Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alveolar Macrophages
2.2. Antiviral Immune Response in Alveolar Macrophages
2.3. Cloning of Porcine Ifns and Anti-Viral Factors
2.4. Quantitative Real-Time PCR
2.5. Microorganisms
2.6. Modulation of Alveolar Macrophages’ Immune Response by Lactobacilli
2.7. Phagocytosis
2.8. Westen Blotting Analysis
2.9. Experimental Aniamls and Treatments
2.10. In Vivo Poly(I:C) Administration
2.11. Alveolar Macrophages Primary Cultures
2.12. Statistical Analysis
3. Results
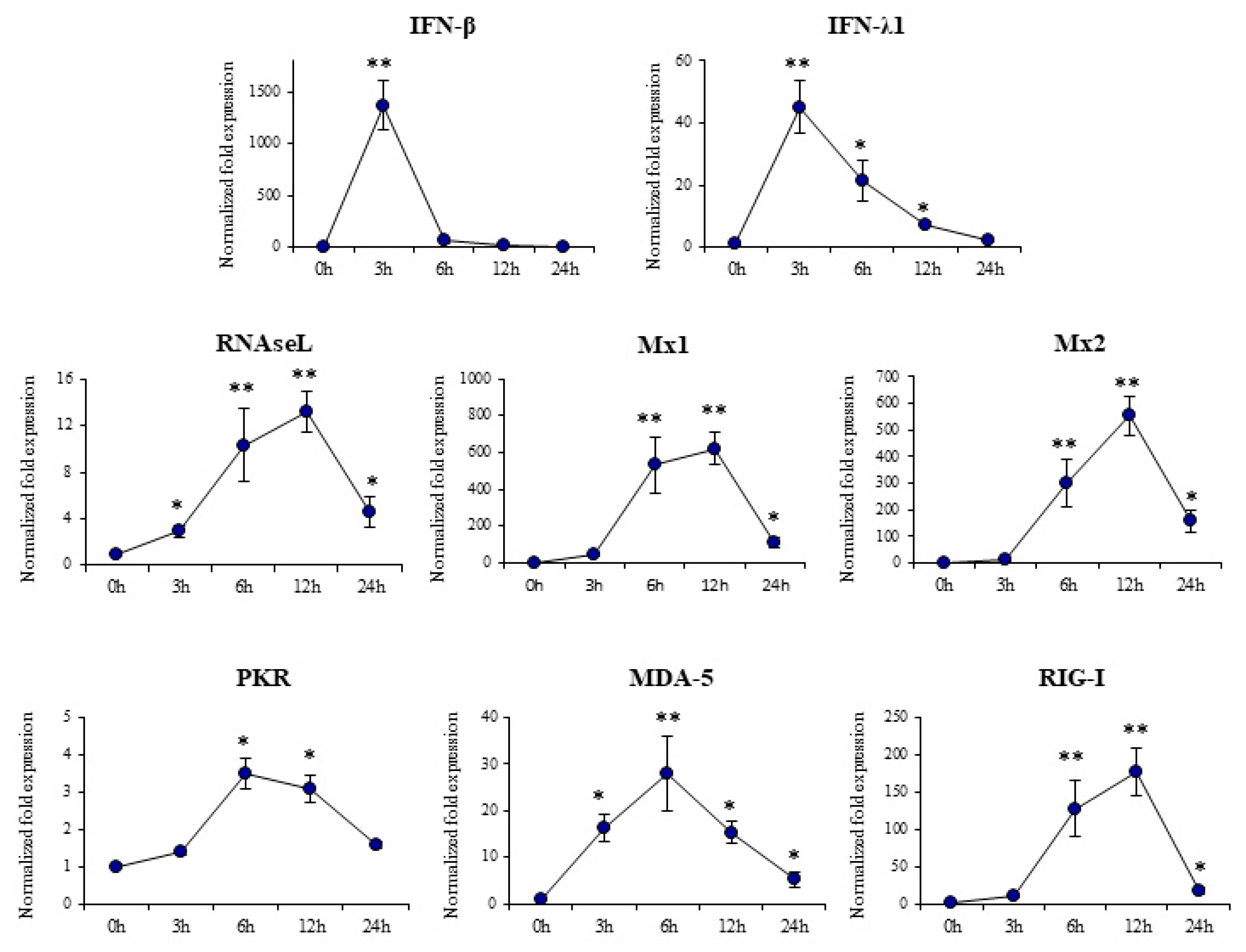
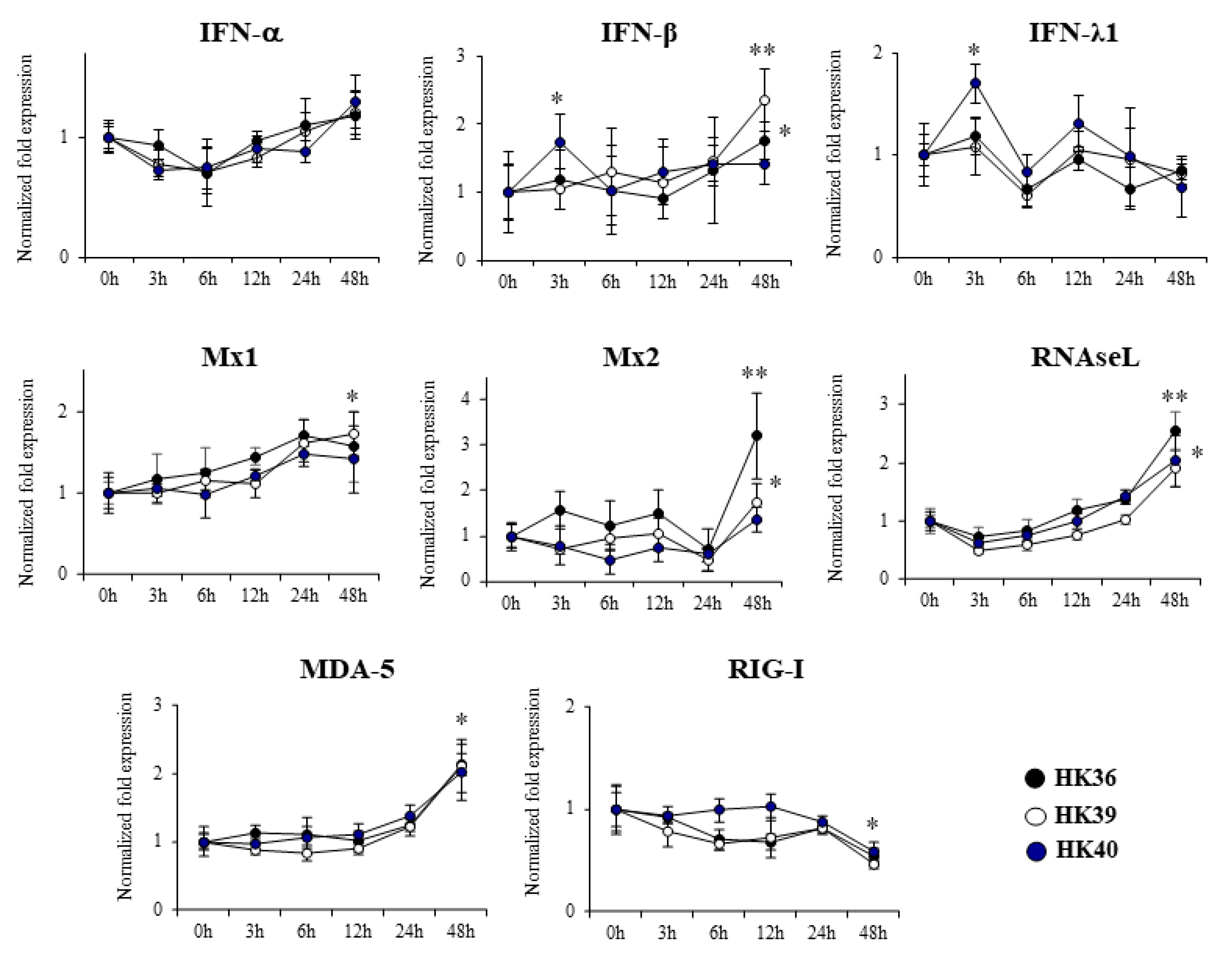
3.1. Response of Porcine Alveolar Macrophages to Poly(I:C) Challenge
3.2. Selection of New Immunobiotics with the Ability to Modulate Porcine Alveolar Macrophages
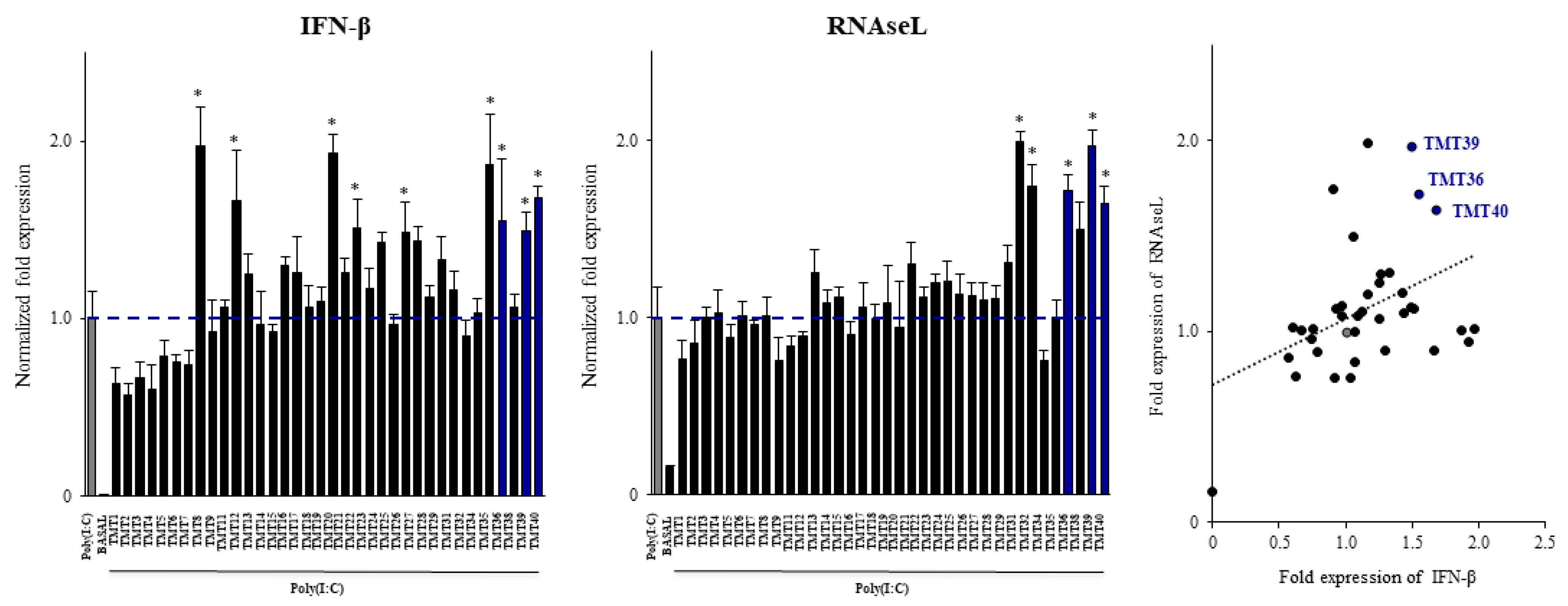
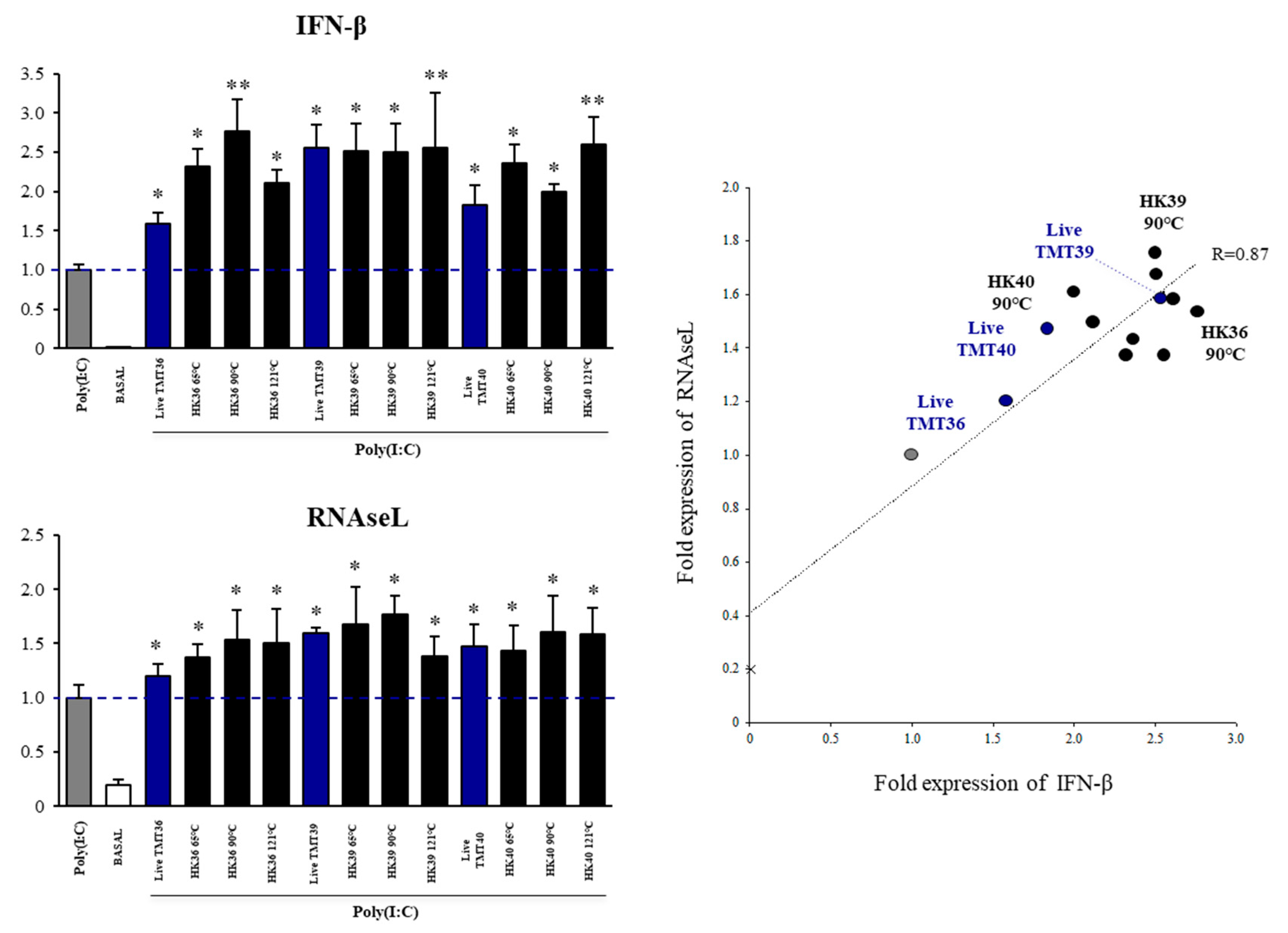
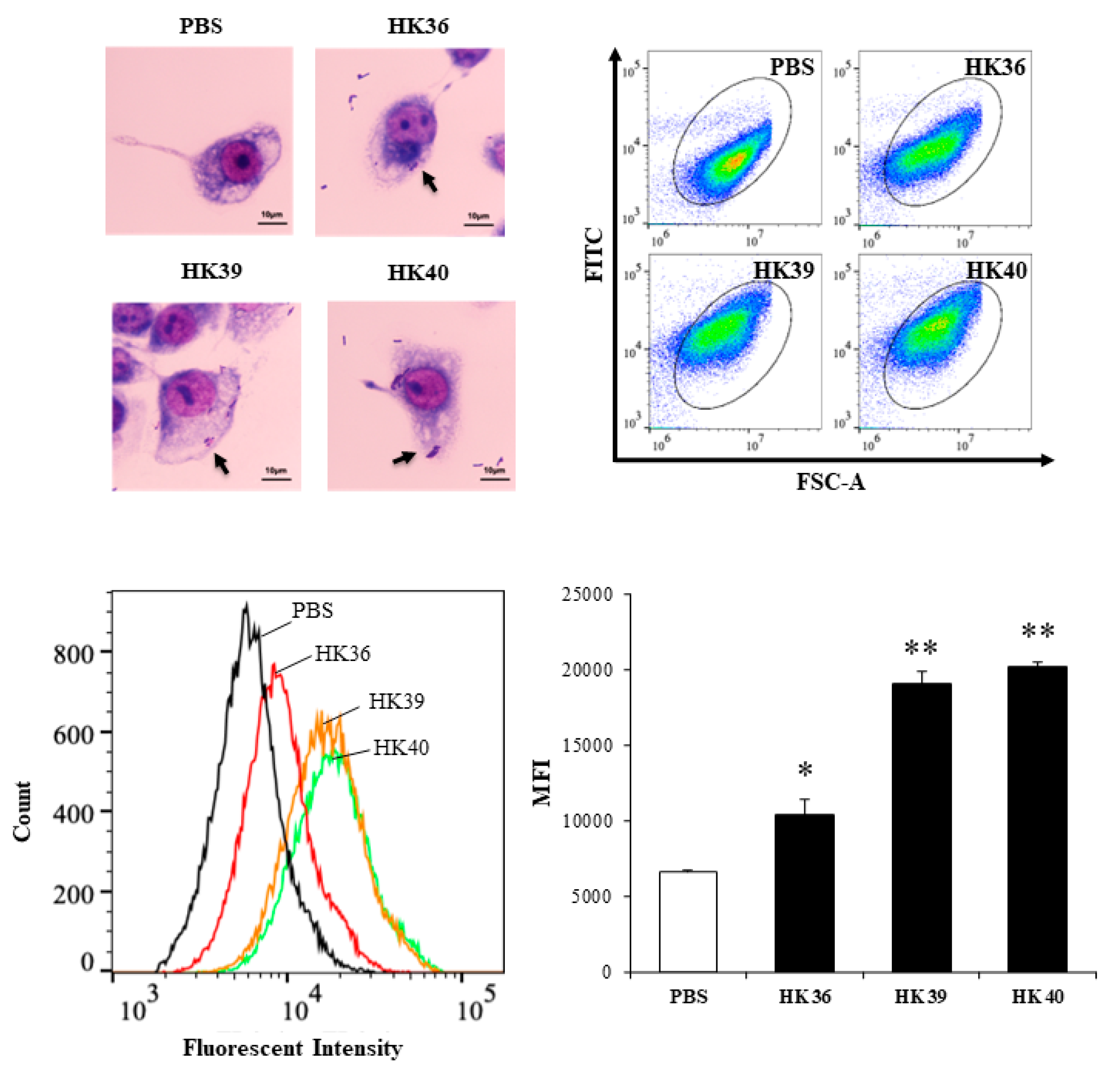
3.3. Selection of Postimmunobiotics with the Ability to Modulate Porcine Alveolar Macrophages
3.4. Effect of Postimmunobiotics in Alveolar Macrophages Activities
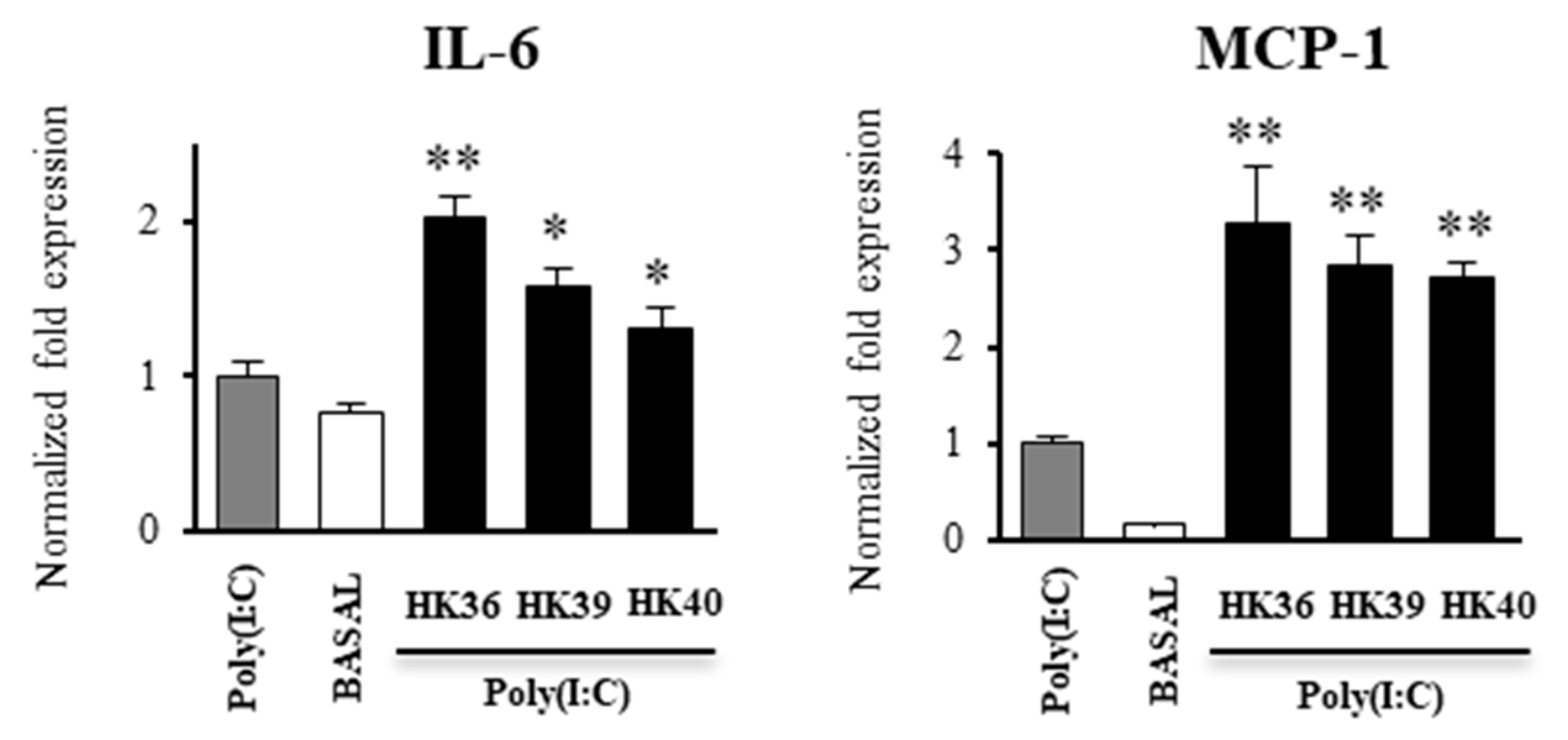
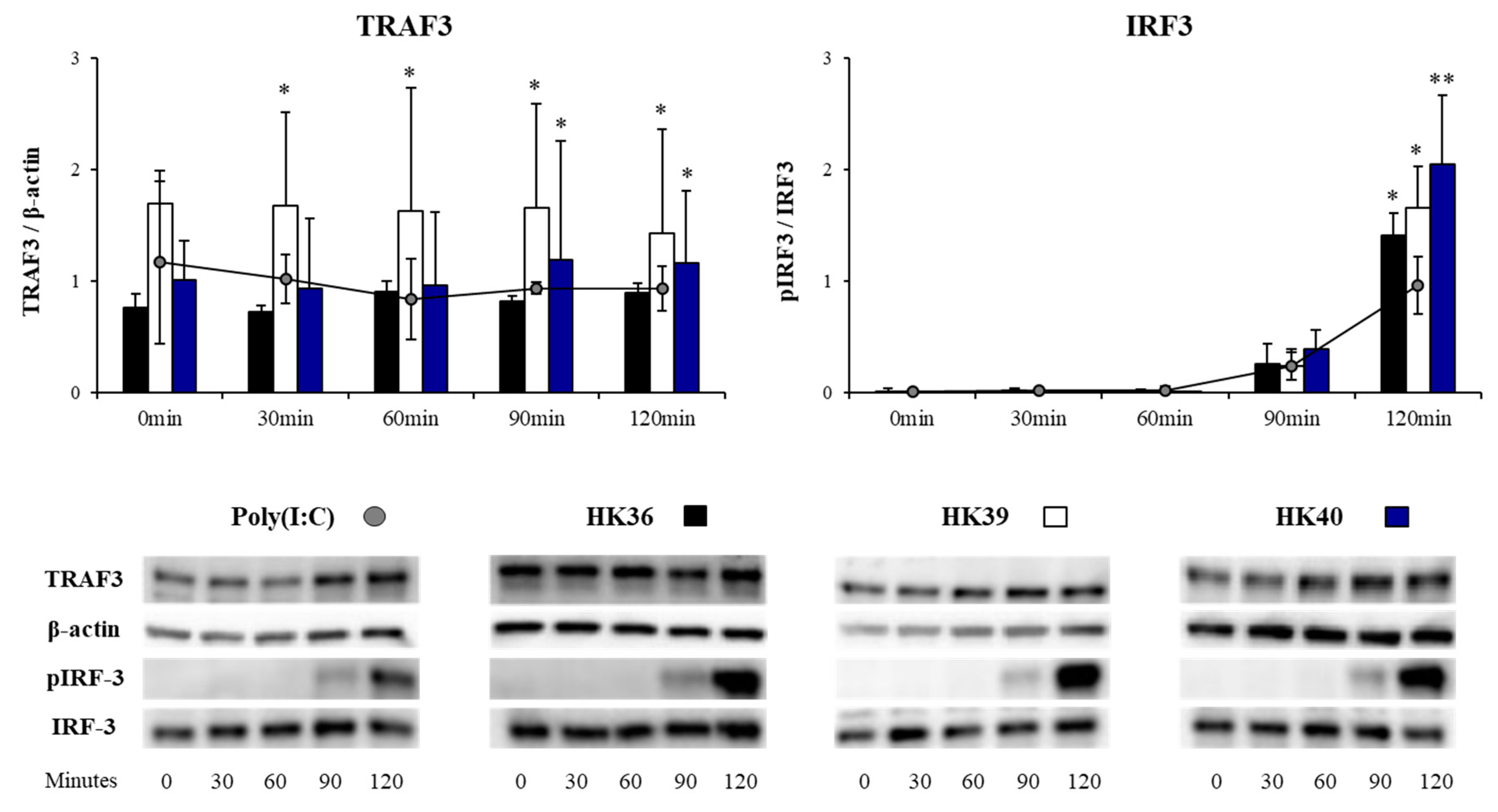
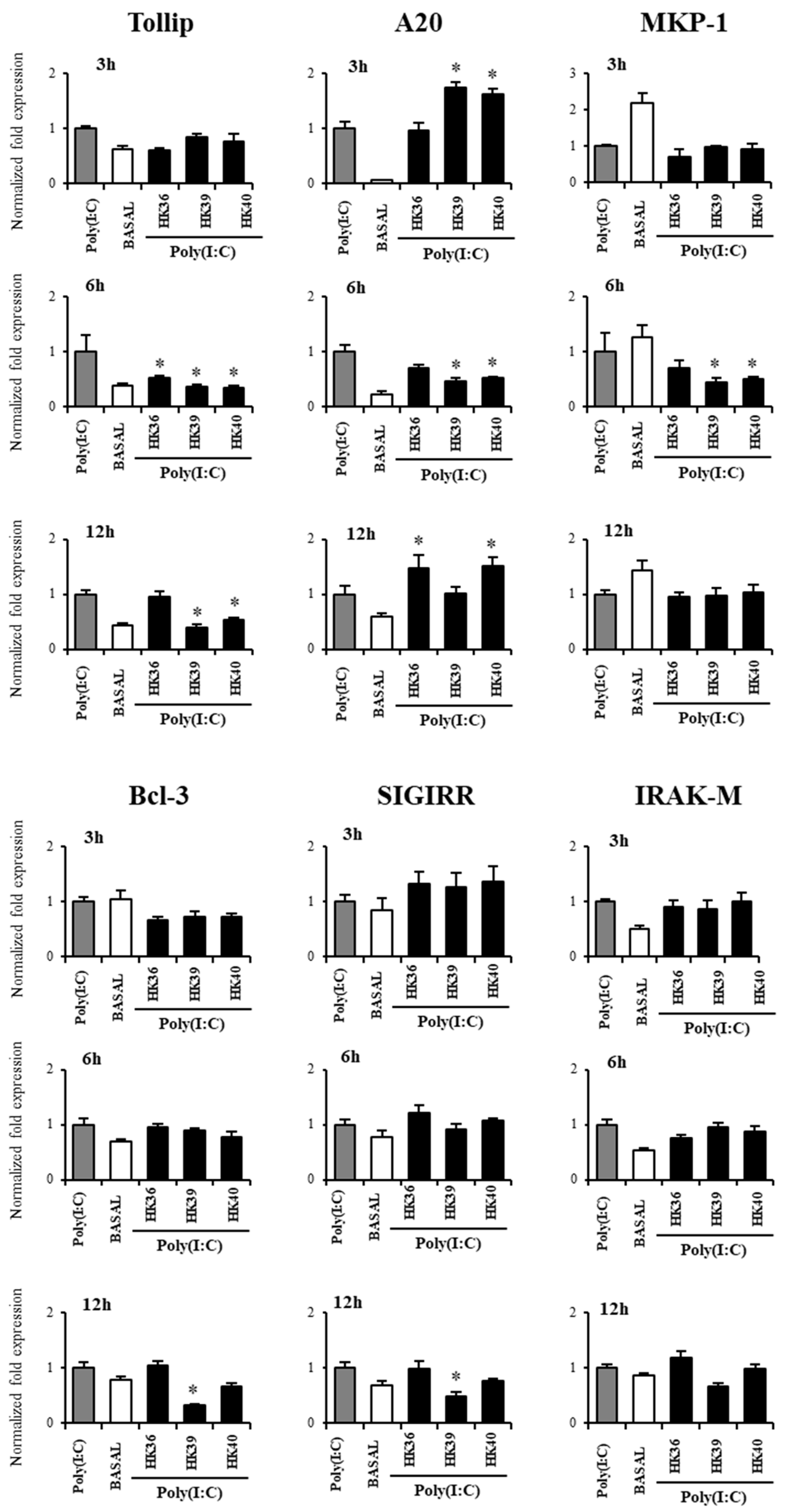
3.5. Effect of Postimmunobiotics in the Response of Alveolar Macrophages to TLR3 Activation
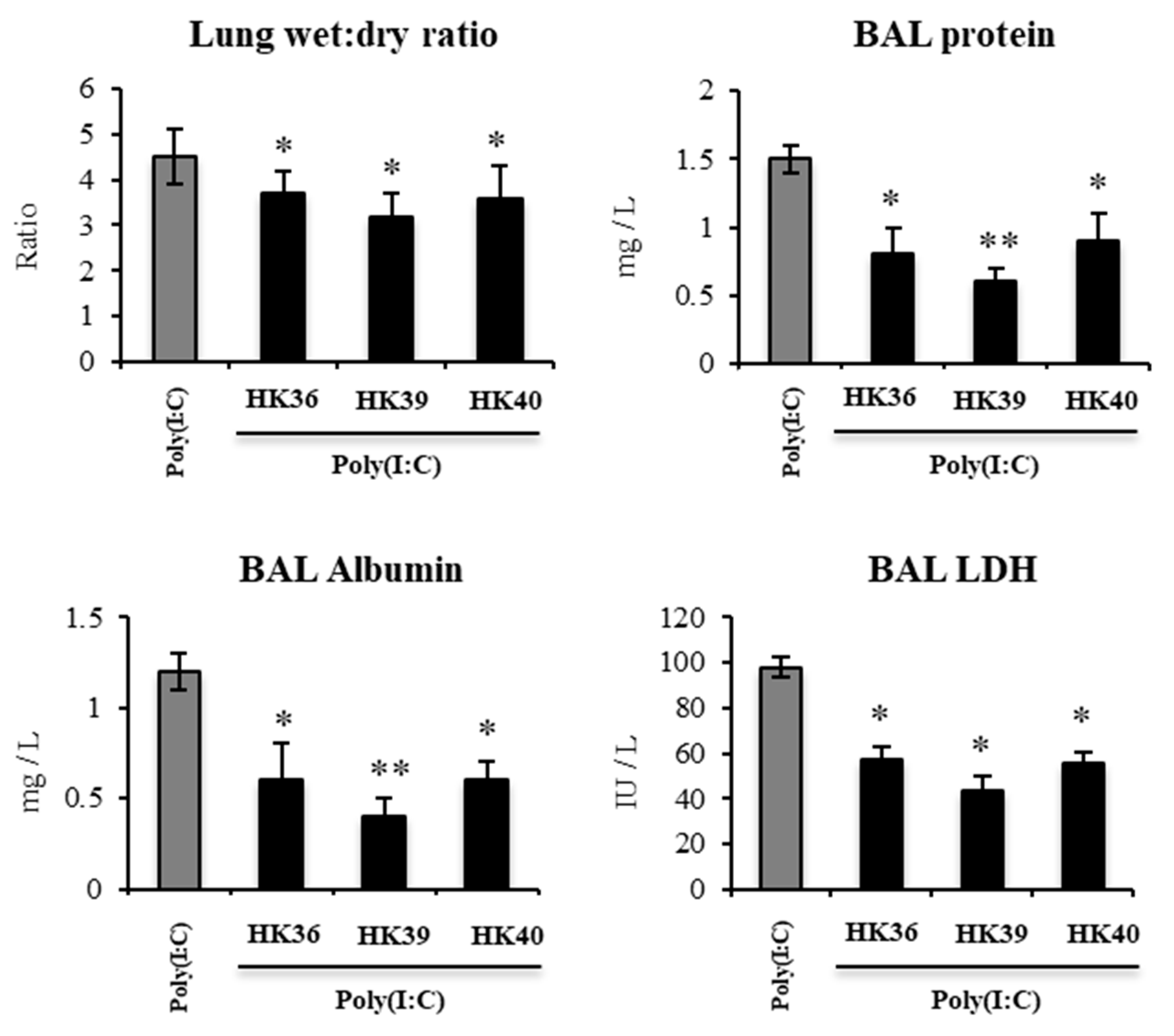
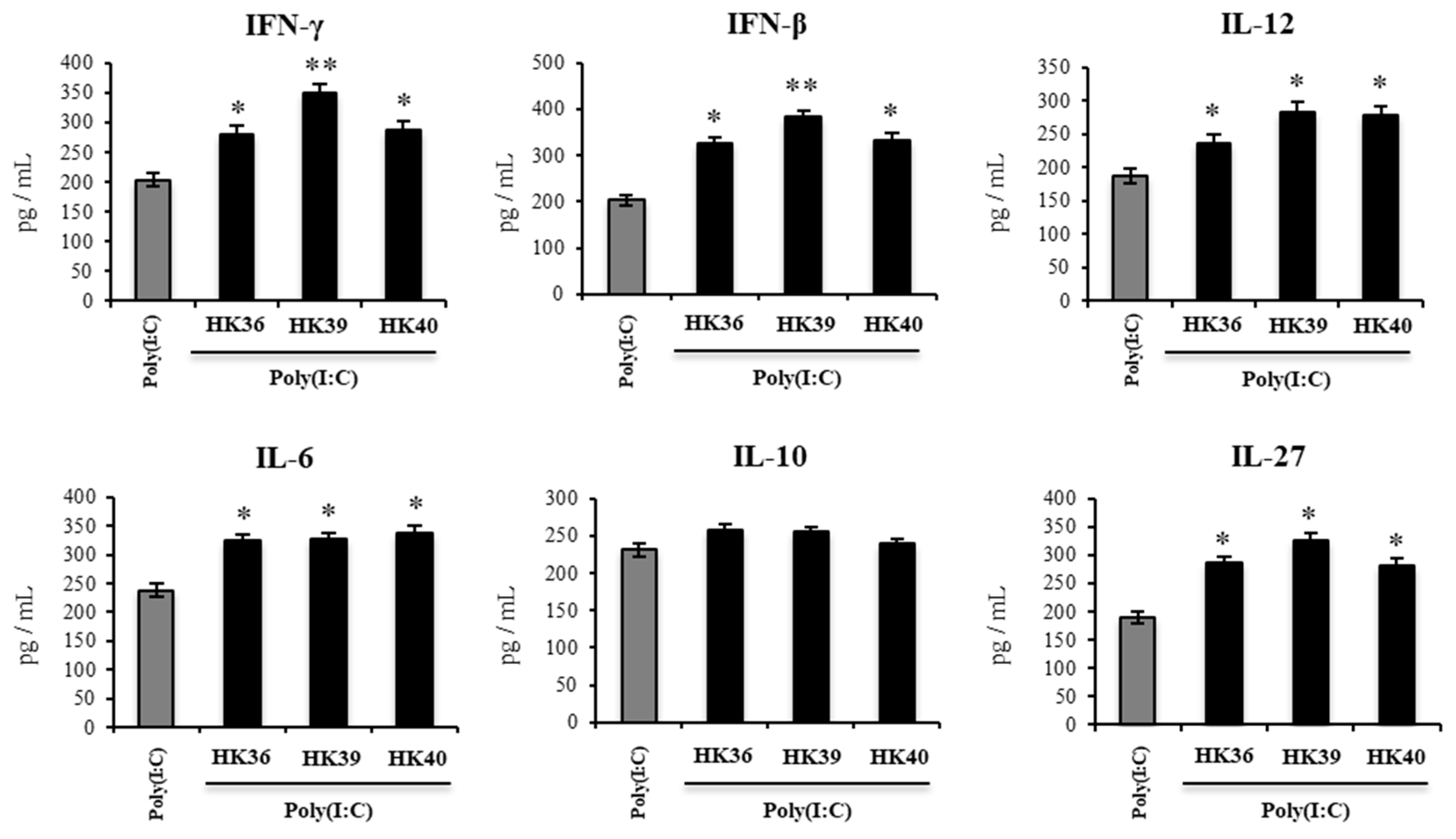
3.6. Effect of Postimmunobiotics in the Respiratory Innate Immune Response Triggered by TLR3 Activation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Aeffner, F.; Traylor, Z.P.; Yu, E.N.; Davis, I.C. Double-stranded RNA induces similar pulmonary dysfunction to respiratory syncytial virus in BALB/c mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L99–L109. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Balloy, V.; Lagranderie, M.; Alexopoulou, L.; Escriou, N.; Flavell, R.; Chignard, M.; Si-Tahar, M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Rudd, B.D.; Burstein, E.; Duckett, C.S.; Li, X.; Lukacs, N.W. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 2005, 79, 3350–3357. [Google Scholar] [CrossRef]
