, 369, 187–196. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, T.; Pang, T.; Du, Z.; Yin, X.; Cui, H.; Fang, G.; Xue, X. MALAT1 promotes gastric adenocarcinoma through the MALAT1/miR-181a-5p/AKT3 axis. Open Biol. 2019, 9, 190095. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, J.; Zhang, Y.; Xie, A.; Yu, L.; Zhang, C.; Lei, J.; Xu, H.; Leng, Z.; Li, T.; et al. LncTarD: A manually-curated database of experimentally-supported functional lncRNA-target regulations in human diseases. Nucleic Acids Res. 2020, 48, D118–D126. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Qin, X.; Geng, H.; Zuo, D.; Zhao, Q. PI3K/AKT/mTOR pathway-related long non-coding RNAs: Roles and mechanisms in hepatocellular carcinoma. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2020, 160, 105195. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Majidpoor, J.; Taheri, M. The emerging role of non-coding RNAs in the regulation of PI3K/AKT pathway in the carcinogenesis process. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 137, 111279. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Wu, J.C.C.; Fang, C.; Chang, A.Y.W. PTEN, a negative regulator of PI3K/Akt signaling, sustains brain stem cardiovascular regulation during mevinphos intoxication. Neuropharmacology 2017, 123, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yang, M.; Wang, X.; Fan, J.; Liu, X.; Zhang, Y.; Shu, Y. Long noncoding RNA FER1L4 inhibits cell proliferation and promotes cell apoptosis via the PTEN/AKT/p53 signaling pathway in lung cancer. Oncol. Rep. 2021, 45, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Zhang, G.; Qiu, S. Up-regulation of LINC00619 promotes apoptosis and inhibits proliferation, migration and invasion while promoting apoptosis of osteosarcoma cells through inactivation of the HGF-mediated PI3K-Akt signalling pathway. Epigenetics Off. J. DNA Methylation Soc. 2022, 17, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Zhang, K.; Zhang, X. LncRNA HOTAIR facilitates proliferation and represses apoptosis of retinoblastoma cells through the miR-20b-5p/RRM2/PI3K/AKT axis. Orphanet J. Rare Dis. 2022, 17, 119. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Zhang, L.; Younis, M.; Yuan, L. The LncRNA RP11-301G19.1/miR-582-5p/HMGB2 axis modulates the proliferation and apoptosis of multiple myeloma cancer cells via the PI3K/AKT signalling pathway. Cancer Gene Ther. 2022, 29, 292–303. [Google Scholar] [CrossRef]
- Chen, H.; Tan, X.; Ding, Y. Knockdown SNHG20 suppresses nonsmall cell lung cancer development by repressing proliferation, migration and invasion, and inducing apoptosis by regulating miR-2467-3p/E2F3. Cancer Biother. Radiopharm. 2021, 36, 360–370. [Google Scholar] [CrossRef]
- Li, F.; Gu, F.; Li, Q.; Zhai, C.; Gong, R.; Zhu, X. ROR1-AS1 knockdown inhibits growth and invasion and promotes apoptosis in NSCLC cells by suppression of the PI3K/Akt/mTOR pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22726. [Google Scholar] [CrossRef]
- Fu, T.; Yang, Y.; Mu, Z.; Sun, R.; Li, X.; Dong, J. Silencing lncRNA LINC01410 suppresses cell viability yet promotes apoptosis and sensitivity to temozolomide in glioblastoma cells by inactivating PTEN/AKT pathway via targeting miR-370-3p. Immunopharmacol. Immunotoxicol. 2021, 43, 680–692. [Google Scholar] [CrossRef]
- Geng, S.; Tu, S.; Fu, W.; Wang, J.; Bai, Z. LncRNA PITPNA-AS1 stimulates cell proliferation and suppresses cell apoptosis in glioblastoma via targeting miR-223-3p/EGFR axis and activating PI3K/AKT signaling pathway. Cell Cycle 2021, 20, 1988–1998. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, K.; Lv, S.C.; Xu, G.W.; Zhang, J.F.; Jia, H.Y. LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway to enhance autophagy and inhibit inflammation in TNF-alpha-treated keratinocytes and psoriatic mice. Cytokine 2021, 148, 155657. [Google Scholar] [CrossRef]
- Yang, G.; Li, Z.; Dong, L.; Zhou, F. lncRNA ADAMTS9-AS1 promotes bladder cancer cell invasion, migration, and inhibits apoptosis and autophagy through PI3K/AKT/mTOR signaling pathway. Int. J. Biochem. Cell Biol. 2021, 140, 106069. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress confers 5-fluorouracil resistance in breast cancer cell via the GRP78/OCT4/lncRNA MIAT/AKT pathway. Am. J. Cancer Res. 2020, 10, 838–855. [Google Scholar] [PubMed]
- Guo, Z.; Wang, Y.H.; Xu, H.; Yuan, C.S.; Zhou, H.H.; Huang, W.H.; Wang, H.; Zhang, W. LncRNA linc00312 suppresses radiotherapy resistance by targeting DNA-PKcs and impairing DNA damage repair in nasopharyngeal carcinoma. Cell Death Dis. 2021, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Shi, Z.; Ma, X.; Xu, D.; Ming, G. lncRNA GAS5/miR-223/NAMPT axis modulates the cell proliferation and senescence of endothelial progenitor cells through PI3K/AKT signaling. J. Cell. Biochem. 2019, 120, 14518–14530. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Huang, X.; Xuan, Y. Pyrroline-5-carboxylate reductase-2 promotes colorectal cancer progression via activating PI3K/AKT/mTOR pathway. Dis. Markers 2021, 2021, 9950663. [Google Scholar] [CrossRef]
- Xie, Z.; Zhong, C.; Shen, J.; Jia, Y.; Duan, S. LINC00963: A potential cancer diagnostic and therapeutic target. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 113019. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.; Chen, D.; Yuwen, D.; Zhang, J.; Wei, X.; Han, X.; Guan, X. SOX2-OT induced by PAI-1 promotes triple-negative breast cancer cells metastasis by sponging miR-942-5p and activating PI3K/Akt signaling. Cell. Mol. Life Sci. 2022, 79, 59. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, F.; Li, Q.; Sun, H.; Li, F.; Jiao, Z.; Lei, J. LncRNA MIR205HG accelerates cell proliferation, migration and invasion in hepatoblastoma through the activation of MAPK signaling pathway and PI3K/AKT signaling pathway. Biol Direct 2022, 17, 2. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, S.; Hu, D.; Chen, L. LncRNA AC099850.3 promotes hepatocellular carcinoma proliferation and invasion through PRR11/PI3K/AKT axis and is associated with patients prognosis. J. Cancer 2022, 13, 1048–1060. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L.; Yu, Y.; Fu, Y. LncRNA RP5-857K21.7 inhibits PDGF-BB-induced proliferation and migration of airway smooth muscle cells through the miR-508-3p/PI3K/AKT/mTOR axis. Autoimmunity 2022, 55, 65–73. [Google Scholar] [CrossRef]
- Glazar, P.; Papavasileiou, P.; Rajewsky, N. circBase: A database for circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yan, C.; Zhang, L.; Li, Y.; Wan, Q. LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine 2018, 97, e0473. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Cheng, J.; Mujahid, H.; Wang, H.; Kong, J.; Yin, Y.; Li, J.; Zhang, Y.; Ji, X.; Chen, W. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS ONE 2014, 9, e90563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Zhang, Y.; Wang, M.; Li, X.; Liu, S.; Xu, D.; Bao, Y.; Jia, P.; Wu, N.; et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox Biol. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Fu, Z.; Luo, W.; Wang, J.; Peng, T.; Sun, G.; Shi, J.; Li, Z.; Zhang, B. Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma. Biochem. Biophys. Res. Commun. 2017, 492, 480–486. [Google Scholar] [CrossRef]
- Jia, Y.; Yi, L.; Li, Q.; Liu, T.; Yang, S. LncRNA MALAT1 aggravates oxygen-glucose deprivation/reoxygenation-induced neuronal endoplasmic reticulum stress and apoptosis via the miR-195a-5p/HMGA1 axis. Biol. Res. 2021, 54, 8. [Google Scholar] [CrossRef]
- Lin, N.; Yao, Z.; Xu, M.; Chen, J.; Lu, Y.; Yuan, L.; Zhou, S.; Zou, X.; Xu, R. Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 244. [Google Scholar] [CrossRef]
- Yang, M.H.; Hu, Z.Y.; Xu, C.; Xie, L.Y.; Wang, X.Y.; Chen, S.Y.; Li, Z.G. MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9. Biochim. Biophys. Acta 2015, 1852, 166–174. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Wang, H.; Jiang, C.; Liu, Z. LncRNA growth arrest specific transcript 5 inhibits the growth of pituitary neuroendocrine tumors via miR-27a-5p/cylindromatosis axis. Bioengineered 2022, 13, 10274–10286. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhan, H.L.; Li, M.K.; Wu, G.D.; Liu, Z.; Wu, L.F. Long noncoding RNA Gas5 induces cell apoptosis and inhibits tumor growth via activating the CHOP-dependent endoplasmic reticulum stress pathway in human hepatoblastoma HepG2 cells. J. Cell. Biochem. 2022, 123, 231–247. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.J.; Meng, T.; Lei, C.; Yang, X.H.; Wang, Q.S.; Jin, B.; Zhu, J.F. lncRNA GAS5 inhibits cell migration and invasion and promotes autophagy by targeting miR-222-3p via the GAS5/PTEN-signaling pathway in CRC. Mol. Ther. Nucleic Acids 2019, 17, 644–656. [Google Scholar] [CrossRef]
- Li, G.; Qian, L.; Tang, X.; Chen, Y.; Zhao, Z.; Zhang, C. Long noncoding RNA growth arrestspecific 5 (GAS5) acts as a tumor suppressor by promoting autophagy in breast cancer. Mol. Med. Rep. 2020, 22, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, C.; Shen, X. LncRNA GAS5 suppresses ER stress induced apoptosis and inflammation by regulating SERCA2b in HG treated retinal epithelial cell. Mol. Med. Rep. 2020, 22, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; Zhang, X.; Dang, Y.; Cheng, Y.; Hua, W.; Teng, M.; Wang, S.; Lu, X. Novel lncRNA-miRNA-mRNA competing endogenous RNA triple networks associated programmed cell death in heart failure. Front. Cardiovasc. Med. 2021, 8, 747449. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, N.; Xia, F.; Liu, S.; Jia, D. Long noncoding RNA GAS5 regulates myocardial ischemiareperfusion injury through the PI3K/AKT apoptosis pathway by sponging miR5325p. Int. J. Mol. Med. 2020, 45, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, L.; Yan, W.; Qiu, L.; Zhang, J.; Jia, X. lncRNA GAS5 sensitizes breast cancer cells to ionizing radiation by inhibiting DNA repair. BioMed Res. Int. 2022, 2022, 1987519. [Google Scholar] [CrossRef]