- Stowell, N.C.; Seideman, J.; Raymond, H.A.; Smalley, K.A.; Lamb, R.J.; Egenolf, D.D.; Bugelski, P.J.; Murray, L.A.; Marsters, P.A.; Bunting, R.A.; et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir. Res. 2009, 10, 43. [Google Scholar] [CrossRef]
- Kamiya, Y.; Fujisawa, T.; Katsumata, M.; Yasui, H.; Suzuki, Y.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Nakamura, Y.; et al. Influenza A virus enhances ciliary activity and mucociliary clearance via TLR3 in airway epithelium. Respir. Res. 2020, 21, 282. [Google Scholar] [CrossRef]
- Londhe, V.A.; Belperio, J.A.; Keane, M.P.; Burdick, M.D.; Xue, Y.Y.; Strieter, R.M. CXCR2 is critical for dsRNA-induced lung injury: Relevance to viral lung infection. J. Inflamm. (Lond.) 2005, 2, 4. [Google Scholar] [CrossRef]
- Sola, I.; Almazan, F.; Zuniga, S.; Enjuanes, L. Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu. Rev. Virol. 2015, 2, 265–288. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef]
- Tripathi, U.; Nchioua, R.; Prata, L.; Zhu, Y.; Gerdes, E.O.W.; Giorgadze, N.; Pirtskhalava, T.; Parker, E.; Xue, A.; Espindola-Netto, J.M.; et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 2021, 13, 21838–21854. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The modulation of mucosal antiviral immunity by immunobiotics: Could they offer any benefit in the SARS-CoV-2 pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a potential adjuvant and delivery system for the development of SARS-CoV-2 oral vaccines. Microorganisms 2021, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.G.N.; Cuadrat, R.R.C.; Tonetti, F.R.; Kitazawa, H.; Villena, J. The role of respiratory microbiota in the protection against viral diseases: Respiratory commensal bacteria as next-generation probiotics for COVID-19. Biosci. Microbiota Food Health 2022, 41, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kiyoshima, J.; Shida, K.; Yasui, H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin. Diagn. Lab. Immunol. 2001, 8, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic lactobacilli improves the adaptive immune response against influenza virus. Int. Immunopharmacol. 2020, 78, 106115. [Google Scholar] [CrossRef]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Aguero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef]