- Chen, T.; Liang, Q.; Xu, J.; Zhang, Y.; Zhang, Y.; Mo, L.; Zhang, L. MiR-665 regulates vascular smooth muscle cell senescence by interacting With LncRNA GAS5/SDC1. Front. Cell Dev. Biol. 2021, 9, 700006. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, L.; Yang, C.; Zhang, W.; Wang, H. Propofol inhibits proliferation and migration of glioma cells by up-regulating lncRNA GAS5. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2022, 80, 105321. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Q.; Li, S.; Jiang, S.; Cui, J.; Dang, G. Interference of the long noncoding RNA CDKN2B-AS1 upregulates miR-181a-5p/TGFbetaI axis to restrain the metastasis and promote apoptosis and senescence of cervical cancer cells. Cancer Med. 2019, 8, 1721–1730. [Google Scholar] [CrossRef]
- Yang, M.; Yin, E.; Xu, Y.; Liu, Y.; Li, T.; Dong, Z.; Tai, W. CDKN2B antisense RNA 1 expression alleviates idiopathic pulmonary fibrosis by functioning as a competing endogenouse RNA through the miR-199a-5p/Sestrin-2 axis. Bioengineered 2022, 13, 7746–7759. [Google Scholar] [CrossRef]
- LIU, Y.; ZHANG, X.; REN, C.; ZHU, W.; DAI, J.; LAI, Y. Protective effect of stress-associated endoplasmic reticulum protein 1 on glucose and oxygen deprivation-induced injury in cardiomyocytes. Chin. J. Geriatr. 2019, 12, 678–682. [Google Scholar]
- Puvvula, P.K. LncRNAs regulatory networks in cellular senescence. Int. J. Mol. Sci. 2019, 20, 2615. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiang, B.; Liu, Y.; Wang, Y.; Kan, H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018, 437, 56–66. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y. Knockdown of lncRNA HULC inhibits proliferation, migration, invasion, and promotes apoptosis by sponging miR-122 in osteosarcoma. J. Cell. Biochem. 2018, 119, 1050–1061. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.Y.; Zhang, J.Q.; Wang, G.G.; He, J.; Chen, Y.Y.; Huang, C.; Li, L.; Li, S.Q. LncRNA HULC promotes non-small cell lung cancer cell proliferation and inhibits the apoptosis by up-regulating sphingosine kinase 1 (SPHK1) and its downstream PI3K/Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8722–8730. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ni, Z.; He, J.; Jiang, S.; Li, X.; He, J.; Gong, W.; Zheng, L.; Chen, S.; Li, B.; et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene 2017, 36, 3528–3540. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Qin, R.; Lu, Y.; Chen, Y.; Xie, S.; Jiang, X.; Lu, D. MiR-26a-1 promotes DNA damage repair by inhibiting Sirt1 and KDM5A in human liver cancer stem cells. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Grammatikakis, I.; Panda, A.C.; Abdelmohsen, K.; Gorospe, M. Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging 2014, 6, 992–1009. [Google Scholar] [CrossRef]
- Feng, H.; Wei, B.; Zhang, Y. Long non-coding RNA HULC promotes proliferation, migration and invasion of pancreatic cancer cells by down-regulating microRNA-15a. Int. J. Biol. Macromol. 2019, 126, 891–898. [Google Scholar] [CrossRef]
- Yan, C.; Wei, S.; Han, D.; Wu, L.; Tan, L.; Wang, H.; Dong, Y.; Hua, J.; Yang, W. LncRNA HULC shRNA disinhibits miR-377-5p to suppress the growth and invasion of hepatocellular carcinoma in vitro and hepatocarcinogenesis in vivo. Ann. Transl. Med. 2020, 8, 1294. [Google Scholar] [CrossRef]
- Shen, Q.; Xu, Z.; Xu, S. Long noncoding RNA LUCAT1 contributes to cisplatin resistance by regulating the miR514a3p/ULK1 axis in human nonsmall cell lung cancer. Int. J. Oncol. 2020, 57, 967–979. [Google Scholar] [CrossRef]
- Xing, X.L.; Yao, Z.Y.; Ou, J.; Xing, C.; Li, F. Development and validation of ferroptosis-related lncRNAs prognosis signatures in kidney renal clear cell carcinoma. Cancer Cell Int. 2021, 21, 591. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Sun, K.; Fan, X.; Jia, K.; Wang, X.; Ma, C.; Wei, L. Necroptosis-related lncRNAs and hepatocellular carcinoma undoubtedly secret. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Huan, L.; Guo, T.; Wu, Y.; Xu, L.; Huang, S.; Xu, Y.; Liang, L.; He, X. Hypoxia induced LUCAT1/PTBP1 axis modulates cancer cell viability and chemotherapy response. Mol. Cancer 2020, 19, 11. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, C.; Xu, C.; Xue, H.; Zhou, H.; Gu, L.; Liu, Y.; Xu, Q. Down-regulation of long non-coding RNA RP11-708H21.4 is associated with poor prognosis for colorectal cancer and promotes tumorigenesis through regulating AKT/mTOR pathway. Oncotarget 2017, 8, 27929–27942. [Google Scholar] [CrossRef]
- Sun, J.; Min, H.; Yu, L.; Yu, G.; Shi, Y.; Sun, J. The knockdown of LncRNA AFAP1-AS1 suppressed cell proliferation, migration, and invasion, and promoted apoptosis by regulating miR-545-3p/hepatoma-derived growth factor axis in lung cancer. Anti-Cancer Drugs 2021, 32, 11–21. [Google Scholar] [CrossRef]
- Wang, R.; Yan, Y.; Li, C. LINC00462 is involved in high glucose-induced apoptosis of renal tubular epithelial cells via AKT pathway. Cell Biol. Int. 2019. ahead of print. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, W.; Sun, C.; Zhang, B.; Zheng, F. Linc00462 promotes pancreatic cancer invasiveness through the miR-665/TGFBR1-TGFBR2/SMAD2/3 pathway. Cell Death Dis. 2018, 9, 706. [Google Scholar] [CrossRef]
- Li, G.H.; Yu, J.H.; Yang, B.; Gong, F.C.; Zhang, K.W. LncRNA LOXL1-AS1 inhibited cell proliferation, migration and invasion as well as induced apoptosis in breast cancer via regulating miR-143-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10400–10409. [Google Scholar] [CrossRef]
- Gu, N.; Wang, X.; Di, Z.; Xiong, J.; Ma, Y.; Yan, Y.; Qian, Y.; Zhang, Q.; Yu, J. Silencing lncRNA FOXD2-AS1 inhibits proliferation, migration, invasion and drug resistance of drug-resistant glioma cells and promotes their apoptosis via microRNA-98-5p/CPEB4 axis. Aging 2019, 11, 10266–10283. [Google Scholar] [CrossRef]
- Guo, L.Y.; Qin, C.F.; Zou, H.X.; Song, M.Y.; Gong, M.L.; Chen, C. LncRNA AB073614 promotes the proliferation and inhibits apoptosis of cervical cancer cells by repressing RBM5. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7201. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhou, H.Y.; Yao, X.M.; Chen, X.D.; Wu, J.; Lu, X.C. Long non-coding RNA AB073614 promotes metastasis of gastric cancer cells by upregulating IGF-2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7207. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/beta-catenin in ox-LDL-stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018, 25, 11. [Google Scholar] [CrossRef]
- Xu, J.; Xia, Y.; Zhang, H.; Guo, H.; Feng, K.; Zhang, C. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed. Pharmacother. 2018, 101, 691–697. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Cheng, L.; Li, C.; Wu, Z.; Luo, Y.; Zhou, K.; Li, Y.; Zhao, Q.; Huang, Y. Modulation of lncRNA H19 enhances resveratrol-inhibited cancer cell proliferation and migration by regulating endoplasmic reticulum stress. J. Cell. Mol. Med. 2022, 26, 2205–2217. [Google Scholar] [CrossRef]
- Sultan, H.K.; El-Ayat, W.M.; AbouGhalia, A.H.; Lasheen, N.N.; Moustafa, A.S. Study of long non-coding RNA and mitochondrial dysfunction in diabetic rats. Tissue Cell 2021, 71, 101516. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Xie, H.; Liang, Z.; Chen, B.; Xu, C.; Wang, J.; Huang, X.; et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater. 2022, 13, 23–36. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Lin, L.; Sang, Y.; Yang, F.; Zhang, J.; Jia, L.; Xu, Z.; Zhang, W. Long non-coding RNA H19 and the underlying epigenetic function in response to DNA damage of lung cancer cells. Am. J. Transl. Res. 2021, 13, 5835–5850. [Google Scholar]
- Zhuang, Y.; Li, T.; Xiao, H.; Wu, J.; Su, S.; Dong, X.; Hu, X.; Hua, Q.; Liu, J.; Shang, W.; et al. LncRNA-H19 drives cardiomyocyte senescence by targeting miR-19a/socs1/p53 axis. Front. Pharmacol. 2021, 12, 631835. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, P.; Chen, L.; Wang, W.; Zhang, J.; Li, Q.; Xu, Y. Upregulated long non-coding RNA SPRY4-IT1 predicts dismal prognosis for pancreatic ductal adenocarcinoma and regulates cell proliferation and apoptosis. Gene 2018, 659, 52–58. [Google Scholar] [CrossRef]
- Zhao, W.; Mazar, J.; Lee, B.; Sawada, J.; Li, J.L.; Shelley, J.; Govindarajan, S.; Towler, D.; Mattick, J.S.; Komatsu, M.; et al. The long noncoding RNA SPRIGHTLY regulates cell proliferation in primary human melanocytes. J. Investig. Dermatol. 2016, 136, 819–828. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Z.; Wang, R.; Liang, Z.; Lin, Z.; Deng, S.; Chen, L.; Liu, Z.; Feng, S. Long non-coding RNA SPRY4-IT1 promotes proliferation and metastasis in nasopharyngeal carcinoma cell. PeerJ 2022, 10, e13221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Lv, T.; Wu, Y.; Shi, X.; Liu, H.; Song, Y. Long non-coding RNA 00312 regulated by HOXA5 inhibits tumour proliferation and promotes apoptosis in non-small cell lung cancer. J. Cell. Mol. Med. 2017, 21, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wu, Z.Y.; Wang, G.C.; Liu, K.; Niu, X.B.; Gu, S.; Meng, J.S. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197-3p. Tumour Biol. 2016, 37, 14553–14563. [Google Scholar] [CrossRef]
- Ruan, Y.; Lin, N.; Ma, Q.; Chen, R.; Zhang, Z.; Wen, W.; Chen, H.; Sun, J. Circulating LncRNAs analysis in patients with type 2 diabetes reveals novel genes influencing glucose metabolism and islet beta-cell function. Cell. Physiol. Biochem. 2018, 46, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, Z.; Wang, J. Long Noncoding RNA FEZF1-AS1 promotes proliferation and inhibits apoptosis in ovarian cancer by activation of JAK-STAT3 pathway. Med. Sci. Monit. 2018, 24, 8088–8095. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Zhao, Z.; Sun, Q.; Shao, G.; Huang, J.; Zhao, W.; Kuang, Y. LncRNA FEZF1-AS1 promotes multi-drug resistance of gastric cancer cells via upregulating ATG5. Front. Cell Dev. Biol. 2021, 9, 749129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.D.; Han, L.; Lee, H.; Zhuang, L.; Zhang, Y.; Baddour, J.; Nagrath, D.; Wood, C.G.; Gu, J.; Wu, X.; et al. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat. Commun. 2017, 8, 783. [Google Scholar] [CrossRef]