- Clua, P.; Kanmani, P.; Zelaya, H.; Tada, A.; Kober, A.H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Peptidoglycan from immunobiotic Lactobacillus rhamnosus improves resistance of infant mice to respiratory syncytial viral infection and secondary pneumococcal pneumonia. Front. Immunol. 2017, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Clua, P.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; García Castillo, V.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.J.C. The role of alveolar macrophages in the improved protection against respiratory syncytial virus and pneumococcal superinfection induced by the peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum Improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic analysis of the innate antiviral immune response in porcine intestinal epithelial cells: Influence of immunobiotic lactobacilli. Front. Immunol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Hebert, E.M.; Saavedra, L.; Komatsu, R.; Gatica, B.; Miyazaki, A.; Ikeda-Ohtsubo, W.; Suda, Y.; et al. Genomic characterization of Lactobacillus delbrueckii TUA4408L and Evaluation of the antiviral activities of its extracellular polysaccharides in porcine intestinal epithelial cells. Front. Immunol. 2018, 9, 2178. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Sabara, M.; Pasick, J.; van Moorlehem, E.; Babiuk, L. Continuous porcine cell lines developed from alveolar macrophages: Partial characterization and virus susceptibility. J. Virol. Methods 2002, 104, 203–216. [Google Scholar] [CrossRef]
- Hou, N.; Du, X.; Wu, S. Advances in pig models of human diseases. Anim. Model Exp. Med. 2022, 5, 141–152. [Google Scholar] [CrossRef]
- Bertho, N.; Meurens, F. The pig as a medical model for acquired respiratory diseases and dysfunctions: An immunological perspective. Mol. Immunol. 2021, 135, 254–267. [Google Scholar] [CrossRef]
- Han, M.; Rajput, C.; Ishikawa, T.; Jarman, C.R.; Lee, J.; Hershenson, M.B. Small Animal models of respiratory viral infection related to asthma. Viruses 2018, 10, 682. [Google Scholar] [CrossRef]