- Chen, Q.B.; Li, Z.H.; Fu, Y.; Lv, N.N.; Tian, N.; Han, L.; Tian, Y. Downregulated long non-coding RNA LINC00899 inhibits invasion and migration of spinal ependymoma cells via RBL2-dependent FoxO pathway. Cell Cycle 2019, 18, 2566–2579. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, X.; Fang, X.; Xin, Z. Construction on of a ferroptosis-related lncRNA-based model to improve the prognostic evaluation of gastric cancer patients based on bioinformatics. Front. Genet. 2021, 12, 739470. [Google Scholar] [CrossRef]
- Angeles, A.K.; Heckmann, D.; Flosdorf, N.; Duensing, S.; Sultmann, H. The ERG-regulated LINC00920 promotes prostate cancer cell survival via the 14-3-3epsilon-FOXO pathway. Mol. Cancer Res. 2020, 18, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wei, J.; Zhao, C.; Xiang, S.; Shi, M.; Wang, Y. Targeting LncRNA EPIC1 to inhibit human colon cancer cell progression. Aging 2020, 12. ahead of print. [Google Scholar] [CrossRef]
- Su, W.; Guo, C.; Wang, L.; Wang, Z.; Yang, X.; Niu, F.; Tzou, D.; Yang, X.; Huang, X.; Wu, J.; et al. LncRNA MIR22HG abrogation inhibits proliferation and induces apoptosis in esophageal adenocarcinoma cells via activation of the STAT3/c-Myc/FAK signaling. Aging 2019, 11, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Lu, P.; Guan, J.; Zhou, Y.; Zou, L.; Yi, X.; Cheng, H. LncRNA KCNQ1OT1 controls cell proliferation, differentiation and apoptosis by sponging miR-326 to regulate c-Myc expression in acute myeloid leukemia. Neoplasma 2020, 67, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Zhao, T.T.; Du, W.W.; Yang, Z.F.; Peng, W.; Cui, Z.J. C-MYC-induced upregulation of LINC01503 promotes progression of non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11120–11127. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Zheng, X.; Liu, N. c-MYC-induced long noncoding RNA MEG3 aggravates kidney ischemia-reperfusion injury through activating mitophagy by upregulation of RTKN to trigger the Wnt/beta-catenin pathway. Cell Death Dis. 2021, 12, 191. [Google Scholar] [CrossRef]
- Capizzi, M.; Strappazzon, F.; Cianfanelli, V.; Papaleo, E.; Cecconi, F. MIR7-3HG, a MYC-dependent modulator of cell proliferation, inhibits autophagy by a regulatory loop involving AMBRA1. Autophagy 2017, 13, 554–566. [Google Scholar] [CrossRef]
- Song, Y.; Du, J.; Lu, P.; Zou, Q.; Zeng, S.; Liu, M.; Hu, X.; Ma, W.; Lin, H.; Liu, X.; et al. LncRNA NFYC-AS1 promotes the development of lung adenocarcinomas through autophagy, apoptosis, and MET/c-Myc oncogenic proteins. Ann. Transl. Med. 2021, 9, 1621. [Google Scholar] [CrossRef]
- Zhang, T.; Li, N.; Sun, C.; Jin, Y.; Sheng, X. MYC and the unfolded protein response in cancer: Synthetic lethal partners in crime? EMBO Mol. Med. 2020, 12, e11845. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Z.; Zhang, J.; Hao, Z.; He, Y.; Wu, Z.; Song, Y.; Yuan, K.; Zheng, S.; Zhao, Q.; et al. lncRNA MALAT1 participates in metformin inhibiting the proliferation of breast cancer cell. J. Cell. Mol. Med. 2021, 25, 7135–7145. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, S.; Xu, M.; Ma, B.; Liu, R.; Xu, Y.; Zhang, Y. TFAP2C-mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front. Oncol. 2022, 12, 862015. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.D.H.; Kessler, C.; Niehus, S.E.; Mahnkopf, M.; Koch, A.; Tamura, T. Myc target gene, long intergenic noncoding RNA, Linc00176 in hepatocellular carcinoma regulates cell cycle and cell survival by titrating tumor suppressor microRNAs. Oncogene 2018, 37, 75–85. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jing, Y.; Wei, F.; Tang, Y.; Yang, L.; Luo, J.; Yang, P.; Ni, Q.; Pang, J.; Liao, Q.; et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Olivero, C.E.; Martinez-Terroba, E.; Zimmer, J.; Liao, C.; Tesfaye, E.; Hooshdaran, N.; Schofield, J.A.; Bendor, J.; Fang, D.; Simon, M.D.; et al. p53 activates the long noncoding RNA Pvt1b to inhibit Myc and suppress tumorigenesis. Mol. Cell 2020, 77, 761–774.e768. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, F.; Jin, Y.; Yang, Y.; Wang, S.; Zhang, J.; Chen, C.; Zeng, Q.; Han, W.; Wang, H.; et al. Downregulated NORAD in neuroblastoma promotes cell proliferation via chromosomal instability and predicts poor prognosis. Acta Biochim. Pol. 2020, 67, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Koziol, K.; Stasiak, M.; Obacz, J.; Guglas, K.; Poter, P.; Mackiewicz, A.; Kolenda, T. The role of NEAT1 lncRNA in squamous cell carcinoma of the head and neck is still difficult to define. Contemp. Oncol. 2020, 24, 96–105. [Google Scholar] [CrossRef]
- Vucicevic, D.; Gehre, M.; Dhamija, S.; Friis-Hansen, L.; Meierhofer, D.; Sauer, S.; Orom, U.A. The long non-coding RNA PARROT is an upstream regulator of c-Myc and affects proliferation and translation. Oncotarget 2016, 7, 33934–33947. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.; Wang, Z.; Yang, Y.; Wang, C.; Fang, D.; Liu, K.; Wang, F.; Mei, Y. c-Myc-activated USP2-AS1 suppresses senescence and promotes tumor progression via stabilization of E2F1 mRNA. Cell Death Dis. 2021, 12, 1006. [Google Scholar] [CrossRef]
- Zhen-Hua, W.; Yi-Wei, G.; Li-Qin, Z.; Jie-Yun, Z.; Zhe, G.; Wei-Jian, G. Silencing of LncRNA C1RL-AS1 suppresses the malignant phenotype in gastric cancer cells via the AKT/beta-Catenin/c-Myc pathway. Front. Oncol. 2020, 10, 1508. [Google Scholar] [CrossRef]
- Sang, B.; Zhang, Y.Y.; Guo, S.T.; Kong, L.F.; Cheng, Q.; Liu, G.Z.; Thorne, R.F.; Zhang, X.D.; Jin, L.; Wu, M. Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence. Proc. Natl. Acad. Sci. USA 2018, 115, E11661–E11670. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, T.; Wei, W.; Wang, H.; Zhang, Z.; Yang, W.; Xia, W.; Mao, Q.; Xu, L.; Jiang, F.; et al. The long noncoding RNA LINC00665 facilitates c-Myc transcriptional activity via the miR-195-5p MYCBP axis to promote progression of lung adenocarcinoma. Front. Oncol. 2021, 11, 666551. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, L.; Xiong, F.; He, Y.; Tang, Y.; Shi, L.; Fan, S.; Li, Z.; Zhang, S.; Gong, Z.; et al. Long non-coding RNA AFAP1-AS1 accelerates lung cancer cells migration and invasion by interacting with SNIP1 to upregulate c-Myc. Signal Transduct. Target. Ther. 2021, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Xie, Y.; Deng, M.; Zhu, L.; Wu, X.; Li, G.; Shi, N.X.; Wen, C.; Huang, W.; Duan, Y.; et al. c-Myc-activated intronic miR-210 and lncRNA MIR210HG synergistically promote the metastasis of gastric cancer. Cancer Lett. 2022, 526, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Tang, T.; Chen, M.; Dong, B.; Sun, W.; Wu, N.; Chen, H.; Feng, Q.; Yang, X.; Jin, R.; et al. C-Myc-activated long non-coding RNA LINC01050 promotes gastric cancer growth and metastasis by sponging miR-7161-3p to regulate SPZ1 expression. J. Exp. Clin. Cancer Res. 2021, 40, 351. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.R.; Yan, L.; Liu, Y.T.; Cao, L.; Guo, Y.H.; Zhang, Y.; Yao, H.; Cai, L.; Shang, H.B.; Rui, W.W.; et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018, 9, 4624. [Google Scholar] [CrossRef]
- Fang, X.; Pan, X.; Mai, H.; Yuan, X.; Liu, S.; Wen, F. LINC00998 functions as a novel tumor suppressor in acute myeloid leukemia via regulating the ZFP36 ring finger protein/mammalian target of rapamycin complex 2 axis. Bioengineered 2021, 12, 10363–10372. [Google Scholar] [CrossRef]
- Li, P.; He, J.; Yang, Z.; Ge, S.; Zhang, H.; Zhong, Q.; Fan, X. ZNNT1 long noncoding RNA induces autophagy to inhibit tumorigenesis of uveal melanoma by regulating key autophagy gene expression. Autophagy 2020, 16, 1186–1199. [Google Scholar] [CrossRef]
- Chen, J.F.; Wu, P.; Xia, R.; Yang, J.; Huo, X.Y.; Gu, D.Y.; Tang, C.J.; De, W.; Yang, F. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol. Cancer 2018, 17, 6. [Google Scholar] [CrossRef]
- Hu, T.J.; Huang, H.B.; Shen, H.B.; Chen, W.; Yang, Z.H. Role of long non-coding RNA MALAT1 in chronic obstructive pulmonary disease. Exp. Ther. Med. 2020, 20, 2691–2697. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Dong, W. LncRNA RHPN1-AS1 promotes the progression of nasopharyngeal carcinoma by targeting CELF2 expression. Exp. Mol. Pathol. 2021, 122, 104671. [Google Scholar] [CrossRef]
- Lan, C.; Wang, Y.; Su, X.; Lu, J.; Ma, S. LncRNA LINC00958 activates mTORC1/P70S6K signalling pathway to promote epithelial-mesenchymal transition process in the hepatocellular carcinoma. Cancer Investig. 2021, 39, 539–549. [Google Scholar] [CrossRef]
- Li, P.; Yan, X.; Xu, G.; Pang, Z.; Weng, J.; Yin, J.; Li, M.; Yu, L.; Chen, Q.; Sun, K. A novel plasma lncRNA ENST00000416361 is upregulated in coronary artery disease and is related to inflammation and lipid metabolism. Mol. Med. Rep. 2020, 21, 2375–2384. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, H.; Gao, Y.; Jin, Y.; Ning, W.; Hou, Y.; Su, J. Long non-coding RNA AC012668 suppresses non-alcoholic fatty liver disease by competing for microRNA miR-380-5p with lipoprotein-related protein LRP2. Bioengineered 2021, 12, 6738–6747. [Google Scholar] [CrossRef]
- Ma, J.; Feng, J.; Zhou, X. Long non-coding RNA HAGLROS regulates lipid metabolism reprogramming in intrahepatic cholangiocarcinoma via the mTOR signaling pathway. Exp. Mol. Pathol. 2020, 115, 104466. [Google Scholar] [CrossRef]
- Fan, H.; Li, J.; Wang, J.; Hu, Z. Long non-coding RNAs (lncRNAs) tumor-suppressive role of lncRNA on chromosome 8p12 (TSLNC8) inhibits tumor metastasis and promotes apoptosis by regulating interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3)/hypoxia-inducible factor 1-alpha (HIF-1alpha) signaling pathway in non-small cell lung cancer. Med. Sci. Monit. 2019, 25, 7624–7633. [Google Scholar] [CrossRef]
- Hall, J.R.; Messenger, Z.J.; Tam, H.W.; Phillips, S.L.; Recio, L.; Smart, R.C. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015, 6, e1700. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Y.; Xu, Z.; Wang, Z. lncRNA nuclear factor of activated T cells knockdown alleviates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by upregulating HIF-1alpha expression. J. Cardiovasc. Pharmacol. 2022, 79, 479–488. [Google Scholar] [CrossRef]
- Yang, H.; Wang, G.; Liu, J.; Lin, M.; Chen, J.; Fang, Y.; Li, Y.; Cai, W.; Zhan, D. LncRNA JPX regulates proliferation and apoptosis of nucleus pulposus cells by targeting the miR-18a-5p/HIF-1alpha/Hippo-YAP pathway. Biochem. Biophys. Res. Commun. 2021, 566, 16–23. [Google Scholar] [CrossRef]
- Choudhry, H. UCA1 overexpression promotes hypoxic breast cancer cell proliferation and inhibits apoptosis via HIF-1alpha activation. J. Oncol. 2021, 2021, 5512156. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Du, Y.; Liu, Y.; Xiong, X. Long non-coding RNA MALAT1 mediates hypoxia-induced pro-survival autophagy of endometrial stromal cells in endometriosis. J. Cell. Mol. Med. 2019, 23, 439–452. [Google Scholar] [CrossRef]
- Liu, H.; Shi, C.; Deng, Y. MALAT1 affects hypoxia-induced vascular endothelial cell injury and autophagy by regulating miR-19b-3p/HIF-1alpha axis. Mol. Cell. Biochem. 2020, 466, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Luo, D.; Li, X.; Li, Z.Q.; Yu, X.; Zhu, H.W. PVT1 knockdown inhibits autophagy and improves gemcitabine sensitivity by regulating the MiR-143/HIF-1alpha/VMP1 axis in pancreatic cancer. Pancreas 2021, 50, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Song, J.; Gao, Y.; Huang, S.; Dou, R.; Zhong, P.; Huang, G.; Han, L.; Zheng, J.; Zhang, X.; et al. Hypoxia-induced HIF-1alpha/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022, 52, 102312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, X.; Xue, X.; Li, L.; Hu, Y. A long noncoding RNA sensitizes genotoxic treatment by attenuating ATM activation and homologous recombination repair in cancers. PLoS Biol. 2020, 18, e3000666. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Chuang, L.L.; Tsai, M.H.; Chen, L.H.; Chuang, E.Y.; Lu, T.P.; Lai, L.C. Hypoxia-induced MALAT1 promotes the proliferation and migration of breast cancer cells by sponging MiR-3064-5p. Front. Oncol. 2021, 11, 658151. [Google Scholar] [CrossRef]
- Song, Y.; Jin, X.; Liu, Y.; Wang, S.; Bian, F.; Zhao, Q.; Shi, H.; Gao, Z. Long noncoding RNA ZFPM2-AS1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by regulating the miR-576-3p/HIF-1alpha axis. Anti-Cancer Drugs 2021, 32, 812–821. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Zhang, L.; Wang, Y.; Zhang, Y.; Li, J. LncRNA LINC00649 promotes the growth and metastasis of triple-negative breast cancer by maintaining the stability of HIF-1alpha through the NF90/NF45 complex. Cell Cycle 2022, 21, 1034–1047. [Google Scholar] [CrossRef]
- Yan, J.; Deng, Y.X.; Cai, Y.L.; Cong, W.D. LncRNA MIR17HG promotes the proliferation, migration, and invasion of retinoblastoma cells by up-regulating HIF-1alpha expression via sponging miR-155-5p. Kaohsiung J. Med. Sci. 2022, 38, 554–564. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, Z.; Liu, Y.; Zhao, J.; Lu, Q.; Guo, J.; Liu, X.; Huang, D.; Xu, Q. HIF-1alpha-activated TM4SF1-AS1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by enhancing TM4SF1 expression. Biochem. Biophys. Res. Commun. 2021, 566, 80–86. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Z.; Sui, Q.; Huang, Y.; Zhao, M.; Li, M.; Liang, J.; Lu, T.; Zhan, C.; Lin, Z.; et al. LncRNA FAM83A-AS1 facilitates tumor proliferation and the migration via the HIF-1alpha/glycolysis axis in lung adenocarcinoma. Int. J. Biol. Sci. 2022, 18, 522–535. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhu, Z.; Gong, J.; Dou, W. Knockdown of lncRNA PVT1 inhibits the proliferation and accelerates the apoptosis of colorectal cancer cells via the miR761/MAPK1 axis. Mol. Med. Rep. 2021, 24, 794. [Google Scholar] [CrossRef] [PubMed]
- Ibrahiem, A.T.; Makhdoom, A.K.; Alanazi, K.S.; Alanazi, A.M.; Mukhlef, A.M.; Elshafey, S.H.; Toraih, E.A.; Fawzy, M.S. Analysis of anti-apoptotic PVT1 oncogene and apoptosis-related proteins (p53, Bcl2, PD-1, and PD-L1) expression in thyroid carcinoma. J. Clin. Lab. Anal. 2022, 36, e24390. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peng, X.; Jin, H.; Liu, J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma. Gene 2019, 697, 94–102. [Google Scholar] [CrossRef]
- He, G.N.; Bao, N.R.; Wang, S.; Xi, M.; Zhang, T.H.; Chen, F.S. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Dev. Ther. 2021, 15, 3965–3978. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, L.; Liu, L.; Feng, Z.; Zeng, Q.; Shu, X.; Cao, Y.; Li, Z. A necroptosis-related lncRNA-based signature to predict prognosis and probe molecular characteristics of stomach adenocarcinoma. Front. Genet. 2022, 13, 833928. [Google Scholar] [CrossRef]
- Tesfaye, E.; Martinez-Terroba, E.; Bendor, J.; Winkler, L.; Olivero, C.; Chen, K.; Feldser, D.M.; Zamudio, J.R.; Dimitrova, N. The p53 transcriptional response across tumor types reveals core and senescence-specific signatures modulated by long noncoding RNAs. Proc. Natl. Acad. Sci. USA 2021, 118, e2025539118. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Fang, Y.; Xu, C. LncRNA PVT1 contributes to invasion and doxorubicin resistance of bladder cancer cells through promoting MDM2 expression and AURKB-mediated p53 ubiquitination. Environ. Toxicol. 2022, 37, 1495–1508. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, Z.; Cui, C. Knockdown of lncRNA HOTTIP inhibits retinoblastoma progression by modulating the miR-101-3p/STC1 axis. Technol. Cancer Res. Treat. 2021, 20, 1533033821997831. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Chen, X.; Liang, C.; Luo, P.; Qin, C.; Zhang, J. Long non-coding RNA HOTTIP affects renal cell carcinoma progression by regulating autophagy via the PI3K/Akt/Atg13 signaling pathway. J. Cancer Res. Clin. Oncol. 2019, 145, 573–588. [Google Scholar] [CrossRef]
- Liang, M.; Hu, K. Involvement of lncRNA-HOTTIP in the repair of ultraviolet light-induced DNA damage in spermatogenic cells. Mol. Cells 2019, 42, 794–803. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Jiang, Y.; Liu, K.; Ran, L.; Song, F. Identification of functional lncRNAs in gastric cancer by integrative analysis of GEO and TCGA data. J. Cell. Biochem. 2019, 120, 17898–17911. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Y.; Liu, J.F.; Luo, Y.; Xu, X.Z.; Bu, J. LncRNA HOTTIP facilitates cell proliferation, invasion, and migration in osteosarcoma by interaction with PTBP1 to promote KHSRP level. Cell Cycle 2021, 20, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Tao, C.; Li, Y.; Cao, S.; Yang, X. CRNDE silencing promotes apoptosis and enhances cisplatin sensitivity of colorectal carcinoma cells by inhibiting the Akt/mTORC1-mediated Warburg effect. Oncol. Lett. 2022, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, L.; Dai, X.; Li, T.; Yan, X.; Zhang, Y.; Xiao, H.; Shen, X.; Huang, G.; Xiang, W.; et al. LncRNA CRNDE promotes ATG4B-mediated autophagy and alleviates the sensitivity of sorafenib in hepatocellular carcinoma cells. Front. Cell Dev. Biol. 2021, 9, 687524. [Google Scholar] [CrossRef]
- Moran, M.; Cheng, X.; Shihabudeen Haider Ali, M.S.; Wase, N.; Nguyen, N.; Yang, W.; Zhang, C.; DiRusso, C.; Sun, X. Transcriptome analysis-identified long noncoding RNA CRNDE in maintaining endothelial cell proliferation, migration, and tube formation. Sci. Rep. 2019, 9, 19548. [Google Scholar] [CrossRef]
- Gao, H.; Song, X.; Kang, T.; Yan, B.; Feng, L.; Gao, L.; Ai, L.; Liu, X.; Yu, J.; Li, H. Long noncoding RNA CRNDE functions as a competing endogenous RNA to promote metastasis and oxaliplatin resistance by sponging miR-136 in colorectal cancer. OncoTargets Ther. 2017, 10, 205–216. [Google Scholar] [CrossRef]
- Gao, P.; Sun, D.; Guo, H.; Wu, Z.; Chen, J. LncRNA CCAT2 promotes proliferation and suppresses apoptosis of colorectal cancer cells. J. BUON 2020, 25, 1840–1846. [Google Scholar]
- Shi, J.; Guo, C.; Ma, J. CCAT2 enhances autophagy-related invasion and metastasis via regulating miR-4496 and ELAVL1 in hepatocellular carcinoma. J. Cell. Mol. Med. 2021, 25, 8985–8996. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, Y.; Guan, B.; Wang, Z.; Peng, D.; Chen, Z.; He, A.; He, S.; Gong, Y.; Li, X.; et al. Long non-coding RNA HNF1A-AS1 promotes proliferation and suppresses apoptosis of bladder cancer cells through upregulating Bcl-2. Oncotarget 2017, 8, 76656–76665. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, X.; Zhang, A.; Li, C.; Bai, J.; Dong, J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem. Biophys. Res. Commun. 2016, 473, 1268–1275. [Google Scholar] [CrossRef]
- Zhang, G.; An, X.; Zhao, H.; Zhang, Q.; Zhao, H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed. Pharmacother. 2018, 98, 594–599. [Google Scholar] [CrossRef]
- Sur, S.; Nakanishi, H.; Steele, R.; Ray, R.B. Depletion of PCAT-1 in head and neck cancer cells inhibits tumor growth and induces apoptosis by modulating c-Myc-AKT1-p38 MAPK signalling pathways. BMC Cancer 2019, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Fu, C.; Wang, C.; Duan, X.; Zou, W.; Zhao, T. Knockdown of long non-coding RNA PCAT1 in glioma stem cells promotes radiation sensitivity. Med. Mol. Morphol. 2019, 52, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Dong, N.; Huang, J.; Ye, B. Long non-coding RNA PCAT1 promotes cell migration and invasion in human laryngeal cancer by sponging miR-210-3p. J. BUON 2019, 24, 2429–2434. [Google Scholar]