- Kadooka, Y.; Tominari, K.; Sakai, F.; Yasui, H. Prevention of rotavirus-induced diarrhea by preferential secretion of IgA in breast milk via maternal administration of Lactobacillus gasseri SBT2055. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 66–71. [Google Scholar] [CrossRef]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; He, F.; Miyazawa, K.; Kawase, M.; Kubota, A.; Hiramatsu, M. Orally administered heat-killed Lactobacillus gasseri TMC0356 alters respiratory immune responses and intestinal microbiota of diet-induced obese mice. J. Appl. Microbiol. 2012, 113, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fiore, W.; Arioli, S.; Guglielmetti, S. The neglected microbial components of commercial probiotic formulations. Microorganisms 2020, 8, 1177. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar macrophages. Cell Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef]
- Dalskov, L.; Mohlenberg, M.; Thyrsted, J.; Blay-Cadanet, J.; Poulsen, E.T.; Folkersen, B.H.; Skaarup, S.H.; Olagnier, D.; Reinert, L.; Enghild, J.J.; et al. SARS-CoV-2 evades immune detection in alveolar macrophages. EMBO Rep. 2020, 21, e51252. [Google Scholar] [CrossRef]
- Galani, I.E.; Triantafyllia, V.; Eleminiadou, E.E.; Koltsida, O.; Stavropoulos, A.; Manioudaki, M.; Thanos, D.; Doyle, S.E.; Kotenko, S.V.; Thanopoulou, K.; et al. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 2017, 46, 875–890 e876. [Google Scholar] [CrossRef]
- Jagannathan, P.; Andrews, J.R.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Balasubramanian, V.; Purington, N.; Kamble, S.; de Vries, C.R.; Quintero, O.; et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: A randomized placebo-controlled trial. Nat. Commun. 2021, 12, 1967. [Google Scholar] [CrossRef]
- Klinkhammer, J.; Schnepf, D.; Ye, L.; Schwaderlapp, M.; Gad, H.H.; Hartmann, R.; Garcin, D.; Mahlakoiv, T.; Staeheli, P. IFN-lambda prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. Elife 2018, 7, e33354. [Google Scholar] [CrossRef]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.R.; Pereira, C.; Kumagai, Y.; Culley, F.J.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Espinoza, J.A.; Pulgar, R.A.; Jara, E.L.; Kalergis, A.M. Functional impairment of mononuclear phagocyte system by the human respiratory syncytial virus. Front. Immunol. 