- Meng, F.; Liu, J.; Lu, T.; Zang, L.; Wang, J.; He, Q.; Zhou, A. SNHG1 knockdown upregulates miR-376a and downregulates FOXK1/Snail axis to prevent tumor growth and metastasis in HCC. Mol. Ther. Oncolytics 2021, 21, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, X.; Xie, J.; Liu, D.; Geng, J.; Ye, L.; Yan, Y.; Yao, X.; Luo, M. Long noncoding RNA SNHG1 activates autophagy and promotes cell invasion in bladder cancer. Front. Oncol. 2021, 11, 660551. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Fujimura, M.; Yamashita, A. Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters. Sci. Rep. 2017, 7, 12390. [Google Scholar] [CrossRef]
- Xie, F.; Xiang, X.; Huang, Q.; Ran, P.; Yuan, Y.; Li, Q.; Qi, G.; Guo, X.; Xiao, C.; Zheng, S. Reciprocal control of lncRNA-BCAT1 and beta-catenin pathway reveals lncRNA-BCAT1 long non-coding RNA acts as a tumor suppressor in colorectal cancer. Oncotarget 2017, 8, 23628–23637. [Google Scholar] [CrossRef]
- Geng, F.; Jia, W.C.; Li, T.; Li, N.; Wei, W. Knockdown of lncRNA NEAT1 suppresses proliferation and migration, and induces apoptosis of cervical cancer cells by regulating the miR377/FGFR1 axis. Mol. Med. Rep. 2022, 25, 10. [Google Scholar] [CrossRef]
- Zhou, Y.; Sha, Z.; Yang, Y.; Wu, S.; Chen, H. lncRNA NEAT1 regulates gastric carcinoma cell proliferation, invasion and apoptosis via the miR500a3p/XBP1 axis. Mol. Med. Rep. 2021, 24, 503. [Google Scholar] [CrossRef]
- Wei, X.B.; Jiang, W.Q.; Zeng, J.H.; Huang, L.Q.; Ding, H.G.; Jing, Y.W.; Han, Y.L.; Li, Y.C.; Chen, S.L. Exosome-derived lncRNA NEAT1 exacerbates sepsis-associated encephalopathy by promoting ferroptosis through regulating miR-9-5p/TFRC and GOT1 axis. Mol. Neurobiol. 2022, 59, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Gallo Cantafio, M.E.; et al. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia 2020, 34, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Xia, W.; Chen, D.; Ye, Y.; Hu, T.; Li, S.; Hou, M. Exosomal LncRNA-NEAT1 derived from MIF-treated mesenchymal stem cells protected against doxorubicin-induced cardiac senescence through sponging miR-221-3p. J. Nanobiotechnol. 2020, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Jiang, L.; Han, Z.; Wang, Z. LncRNA CERNA2 is an independent predictor for clinical prognosis and is related to tumor development in gastric cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 5783–5791. [Google Scholar]
- Wang, M.; Ouyang, J.; Li, H. CERNA2: A predictor for clinical progression and poor prognosis in cervical carcinoma. J. Cell. Biochem. 2019, 120, 11216–11221. [Google Scholar] [CrossRef]
- Huang, W.; Su, G.; Huang, X.; Zou, A.; Wu, J.; Yang, Y.; Zhu, Y.; Liang, S.; Li, D.; Ma, F.; et al. Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle 2019, 18, 69–83. [Google Scholar] [CrossRef]
- Xu, G.; Yang, M.; Wang, Q.; Zhao, L.; Zhu, S.; Zhu, L.; Xu, T.; Cao, R.; Li, C.; Liu, Q.; et al. A novel prognostic prediction model for colorectal cancer based on nine autophagy-related long noncoding RNAs. Front. Oncol. 2021, 11, 613949. [Google Scholar] [CrossRef]
- Fang, C.; Liu, S.; Feng, K.; Huang, C.; Zhang, Y.; Wang, J.; Lin, H.; Wang, J.; Zhong, C. Ferroptosis-related lncRNA signature predicts the prognosis and immune microenvironment of hepatocellular carcinoma. Sci. Rep. 2022, 12, 6642. [Google Scholar] [CrossRef]
- Han, L.; Sun, Y.; Sun, D. LncRNA PCAT6 as a predictor of poor colorectal cancer patient prognosis: A TCGA dataset analysis. Res. Sq. 2021; preprint. [Google Scholar]
- Wan, L.; Zhang, L.; Fan, K.; Cheng, Z.X.; Sun, Q.C.; Wang, J.J. Knockdown of long noncoding RNA PCAT6 inhibits proliferation and invasion in lung cancer cells. Oncol. Res. 2016, 24, 161–170. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Lin, X.; Hong, Y.; Wang, X.; Zheng, C.; Wu, Z.; Hong, Y.; Lv, Y. Long non-coding RNA TUG1 sponges microRNA-381-3p to facilitate cell viability and attenuate apoptosis in cervical cancer by elevating MDM2 expression. Life Sci. 2021, 267, 118902. [Google Scholar] [CrossRef]
- Xia, C.; Li, Q.; Cheng, X.; Wu, T.; Gao, P.; Gu, Y. Insulin-like growth factor 2 mRNA-binding protein 2-stabilized long non-coding RNA Taurine up-regulated gene 1 (TUG1) promotes cisplatin-resistance of colorectal cancer via modulating autophagy. Bioengineered 2022, 13, 2450–2469. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Wu, L.; Li, Z.; Ma, X.; Zhao, S.; Zhao, D.; Qin, G. LncRNA TUG1 ameliorates diabetic nephropathy via inhibition of PU.1/RTN1 signaling pathway. J. Leukoc. Biol. 2022, 111, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, J.; Bi, Q.; Wang, W. Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4 mediated ferroptosis. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Wang, Y.; Peng, S.; Guo, W.; Li, F.; Xu, S. P53 and taurine upregulated gene 1 promotes the repair of the deoxyribonucleic acid damage induced by bupivacaine in murine primary sensory neurons. Bioengineered 2022, 13, 7439–7456. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Zhang, Y.; Wu, Z.; He, D.; Li, X.; Wang, Z. Long non-coding RNA TUG1 enhances chemosensitivity in non-small cell lung cancer by impairing microRNA-221-dependent PTEN inhibition. Aging 2019, 11, 7553–7569. [Google Scholar] [CrossRef]
- Yao, Q.; Li, Y.; Pei, Y.; Xie, B. Long non-coding RNA taurine up regulated 1 promotes osteosarcoma cell proliferation and invasion through upregulating Ezrin expression as a competing endogenous RNA of micro RNA-377-3p. Bioengineered 2022, 13, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, Y.; Wei, H.Y.; Lv, K.Z.; Fu, P.F. Large intergenic non-coding RNA-ROR reverses gemcitabine-induced autophagy and apoptosis in breast cancer cells. Oncotarget 2016, 7, 59604–59617. [Google Scholar] [CrossRef]
- Li, X.; Zuo, C.; Sun, D.; Zhao, T.; Zhang, Z. Arsenite increases linc-ROR in human bronchial epithelial cells that can be inhibited by antioxidant factors. Biol. Trace Elem. Res. 2020, 198, 131–141. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Liu, Y.; Xu, W.; Ling, C.; Li, Y.; Liu, J.; Chen, M.; Zhang, Y.; Chen, B.; et al. Linc-RoR promotes proliferation, migration, and invasion via the Hippo/YAP pathway in pancreatic cancer cells. J. Cell. Biochem. 2020, 121, 632–641. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.H.; Zhang, J.; Zhou, Y.X.; Ying, J.; Wu, G.Q.; Qian, J.H. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem. Biophys. Res. Commun. 2015, 468, 561–567. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, K.; Tang, Y.; Luan, X.; Zheng, X.; Lu, X.; Mao, J.; Hu, L.; Zhang, S.; Zhang, X.; et al. LncRNA-HOTAIR activates autophagy and promotes the imatinib resistance of gastrointestinal stromal tumor cells through a mechanism involving the miR-130a/ATG2B pathway. Cell Death Dis. 2021, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiao, H.; Guo, S.; Li, J.; Wang, Y.; Chen, J.; Lou, G. Long noncoding RNA HOTAIR knockdown inhibits autophagy and epithelial-mesenchymal transition through the Wnt signaling pathway in radioresistant human cervical cancer HeLa cells. J. Cell. Physiol. 2019, 234, 3478–3489. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.L.; Gao, W.Y.; Liao, S.J.; Yu, T.; Shi, Q.; Yu, S.Z.; Cai, Y.F. Paeonol inhibits the progression of intracerebral haemorrhage by mediating the HOTAIR/UPF1/ACSL4 axis. ASN Neuro 2021, 13, 17590914211010647. [Google Scholar] [CrossRef] [PubMed]
- Ozes, A.R.; Miller, D.F.; Ozes, O.N.; Fang, F.; Liu, Y.; Matei, D.; Huang, T.; Nephew, K.P. NF-kappaB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016, 35, 5350–5361. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Chen, J.; Zhang, B.R.; Lu, S.J.; Wang, F.; Peng, L.; Dai, J.H.; Sun, Y.Z. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Die Pharm. 2018, 73, 402–407. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, X.F.; Wang, M.; Zhang, P.P.; Zhang, F.; Zhang, J.J. Long non-coding RNA UCA1 promotes autophagy by targeting miR-96-5p in acute myeloid leukaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 877–885. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Q.; Zhang, B.F.; Liu, X.P.; Yang, S.; Jiang, H. Long noncoding RNA UCA1 inhibits ischaemia/reperfusion injury induced cardiomyocytes apoptosis via suppression of endoplasmic reticulum stress. Genes Genom. 2019, 41, 803–810. [Google Scholar] [CrossRef]
- Teng, B.; Feng, T.; Li, W.; Wang, Z. Abnormal expression of lncRNA UCA1 disturbed cell apoptosis through mediating mitochondrial dynamics in PDAC. Neoplasma 2021, 68, 334–341. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, Q.; Chen, L.; Zhao, D.; Tang, J.; Xu, J.; He, Z. LncRNA UCA1/miR-182-5p/MGMT axis modulates glioma cell sensitivity to temozolomide through MGMT-related DNA damage pathways. Hum. Pathol. 2022, 123, 59–73. [Google Scholar] [CrossRef]
- Kumar, P.P.; Emechebe, U.; Smith, R.; Franklin, S.; Moore, B.; Yandell, M.; Lessnick, S.L.; Moon, A.M. Coordinated control of senescence by lncRNA and a novel T-box3 co-repressor complex. eLife 2014, 3, e02805. [Google Scholar] [CrossRef]
- Yang, G.; Tian, Y.; Li, C.; Xia, J.; Qi, Y.; Yao, W.; Hao, C. LncRNA UCA1 regulates silicosis-related lung epithelial cell-to-mesenchymal transition through competitive adsorption of miR-204-5p. Toxicol. Appl. Pharmacol. 2022, 441, 115977. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Deng, G.; Xiao, S.; Li, F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates il-1beta-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Investig. 2021, 51, 1–18. [Google Scholar] [CrossRef]
- Han, Z.; He, J.; Zou, M.; Chen, W.; Lv, Y.; Li, Y. Small interfering RNA target for long noncoding RNA PCGEM1 increases the sensitivity of LNCaP cells to baicalein. Anat. Rec. 2020, 303, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, J.; Liu, L. The long noncoding RNA PCGEM1 promotes cell proliferation, migration and invasion via targeting the miR-182/FBXW11 axis in cervical cancer. Cancer Cell Int. 2019, 19, 304. [Google Scholar] [CrossRef]
- Shen, P.; Cheng, Y. Long noncoding RNA lncARSR confers resistance to Adriamycin and promotes osteosarcoma progression. Cell Death Dis. 2020, 11, 362. [Google Scholar] [CrossRef]
- Shu, C.; Yan, D.; Mo, Y.; Gu, J.; Shah, N.; He, J. Long noncoding RNA lncARSR promotes epithelial ovarian cancer cell proliferation and invasion by association with HuR and miR-200 family. Am. J. Cancer Res. 2018, 8, 981–992. [Google Scholar]
- Zhang, W.; Yuan, W.; Song, J.; Wang, S.; Gu, X. LncRna CPS1-IT1 suppresses cell proliferation, invasion and metastasis in colorectal cancer. Cell. Physiol. Biochem. 2017, 44, 567–580. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, W.; Song, J.; Wang, S.; Gu, X. LncRNA CPS1-IT1 suppresses EMT and metastasis of colorectal cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1alpha. Biochimie 2018, 144, 21–27. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Liang, J.; Jin, M.; Lu, A. LncRNA CPS1-IT1 serves as anti-oncogenic role in glioma. Biomed. Pharmacother. 2019, 118, 109277. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Z.; Feng, L.; Yang, Y.; Tan, C.; Shi, Q.; Lian, M.; He, S.; Ma, H.; Fang, J. LncRNA MIR31HG targets HIF1A and P21 to facilitate head and neck cancer cell proliferation and tumorigenesis by promoting cell-cycle progression. Mol. Cancer 2018, 17, 162. [Google Scholar] [CrossRef]
- Montes, M.; Nielsen, M.M.; Maglieri, G.; Jacobsen, A.; Hojfeldt, J.; Agrawal-Singh, S.; Hansen, K.; Helin, K.; van de Werken, H.J.G.; Pedersen, J.S.; et al. The lncRNA MIR31HG regulates p16(INK4A) expression to modulate senescence. Nat. Commun. 2015, 6, 6967. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Li, H.; Huo, W.; Zhu, H.; Xu, C.; Zang, R.; Lv, W.; Xia, Y.; Tang, W. Aberrant expression of LncRNA-MIR31HG regulates cell migration and proliferation by affecting miR-31 and miR-31* in Hirschsprung’s disease. J. Cell. Biochem. 2018, 119, 8195–8203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-L.; Chen, R.-P.; Zhou, X.-T.; Zhan, H.-L.; Hu, M.-M.; Liu, B.; Wu, G.-D.; Wu, L.-F. Long non-coding RNA MEG3 induces cell apoptosis in esophageal cancer through endoplasmic reticulum stress. Oncol. Rep. 2017, 37, 3093–3099. [Google Scholar] [CrossRef]
- Xiu, Y.L.; Sun, K.X.; Chen, X.; Chen, S.; Zhao, Y.; Guo, Q.G.; Zong, Z.H. Upregulation of the lncRNA Meg3 induces autophagy to inhibit tumorigenesis and progression of epithelial ovarian carcinoma by regulating activity of ATG3. Oncotarget 2017, 8, 31714–31725. [Google Scholar] [CrossRef]
- Deng, Q.; Wen, R.; Liu, S.; Chen, X.; Song, S.; Li, X.; Su, Z.; Wang, C. Increased long noncoding RNA maternally expressed gene 3 contributes to podocyte injury induced by high glucose through regulation of mitochondrial fission. Cell Death Dis. 2020, 11, 814. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Xia, P.; Zhang, F.; Li, L.; Wang, E.; Guo, Q.; Ye, Z. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur. J. Histochem. 2021, 65, 3224. [Google Scholar] [CrossRef] [PubMed]
- Balusu, S.; Horré, K.; Thrupp, N.; Snellinx, A.; Serneels, L.; Chrysidou, I.; Arranz, A.M.; Sierksma, A.; Simrén, J.; Karikari, T.K. Long noncoding RNA MEG3 activates neuronal necroptosis in Alzheimer’s disease. BioRxiv, 2022; preprint. [Google Scholar] [CrossRef]
- Shihabudeen Haider Ali, M.S.; Cheng, X.; Moran, M.; Haemmig, S.; Naldrett, M.J.; Alvarez, S.; Feinberg, M.W.; Sun, X. LncRNA Meg3 protects endothelial function by regulating the DNA damage response. Nucleic Acids Res. 2019, 47, 1505–1522. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Y.J.; Li, D.J.; Li, P.; Wang, J.Y.; Diao, Y.P.; Ye, G.D.; Li, Y.F. Long noncoding RNA MEG3 prevents vascular endothelial cell senescence by impairing miR-128-dependent Girdin downregulation. Am. J. Physiol. Cell Physiol. 2019, 316, C830–C843. [Google Scholar] [CrossRef]
- Wang, G.; Ye, Q.; Ning, S.; Yang, Z.; Chen, Y.; Zhang, L.; Huang, Y.; Xie, F.; Cheng, X.; Chi, J.; et al. LncRNA MEG3 promotes endoplasmic reticulum stress and suppresses proliferation and invasion of colorectal carcinoma cells through the MEG3/miR-103a-3p/PDHB ceRNA pathway. Neoplasma 2021, 68, 362–374. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Tang, S.; Yan, J.; Chen, H.; Li, D.; Yan, X. Quercetin regulates inflammation, oxidative stress, apoptosis, and mitochondrial structure and function in H9C2 cells by promoting PVT1 expression. Acta Histochem. 2021, 123, 151819. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Tsuchiya, H.; Kitagawa, Y.; Tanino, T.; Yoshida, K.; Uchida, N.; Shiota, G. NEAT1 confers radioresistance to hepatocellular carcinoma cells by inducing autophagy through GABARAP. Int. J. Mol. Sci. 2022, 23, 711. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Paez, J.; Sellers, W.R. PI3K/PTEN/Akt Pathway. In Signal Transduction in Cancer; Frank, D.A., Ed.; Springer: Boston, MA, USA, 2004; pp. 145–167. [Google Scholar] [CrossRef]
- Chang, T.M.; Shan, Y.S.; Chu, P.Y.; Jiang, S.S.; Hung, W.C.; Chen, Y.L.; Tu, H.C.; Lin, H.Y.; Tsai, H.J.; Chen, L.T. The regulatory role of aberrant Phosphatase and Tensin Homologue and Liver Kinase B1 on AKT/mTOR/c-Myc axis in pancreatic neuroendocrine tumors. Oncotarget 2017, 8, 98068–98083. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics 2017, 7, 3842–3855. [Google Scholar] [CrossRef]
- Tu, F.L.; Guo, X.Q.; Wu, H.X.; He, Z.Y.; Wang, F.; Sun, A.J.; Dai, X.D. Circ-0001313/miRNA-510-5p/AKT2 axis promotes the development and progression of colon cancer. Am. J. Transl. Res. 2020, 12, 281–291. [Google Scholar] [PubMed]
- Yue, B.; Wang, J.; Ru, W.; Wu, J.; Cao, X.; Yang, H.; Huang, Y.; Lan, X.; Lei, C.; Huang, B.; et al. The circular RNA circHUWE1 sponges the miR-29b-AKT3 axis to regulate myoblast development. Mol. Ther. Nucleic Acids 2020, 19, 1086–1097. [Google Scholar] [CrossRef]
- Peng, Y.K.; Pu, K.; Su, H.X.; Zhang, J.; Zheng, Y.; Ji, R.; Guo, Q.H.; Wang, Y.P.; Guan, Q.L.; Zhou, Y.N. Circular RNA hsa_circ_0010882 promotes the progression of gastric cancer via regulation of the PI3K/Akt/mTOR signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1142–1151. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Taheri, M.; Jamali, E. CircITCH: A circular RNA with eminent roles in the carcinogenesis. Front. Oncol. 2021, 11, 774979. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Lu, J.; Li, L. Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct. Target. Ther. 2021, 6, 400. [Google Scholar] [CrossRef]
- Shi, W.; Wang, F. circ_AKT3 knockdown suppresses cisplatin resistance in gastric cancer. Open Med. 2022, 17, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xie, H.; Wu, W.; Wen, X.; Zeng, Z.; Shi, Y. CircRNA PIP5K1A promotes the progression of glioma through upregulation of the TCF12/PI3K/AKT pathway by sponging miR-515-5p. Cancer Cell Int. 2021, 21, 27. [Google Scholar] [CrossRef]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein represses cervical cancer cell growth, cell cycle progression and promotes apoptosis via blocking AKT/mTOR pathway by the regulation of circHIAT1/miR-19a-3p axis. OncoTargets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, G.; Huang, C.; Zhao, X. Upregulation of circRNA_100395 sponges miR-142-3p to inhibit gastric cancer progression by targeting the PI3K/AKT axis. Oncol. Lett. 2021, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, C.; Zhang, X.; Sun, L.; Li, J.; Zuo, J. CircRNA HIPK3 promotes the progression of oral squamous cell carcinoma through upregulation of the NUPR1/PI3K/AKT pathway by sponging miR-637. Ann. Transl. Med. 2021, 9, 860. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Peng, F.; Yin, S.; Liang, H.; Su, J.; Li, L.; Yang, A.; Liu, H.; Yang, C.; et al. Downregulation of hsa_circRNA_0001400 helps to promote cell apoptosis through disruption of the circRNA_0001400-miR-326 sponge in cervical cancer cells. Front. Genet. 2021, 12, 779195. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, B.; Cheng, H.; Wu, J.; Chen, Q.; Duan, Y.; Zhang, P.; Zheng, D.; Lin, G.; Zhuo, Y. Knockdown circ_0040414 inhibits inflammation, apoptosis and promotes the proliferation of cardiomyocytes via miR-186-5p/PTEN/AKT axis in chronic heart failure. Cell Biol. Int. 2021, 45, 2304–2315. [Google Scholar] [CrossRef]
- Ling, Z.; Fang, Z.G.; Wu, J.Y.; Liu, J.J. The depletion of Circ-PRKDC enhances autophagy and apoptosis in T-cell acute lymphoblastic leukemia via microRNA-653-5p/Reelin mediation of the PI3K/AKT/mTOR signaling pathway. Kaohsiung J. Med. Sci. 2021, 37, 392–401. [Google Scholar] [CrossRef]
- Gao, L.; Dou, Z.C.; Ren, W.H.; Li, S.M.; Liang, X.; Zhi, K.Q. CircCDR1as upregulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(1/2)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 2019, 10, 745. [Google Scholar] [CrossRef]
- Yang, T.; Shen, P.; Chen, Q.; Wu, P.; Yuan, H.; Ge, W.; Meng, L.; Huang, X.; Fu, Y.; Zhang, Y.; et al. FUS-induced circRHOBTB3 facilitates cell proliferation via miR-600/NACC1 mediated autophagy response in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 261. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tong, F.; Ye, Y.; Hu, T.; Xu, L.; Zhang, L.; Zhu, J.; Pang, Z. Hsa_circRNA_103124 upregulation in Crohn’s disease promotes cell proliferation and inhibits autophagy by regulating the Hsa-miR-650/AKT2 signaling pathway. Front. Genet. 2021, 12, 753161. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, S.; Ding, P.; Chang, S.; Sang, M. Circular RNA ciRS-7 inhibits autophagy of ESCC cells by functioning as miR-1299 sponge to target EGFR signaling. J. Cell. Biochem. 2020, 121, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, H.; Niu, M.; Zheng, X.; Zhang, Y.; Xue, X.; Bo, Y.; Guan, X.; Li, Z.; Guo, Y.; et al. circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol. Cancer 2020, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, H.; Zhang, L. Identification of the tumorsuppressive function of circular RNA FOXO3 in nonsmall cell lung cancer through sponging miR155. Mol. Med. Rep. 2018, 17, 7692–7700. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Zhao, H.; Yan, B.; Zhang, D.; Liang, J. S100A4 regulates motility and invasiveness of human esophageal squamous cell carcinoma through modulating the AKT/Slug signal pathway. Dis. Esophagus 2012, 25, 731–739. [Google Scholar] [CrossRef]