2017, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Chao, H.; Casola, A.; Garofalo, R.P. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, K.M.; Egana, L.; Orend, J.G.; Resetar, E.; Anderson, K.B.; Patel, R.; Empey, K.M. Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice. Respir. Res. 2015, 16, 122. [Google Scholar] [CrossRef]
- Harker, J.A.; Yamaguchi, Y.; Culley, F.J.; Tregoning, J.S.; Openshaw, P.J. Delayed sequelae of neonatal respiratory syncytial virus infection are dependent on cells of the innate immune system. J. Virol. 2014, 88, 604–611. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Zhao, F.; Luo, R.; Chen, H.; Xiao, S. Molecular cloning, expression and antiviral activity of porcine interleukin-29 (poIL-29). Dev. Comp. Immunol. 2011, 35, 378–384. [Google Scholar] [CrossRef]
- Wang, L.; Hu, S.; Liu, Q.; Li, Y.; Xu, L.; Zhang, Z.; Cai, X.; He, X. Porcine alveolar macrophage polarization is involved in inhibition of porcine reproductive and respiratory syndrome virus (PRRSV) replication. J. Vet. Med. Sci. 2017, 79, 1906–1915. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.L.; Hu, W.; Hu, S.P.; Zhang, Z.; Cai, X.H.; He, X.J. Transcriptome of porcine alveolar macrophages activated by interferon-gamma and lipopolysaccharide. Biochem. Biophys. Res. Commun. 2018, 503, 2666–2672. [Google Scholar] [CrossRef]
- Sang, Y.; Rowland, R.R.; Blecha, F. Antiviral regulation in porcine monocytic cells at different activation states. J. Virol. 2014, 88, 11395–11410. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Bi, J.; Liu, J.; Tong, L.; Song, Y.; Bai, C.; Zhu, X. A20 protein regulates lipopolysaccharide-induced acute lung injury by downregulation of NF-kappaB and macrophage polarization in rats. Mol. Med. Rep. 2017, 16, 4964–4972. [Google Scholar] [CrossRef] [Green Version]