- Yao, J.; Qian, K.; Chen, C.; Liu, X.; Yu, D.; Yan, X.; Liu, T.; Li, S. ZNF139/circZNF139 promotes cell proliferation, migration and invasion via activation of PI3K/AKT pathway in bladder cancer. Aging 2020, 12, 9915–9934. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Hou, X.; Zhang, G.; Huang, Y. Circular RNA circ_0002984 promotes cell proliferation and migration by regulating miR-181b-5p/vascular endothelial growth factor axis and PI3K-AKT signaling pathway in oxidized low-density lipoprotein-treated vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2022, 79, 501–511. [Google Scholar] [CrossRef]
- Chen, L.; Zhuo, D.; Yuan, H. Circ_100395 impedes malignancy and glycolysis in papillary thyroid cancer: Involvement of PI3K/AKT/mTOR signaling pathway. Immunol. Lett. 2022, 246, 10–17. [Google Scholar] [CrossRef]
- Ou, R.; Mo, L.; Tang, H.; Leng, S.; Zhu, H.; Zhao, L.; Ren, Y.; Xu, Y. circRNA-AKT1 sequesters miR-942-5p to upregulate AKT1 and promote cervical cancer progression. Mol. Ther. Nucleic Acids 2020, 20, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Wang, G. Hsa_circ_0051079 functions as an oncogene by regulating miR-26a-5p/TGF-β1 in osteosarcoma. Cell Biosci. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Li, J.F. Circular RNA hsa_circ_0001564 regulates osteosarcoma proliferation and apoptosis by acting miRNA sponge. Biochem. Biophys. Res. Commun. 2018, 495, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kang, K.; Chen, S.; Wang, S.; Zhang, J.; Zhang, X.; Chen, Z. Circular RNA circ_0017247 promotes melanoma migration and invasion via targeting miR-145. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1932–1938. [Google Scholar]
- Li, C.; Wang, Y.; Chen, K. Circ_0017247 accelerates epithelial mesenchymal transition in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 256–263. [Google Scholar]
- Luo, Y.; Liu, F.; Guo, J.; Gui, R. Upregulation of circ_0000199 in circulating exosomes is associated with survival outcome in OSCC. Sci. Rep. 2020, 10, 13739. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, Q.; Gu, Y.; Ma, J.; McGrath, M.; Qiao, F.; Chen, B.; Song, C.; Ge, Z. Circular RNA PVT1 expression and its roles in acute lymphoblastic leukemia. Epigenomics 2018, 10, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yu, J.; Guo, L.; Ma, H. Circular RNA circRHOT1 contributes to pathogenesis of non-small cell lung cancer by epigenetically enhancing C-MYC expression through recruiting KAT5. Aging 2021, 13, 20372–20382. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Zou, H.; Zheng, Y.W.; Wang, K.P. circZFR promotes cell proliferation and migration by regulating miR-511/AKT1 axis in hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 1446–1455. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, X.F.; Ling, X.Y.; Yang, Q.; Yu, Y.; Xiao, J.; Wang, Z.N. CircRNA_010763 promotes growth and invasion of lung cancer through serving as a molecular sponge of miR-715 to induce c-Myc expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7310–7319. [Google Scholar] [CrossRef]
- Huang, W.; Song, W.; Jiang, Y.; Chen, L.; Lu, H. c-Myc-induced circ-NOTCH1 promotes aggressive phenotypes of nasopharyngeal carcinoma cells by regulating the miR-34c-5p/c-Myc axis. Cell Biol. Int. 2021, 45, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, Q.; Cheng, X.; Wang, S.; Wu, R.; Leng, X.; Shao, L. miR-449c-5p availability is antagonized by circ-NOTCH1 for MYC-induced NOTCH1 upregulation as well as tumor metastasis and stemness in gastric cancer. J. Cell. Biochem. 2020, 121, 4052–4063. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Chen, Y.; Fu, M.; Zang, X.; Cang, M.; Niu, Y.; Zhang, W.; Zhang, Y.; Mao, Z.; Shao, M.; et al. Circular RNA CCDC66 promotes gastric cancer progression by regulating c-Myc and TGF-beta signaling pathways. J. Cancer 2020, 11, 2759–2768. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Tao, D.; Xie, F.; Liu, F.; Gu, C.; Wang, M.; Wang, L.; Jiang, G.; Wang, Z.; et al. CircCDYL inhibits the expression of C-MYC to suppress cell growth and migration in bladder cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shu, L.; Zhang, H.; Zhu, X.; Huang, G.; Xu, J. Circ_ZNF512-mediated miR-181d-5p inhibition limits cardiomyocyte autophagy and promotes myocardial ischemia/reperfusion injury through an EGR1/mTORC1/TFEB-based mechanism. J. Med. Chem. 2022, 65, 1808–1821. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, C.; Wen, X.; Liu, W.; Huang, X.; Li, Y.; Zang, J.; Weng, Z.; Lu, D.; Tsang, C.K.; et al. Circular RNA circ-FoxO3 attenuates blood-brain barrier damage by inducing autophagy during ischemia/reperfusion. Mol. Ther. 2022, 30, 1275–1287. [Google Scholar] [CrossRef]
- Li, Y.; Qin, G.; Du, J.; Yue, P.; Zhang, Y.; Hou, N. circRNA LDLRAD3 enhances the malignant behaviors of NSCLC Cells via the miR-20a-5p-SLC7A5 axis activating the mTORC1 signaling pathway. J. Healthc. Eng. 2022, 2022, 2373580. [Google Scholar] [CrossRef]
- Wei, H.; Cao, C.; Wei, X.; Meng, M.; Wu, B.; Meng, L.; Wei, X.; Gu, S.; Li, H. Circular RNA circVEGFC accelerates high glucose-induced vascular endothelial cells apoptosis through miR-338-3p/HIF-1alpha/VEGFA axis. Aging 2020, 12, 14365–14375. [Google Scholar] [CrossRef]
- Dang, R.Y.; Liu, F.L.; Li, Y. Circular RNA hsa_circ_0010729 regulates vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1alpha axis. Biochem. Biophys. Res. Commun. 2017, 490, 104–110. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Huang, H.L.; Huang, R.Q.; Tian, C.; Zhu, H.B.; Dai, Y.H.; Li, Z.Y. circRNA_100859 functions as an oncogene in colon cancer by sponging the miR-217-HIF-1alpha pathway. Aging 2020, 12, 13338–13353. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Chen, J.; Wei, D.; Yu, F.; Sun, J. circAGFG1 sponges miR-28-5p to promote non-small-cell lung cancer progression through modulating HIF-1alpha level. Open Med. 2021, 16, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, J.; Peng, B.; Cai, P.; Zhao, B.; Chen, Y.; Zhu, H. CircASXL1 knockdown restrains hypoxia-induced DDP resistance and NSCLC progression by sponging miR-206. Cancer Manag. Res. 2021, 13, 5077–5089. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Fu, Q.; Liu, C.; Zhang, X.; Jia, P.; Xia, P.; Liu, P.; Liao, S.; Qin, T.; Zhang, H. Emerging roles of hsa-circ-0046600 targeting the miR-640/HIF-1alpha signalling pathway in the progression of HCC. OncoTargets Ther. 2019, 12, 9291–9302. [Google Scholar] [CrossRef]
- Wang, J.; Huang, K.; Shi, L.; Zhang, Q.; Zhang, S. CircPVT1 promoted the progression of breast cancer by regulating MiR-29a-3p-Mediated AGR2-HIF-1alpha pathway. Cancer Manag. Res. 2020, 12, 11477–11490. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Du, B.; Zhan, Y.; Wang, K.; Wang, X.; Chen, B.; Wei, X.; Xiao, J. Antitumor effects of circ-EPHB4 in hepatocellular carcinoma via inhibition of HIF-1alpha. Mol. Carcinog. 2019, 58, 875–886. [Google Scholar] [CrossRef]
- Lin, X.; Sun, R.; Zhao, X.; Zhu, D.; Zhao, X.; Gu, Q.; Dong, X.; Zhang, D.; Zhang, Y.; Li, Y.; et al. C-myc overexpression drives melanoma metastasis by promoting vasculogenic mimicry via c-myc/snail/Bax signaling. J. Mol. Med. 2017, 95, 53–67. [Google Scholar] [CrossRef]
- Bai, Y.; Rao, H.; Chen, W.; Luo, X.; Tong, C.; Qi, H. Profiles of circular RNAs in human placenta and their potential roles related to preeclampsia. Biol. Reprod. 2018, 98, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Pu, Z.; Lu, J.; Yang, X. Emerging roles of circular RNAs in vascular smooth muscle cell dysfunction. Front. Genet. 2021, 12, 749296. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Shang, H.; Chen, X.; Yang, S.; Qu, Y.; Ding, J.; Li, X. Circular RNA circ_0000950 promotes neuron apoptosis, suppresses neurite outgrowth and elevates inflammatory cytokines levels via directly sponging miR-103 in Alzheimer’s disease. Cell Cycle 2019, 18, 2197–2214. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, Z. The emerging role of circular RNAs in Alzheimer’s disease and Parkinson’s disease. Front. Aging Neurosci. 2021, 13, 691512. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Li, C.; Zhang, H.; Liu, Y.; Han, D.; Li, Y.; Li, Z.; Luo, D.; Zhang, N.; et al. CircHIF1A regulated by FUS accelerates triple-negative breast cancer progression by modulating NFIB expression and translocation. Oncogene 2021, 40, 2756–2771. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Li, P.B.; Guo, S.F.; Yang, Q.S.; Chen, Z.X.; Wang, D.; Shi, S.B. circRNA_0006393 promotes osteogenesis in glucocorticoidinduced osteoporosis by sponging miR1455p and upregulating FOXO1. Mol. Med. Rep. 2019, 20, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
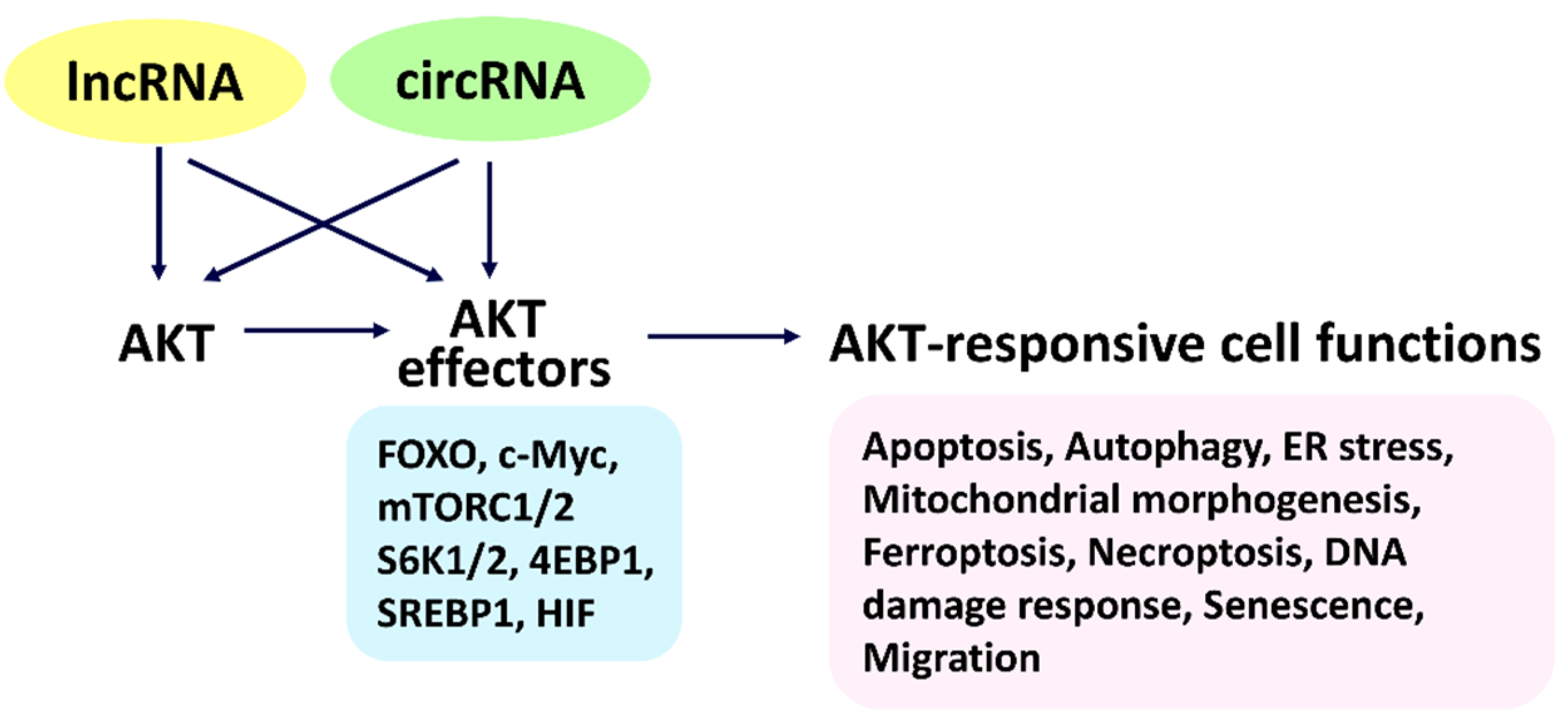


| AKT1 | AKT2 | AKT3 | ||
|---|---|---|---|---|
| Upregulate | ENST00113 MALAT1 CDKN2B-AS1 HULC LUCAT1 AFAP1-AS1 | LINC00462 LOXL1-AS1 AB073614 H19 SPRY4-IT1 | lncRNA-p3134 | FEZF1-AS1 |
| Downregulate | GAS5 RP11-708H21.4 FOXD2-AS1 LINC00312 | - | - | |
| Cell Functions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AKT1/2/3-Targeting lncRNAs | Apoptosis | Autophagy | ER Stress | Mitochondrial Morphogenesis | Ferroptosis | Necroptosis | DNA Damage Response | Senescence | Migration | |
| AKT1 | ENST00113 | [62] | [63] | ○ | ○ | ○ | ○ | ○ | ○ | [62] |
| MALAT1 | [64] | [65] | [66] | [64] | ○ | ○ | ○ | [67] | [68] | |
| GAS5 | [69,70] | [71,72] | [73] | ○ | [74] | [75] | [76] | [77] | [78] | |
| CDKN2B-AS1 | [79] | [80] | [81] | ○ | ○ | ○ | [82] | [79,82] | [83] | |
| HULC | [84,85] | [86] | ○ | ○ | ○ | ○ | [87] | [88] | [89,90] | |
| LUCAT1 | [91] | [91] | ○ | ○ | [92] | [93] | [94] | ○ | [91] | |
| RP11-708H21.4 | [95] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [95] | |
| AFAP1-AS1 | [96] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [96] | |
| LINC00462 | [97] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [98] | |
| LOXL1-AS1 | [99] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [99] | |
| FOXD2-AS1 | [100] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [100] | |
| AB073614 | [101] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [102] | |
| H19 | [103] | [104] | [105] | [106] | [107] | [105] | [108] | [109] | [104] | |
| SPRY4-IT1 | [110] | ○ | ○ | ○ | ○ | ○ | [111] | ○ | [112] | |
| LINC00312 | [113] | ○ | ○ | ○ | ○ | ○ | [53] | ○ | [114] | |
| AKT2 | lncRNA-p3134 | [115] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| AKT3 | FEZF1-AS1 | [116] | [117] | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Cell Functions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Apoptosis | Autophagy | ER Stress | Mitochondrial Morphogenesis | Ferroptosis | Necroptosis | DNA Damage Response | Senescence | Migration |
| FOXO c-Myc mTORC1 mTORC2 SREBP1 HIF | c-Myc mTORC1 SREBP1 HIF | c-Myc | ○ | FOXO c-Myc HIF | c-Myc | c-Myc HIF | c-My cmTORC1 | FOXO c-Myc mTORC1 |
| AKT Effectors | |||||||
|---|---|---|---|---|---|---|---|
| LncRNAs | c-Myc | mTOR | S6K1 | SREBP1 | HIF | ||
| Upregulation | PVT1 HOTTIP CRNDE CCAT2 HNF1A-AS1 SNHG1 NEAT1H19 | CERNA2 TUG1 PCAT1 LINC-ROR FILNC1 THORLNC | MALAT1 ENST00113 HOTAIR PVT1 H19 | CRNDE HULC lncRNA-p3134 UCA1 | HOTAIR PCGEM1 | LNCARSR | HOTAIR RAB4B-EGLN2 MEG3 RPL13AP23 |
| Downregulation | HULC PCAT1 lncRNA-BCAT1 PCAT6 | UCA1 RP11-708H21.4 | GAS5 HOTAIR | RP11-708H21.4 | CPS1-IT1 MIR31HG MALAT1 | ||
| Cell Functions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AKT Effectors | lncRNAs | Apoptosis | Autophagy | ER Stress | Mitochondrial Morphogenesis | Ferroptosis | Necroptosis | DNA Damage Response | Senescence | Migration |
| c-Myc | PVT1 | [171,172] | [173] | ○ | ○ | [174] | [175] | [133] | [176] | [177] |
| HOTTIP | [178] | [179] | ○ | ○ | ○ | ○ | [180] | [181] | [179,182] | |
| CRNDE | [183] | [184] | [185] | ○ | ○ | ○ | [186] | ○ | [185] | |
| HULC | [84,85] | [86] | ○ | ○ | ○ | ○ | [87] | [88] | [89,90] | |
| CCAT2 | [187] | [188] | ○ | ○ | ○ | ○ | ○ | ○ | [188] | |
| HNF1A-AS1 | [189] | [190] | ○ | ○ | ○ | ○ | ○ | ○ | [191] | |
| PCAT1 | [192] | ○ | ○ | ○ | [131] | ○ | [193] | ○ | [194] | |
| SNHG1 | [195] | [196] | [197] | ○ | ○ | ○ | ○ | ○ | [195] | |
| lncRNA-BCAT1 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [198] | |
| NEAT1 | [199,200] | [199] | [200] | ○ | [201] | ○ | [202] | [203] | [199,200] | |
| H19 | [103] | [104] | [105] | [106] | [107] | [105] | [108] | [109] | [104] | |
| CERNA2 | [204] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [205] | |
| PCAT6 | [206] | [207] | ○ | [208]. | ○ | ○ | [209] | [209] | [210] | |
| TUG1 | [211] | [212] | [213] | ○ | [214] | ○ | [215] | [216] | [217] | |
| LINC-ROR | [218] | [218] | ○ | ○ | ○ | ○ | [219] | ○ | [220] | |
| FILNC1 | [118] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| THORLNC | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| mTORC1/2 | MALAT1 | [64] | [65] | [66] | [64] | ○ | ○ | ○ | [67] | [68] |
| (mTOR) | ENST00113 | [62] | [63] | ○ | ○ | ○ | ○ | ○ | ○ | [62] |
| HOTAIR | [221] | [222,223] | ○ | ○ | [224] | ○ | [225] | [225] | [223] | |
| PVT1 | [171,172] | [173] | ○ | ○ | [174] | [175] | [133] | [176] | [177] | |
| UCA1 | [226] | [227] | [228] | [229] | ○ | ○ | [230] | [231] | [232] | |
| RP11-708H21.4 | [95] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [95] | |
| GAS5 | [69,70] | [71,72] | [73] | ○ | [74] | [75] | [76] | [77] | [78] | |
| H19 | [103] | [104] | [105] | [106] | [107] | [105] | [108] | [109] | [104] | |
| lncRNA-p3134 | [115] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| S6K1/2 | HOTAIR | [221] | [222,223] | ○ | ○ | [224] | ○ | [225] | [225] | [223] |
| RP11-708H21.4 | [95] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [95] | |
| PCGEM1 | [233] | [234] | ○ | ○ | ○ | ○ | ○ | ○ | [235] | |
| SREBP1 | LNCARSR | [236] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | [237] |
| HIF | HOTAIR | [221] | [222,223] | ○ | ○ | [224] | ○ | [225] | [225] | [223] |
| CPS1-IT1 | [238] | [239] | ○ | ○ | ○ | ○ | ○ | ○ | [240] | |
| MIR31HG | [241] | ○ | ○ | ○ | ○ | ○ | ○ | [242] | [243] | |
| MEG3 | [244] | [245] | [244] | [246] | [247] | [248] | [249] | [250] | [251] | |
| RPL13AP23 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| AKT1-, AKT2-, AKT3-Targeting CircRNAs | ||||||
|---|---|---|---|---|---|---|
| AKT1 | circ_0101403 | circ_0101404 | circ_0033555 | circ_0033560 | circ_0033559 | circ_0033546 |
| circ_0033552 | circ_0033558 | circ_0033557 | circ_0033553 | circ_0033547 | circ_0033551 | |
| circ_0033548 | circ_0033556 | circ_0033549 | circ_0033554 | circ_0033550 | ||
| AKT2 | circ_0051068 | circ_0051082 | circ_0051074 | circ_0051077 | circ_0051071 | circ_0051081 |
| circ_0051073 | circ_0051080 | circ_0051070 | circ_0051075 | circ_0051069 | circ_0051072 | |
| circ_0051078 | circ_0051079 | circ_0051076 | circ_0008719 | circ_0005812 | ||
| AKT3 | circ_0017242 | circ_0112774 | circ_0017251 | circ_0112785 | circ_0017249 | circ_0112773 |
| circ_0006696 | circ_0017252 | circ_0017243 | circ_0112797 | circ_0112778 | circ_0112800 | |
| circ_0004649 | circ_0112777 | circ_0017254 | circ_0017246 | circ_0112788 | circ_0112798 | |
| circ_0017250 | circ_0000199 | circ_0112770 | circ_0112782 | circ_0112787 | circ_0017247 | |
| circ_0017244 | circ_0112767 | circ_0112775 | circ_0017253 | circ_0112799 | circ_0112776 | |
| circ_0002240 | circ_0112801 | circ_0112802 | circ_0112772 | circ_0112780 | circ_0112771 | |
| circ_0112791 | circ_0112766 | circ_0112783 | circ_0017245 | circ_0112790 | circ_0112786 | |
| circ_0112792 | circ_0112768 | circ_0112784 | circ_0017248 | circ_0112779 | circ_0112796 | |
| circ_0112769 | circ_0112789 | circ_0112781 | circ_0112794 | circ_0112793 | circ_0112795 | |
| AKT Effectors | AKT Effector-Targeting CircRNAs | |||||||
|---|---|---|---|---|---|---|---|---|
| FOXO | ○ | |||||||
| c-Myc | circ_0085535 | circ_0085533 | circ_0085534 | |||||
| mTOR | circ_0110437 | circ_0009803 | circ_0009829 | circ_0110441 | circ_0009793 | circ_0009810 | circ_0110442 | circ_0009787 |
| circ_0009779 | circ_0009834 | circ_0009801 | circ_0009795 | circ_0009813 | circ_0009809 | circ_0110447 | circ_0110418 | |
| circ_0009837 | circ_0009820 | circ_0009822 | circ_0009831 | circ_0009844 | circ_0009825 | circ_0009776 | circ_0009835 | |
| circ_0009845 | circ_0009847 | circ_0009782 | circ_0009842 | circ_0009788 | circ_0009819 | circ_0110416 | circ_0110435 | |
| circ_0009823 | circ_0009832 | circ_0009839 | circ_0009785 | circ_0009830 | circ_0009840 | circ_0009808 | circ_0110438 | |
| circ_0009815 | circ_0009804 | circ_0009799 | circ_0009800 | circ_0009811 | circ_0009784 | circ_0009778 | circ_0009821 | |
| circ_0009786 | circ_0110414 | circ_0110424 | circ_0009789 | circ_0009828 | circ_0009838 | circ_0009833 | circ_0110440 | |
| circ_0009780 | circ_0009777 | circ_0009802 | circ_0009806 | circ_0009796 | circ_0009798 | circ_0009794 | circ_0009791 | |
| circ_0110417 | circ_0006576 | circ_0009826 | circ_0110420 | circ_0110439 | circ_0009797 | circ_0009805 | circ_0009790 | |
| circ_0009846 | circ_0009807 | circ_0009792 | circ_0009814 | circ_0009824 | circ_0009843 | circ_0009818 | circ_0009841 | |
| circ_0110415 | circ_0009817 | circ_0009783 | circ_0110443 | circ_0009812 | circ_0009781 | circ_0110419 | circ_0009827 | |
| circ_0009816 | circ_0009836 | |||||||
| RPTOR | ○ | |||||||
| MLST8 | circ_0105204 | circ_0037498 | ||||||
| AKT1S1 | circ_0051983 | circ_0000950 | circ_0051984 | |||||
| DEPTOR | circ_0135616 | circ_0085412 | circ_0135615 | circ_0135617 | ||||
| S6K1 | circ_0008625 | circ_0044904 | circ_0044907 | circ_0044900 | circ_0044903 | circ_0107292 | circ_0107290 | circ_0044902 |
| circ_0044905 | circ_0044899 | circ_0044906 | circ_0107291 | circ_0044901 | ||||
| S6K2 | circ_0023096 | circ_0023090 | circ_0023094 | circ_0023095 | circ_0023091 | circ_0023092 | circ_0023093 | circ_0023089 |
| SREBP1 | ○ | |||||||
| HIF | circ_0102309 | circ_0102321 | circ_0102322 | circ_0102317 | circ_0004817 | circ_0102315 | circ_0004623 | circ_0102323 |
| circ_0102313 | circ_0006326 | circ_0005205 | circ_0032132 | circ_0032139 | circ_0102310 | circ_0102327 | circ_0032136 | |
| circ_0102326 | circ_0102311 | circ_0102314 | circ_0032135 | circ_0102318 | circ_0032137 | circ_0032140 | circ_0032138 | |
| circ_0007976 | circ_0032133 | circ_0102325 | circ_0032134 | circ_0102312 | circ_0102320 | circ_0102316 | circ_0006393 | |
| circ_0102319 | circ_0102324 | |||||||
| Cell Functions | |||
|---|---|---|---|
| AKT Effectors | circRNAs | Apoptosis | Migration |
| c-Myc | circ_0085533 [308] | downregulate | ○ |
| mTORC1/2 (mTOR) | circ_0009805 [309], circ_0009792 [310] | ○ | ○ |
| mTORC1/2 (AKT1S1) | circ_0000950 [311,312] | upregulate | ○ |
| HIF | circ_0032138 [313], circ_0006393 [314] | ○ | upregulate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.-Y.; Chuang, Y.-T.; Shiau, J.-P.; Yang, K.-H.; Chang, F.-R.; Hou, M.-F.; Farooqi, A.A.; Chang, H.-W. Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells. Cells 2022, 11, 2940. https://doi.org/10.3390/cells11192940
Tang J-Y, Chuang Y-T, Shiau J-P, Yang K-H, Chang F-R, Hou M-F, Farooqi AA, Chang H-W. Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells. Cells. 2022; 11(19):2940. https://doi.org/10.3390/cells11192940
Chicago/Turabian StyleTang, Jen-Yang, Ya-Ting Chuang, Jun-Ping Shiau, Kun-Han Yang, Fang-Rong Chang, Ming-Feng Hou, Ammad Ahmad Farooqi, and Hsueh-Wei Chang. 2022. "Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells" Cells 11, no. 19: 2940. https://doi.org/10.3390/cells11192940