- Beura, L.K.; Sarkar, S.N.; Kwon, B.; Subramaniam, S.; Jones, C.; Pattnaik, A.K.; Osorio, F.A. Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. J. Virol. 2010, 84, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; Laegreid, W.W.; Bono, J.L.; Chitko-McKown, C.G.; Fox, J.M. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol. 2004, 149, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Overend, C.; Mitchell, R.; He, D.; Rompato, G.; Grubman, M.J.; Garmendia, A.E. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007, 88, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Li, C.; Li, Z.; Chen, W.; Zeng, Y. Genetic diversities and differentially selected regions between Shandong indigenous pig breeds and western pig breeds. Front. Genet. 2019, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Chen, W.; Li, Z.; Wang, L.; Ma, L.; Geng, J.; Zhang, Y.; Zhao, J.; Zeng, Y. Role of IFNLR1 gene in PRRSV infection of PAM cells. J. Vet. Sci. 2021, 22, e39. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Fang, L.; Jin, H.; Jiang, Y.; Wang, D.; Chen, H.; Xiao, S. Antiviral activity of type I and type III interferons against porcine reproductive and respiratory syndrome virus (PRRSV). Antivir. Res. 2011, 91, 99–101. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, L.; Xu, L.; Huang, J.; Sun, X.; Xu, Z. Porcine interferon lambda 3 (IFN-λ3) shows potent anti-PRRSV activity in primary porcine alveolar macrophages (PAMs). BMC Vet. Res. 2020, 16, 408. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Qiu, M.; Li, J.; Xiao, Y.; Lin, H.; Zheng, W.; Zhu, J.; Chen, N. Transcriptomic profiling reveals different innate immune responses in primary alveolar macrophages infected by two highly homologous porcine reproductive and respiratory syndrome viruses with distinct virulence. Microb. Pathog. 2021, 158, 105102. [Google Scholar] [CrossRef]
- Qiao, S.; Feng, L.; Bao, D.; Guo, J.; Wan, B.; Xiao, Z.; Yang, S.; Zhang, G. Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Vet. Microbiol. 2011, 149, 213–220. [Google Scholar] [CrossRef]
- Renson, P.; Rose, N.; Le Dimna, M.; Mahe, S.; Keranflec’h, A.; Paboeuf, F.; Belloc, C.; Le Potier, M.F.; Bourry, O. Dynamic changes in bronchoalveolar macrophages and cytokines during infection of pigs with a highly or low pathogenic genotype 1 PRRSV strain. Vet. Res. 2017, 48, 15. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Nagata, D.E.; Demoor, T.; Ptaschinski, C.; Ting, H.A.; Jang, S.; Reed, M.; Mukherjee, S.; Lukacs, N.W. IL-27R-mediated regulation of IL-17 controls the development of respiratory syncytial virus-associated pathogenesis. Am. J. Pathol. 2014, 184, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Pyle, C.J.; Uwadiae, F.I.; Swieboda, D.P.; Harker, J.A. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017, 13, e1006640. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, R.A.; Roberts, N.; Mason, R.J.; Alper, S.; Chu, H.W. Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages. Respir. Res. 2018, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Maelfait, J.; Roose, K.; Bogaert, P.; Sze, M.; Saelens, X.; Pasparakis, M.; Carpentier, I.; van Loo, G.; Beyaert, R. A20 (Tnfaip3) deficiency in myeloid cells protects against influenza A virus infection. PLoS Pathog. 2012, 8, e1002570. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Pottle, J.; Ni, M.; He, Y.; Zhang, Z.Y.; Buckner, J.H. Negative regulation of TLR signaling in myeloid cells--implications for autoimmune diseases. Immunol. Rev. 2016, 269, 212–227. [Google Scholar] [CrossRef]
- Sun, K.Y.; Xu, D.H.; Xie, C.; Plummer, S.; Tang, J.; Yang, X.F.; Ji, X.H. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 2017, 92, 1–11. [Google Scholar] [CrossRef]
- Kawano, M.; Miyoshi, M.; Miyazaki, T. Lactobacillus helveticus SBT2171 induces A20 expression via Toll-like receptor 2 signaling and inhibits the lipopolysaccharide-induced activation of nuclear factor-kappa B and mitogen-activated protein kinases in peritoneal macrophages. Front. Immunol. 2019, 10, 845. [Google Scholar] [CrossRef]
- Burns, K.; Clatworthy, J.; Martin, L.; Martinon, F.; Plumpton, C.; Maschera, B.; Lewis, A.; Ray, K.; Tschopp, J.; Volpe, F. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2000, 2, 346–351. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Watanabe, Y.; Kubota, Y.; Yoshio, S.; Kanto, T.; Motohashi, S.; Shimojo, N.; Tsuji, N.M. Double-stranded RNA derived from lactic acid bacteria augments Th1 immunity via interferon-β from human dendritic cells. Front. Immunol. 2018, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Bersch, K.L.; DeMeester, K.E.; Zagani, R.; Chen, S.; Wodzanowski, K.A.; Liu, S.; Mashayekh, S.; Reinecker, H.C.; Grimes, C.L. Bacterial peptidoglycan fragments differentially regulate innate immune signaling. ACS Cent. Sci. 2021, 7, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Tsukida, K.; Takahashi, T.; Iida, H.; Kanmani, P.; Suda, Y.; Nochi, T.; Ohwada, S.; Aso, H.; Ohkawara, S.; Makino, S.; et al. Immunoregulatory effects triggered by immunobiotic Lactobacillus jensenii TL2937 strain involve efficient phagocytosis in porcine antigen presenting cells. BMC Immunol. 2016, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Suzuki, R.; Fujie, H.; Chiba, E.; Takahashi, T.; Tomosada, Y.; Shimazu, T.; Aso, H.; Ohwada, S.; Suda, Y.; et al. Immunobiotic Lactobacillus jensenii modulates the Toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen-presenting cells. Clin. Vaccine Immunol. 2012, 19, 1038–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomokiyo, M.; Tonetti, F.R.; Yamamuro, H.; Shibata, R.; Fukuyama, K.; Gobbato, N.; Albarracin, L.; Rajoka, M.S.R.; Kober, A.K.M.H.; Ikeda-Ohtsubo, W.; et al. Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity. Cells 2022, 11, 2986. https://doi.org/10.3390/cells11192986
Tomokiyo M, Tonetti FR, Yamamuro H, Shibata R, Fukuyama K, Gobbato N, Albarracin L, Rajoka MSR, Kober AKMH, Ikeda-Ohtsubo W, et al. Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity. Cells. 2022; 11(19):2986. https://doi.org/10.3390/cells11192986
Chicago/Turabian StyleTomokiyo, Mikado, Fernanda Raya Tonetti, Hikari Yamamuro, Ryoko Shibata, Kohtaro Fukuyama, Nadia Gobbato, Leonardo Albarracin, Muhammad Shahid Riaz Rajoka, A. K. M. Humayun Kober, Wakako Ikeda-Ohtsubo, and et al. 2022. "Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity" Cells 11, no. 19: 2986. https://doi.org/10.3390/cells11192986
APA StyleTomokiyo, M., Tonetti, F. R., Yamamuro, H., Shibata, R., Fukuyama, K., Gobbato, N., Albarracin, L., Rajoka, M. S. R., Kober, A. K. M. H., Ikeda-Ohtsubo, W., Villena, J., & Kitazawa, H. (2022). Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity. Cells, 11(19), 2986. https://doi.org/10.3390/cells11192986








