STAT3 and Its Pathways’ Dysregulation—Underestimated Role in Urological Tumors
Abstract
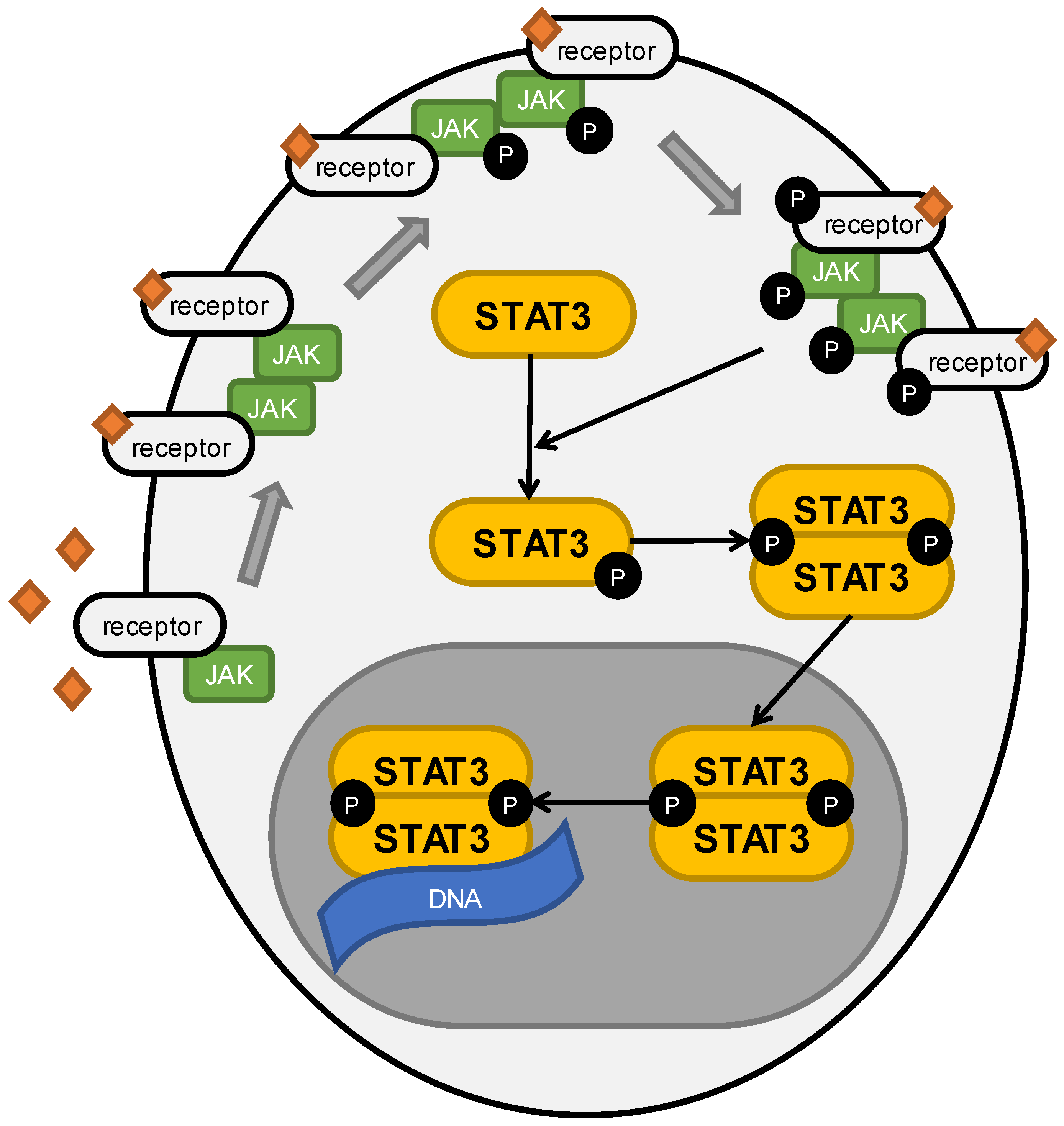
:1. Introduction
2. Role of STAT3 in Cancers
3. Role of STAT3 in Prostate Cancer
3.1. Description and Epidemiology of Prostate Cancer
3.2. Why Is STAT3 Crucial in Prostate Cancer Development and Progression?
3.3. TME Is Crucial for Prostate Cancer Progression
3.4. Attempts at Breaking CRPC
3.5. Breaking the Chemoresistance
3.6. Targeting NF-κB/IL-6/STAT3 Axis
3.7. Can We Treat Prostate Cancer Patients with RNA?
3.8. Importance of the Environment on the Molecular Level
3.9. Different Approaches to Manage PCa
3.10. Should We Pin Our Hopes on Nature?
3.11. Role of STAT3 in Prostate Cancer Diagnostics
4. Role of STAT3 in Bladder Cancer
4.1. Description and Epidemiology of Bladder Cancer
4.2. STAT3 Levels in Bladder Cancer
4.3. Bladder Cancer Cells’ Viability and Apoptosis
4.4. EMT in Bladder Cancer
4.5. Angiogenesis in Bladder Cancer
4.6. Impact of STAT3 on Bladder Cancer Cells’ Metabolism
4.7. Drug Resistance in Bladder Cancer
4.8. Role of STAT3 in Bladder Cancer Diagnostics
5. Role of STAT3 in Upper Tract Urothelial Carcinoma
6. Role of STAT3 in Renal Cell Carcinoma
6.1. Description and Epidemiology of Renal Cell Carcinoma
6.2. Role of IL-6 in Renal Cell Carcinoma and STAT3 Levels
6.3. Impact of STAT3 on Survival, Apoptosis, and Angiogenesis of Renal Cell Carcinoma
6.4. EMT in Renal Cell Carcinoma
6.5. Drug Resistance in Renal Cell Carcinoma
6.6. Impact of STAT3 on Immune System
6.7. Role of STAT3 in Renal Cell Carcinoma Diagnostics
7. Role of STAT3 in Penile Cancer
8. Role of STAT3 in Testicular Cancer
9. STAT3 Inhibitors as Viable Targets
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 27HC | 27-hydroxycholesterol |
| 4-OHE2 | 4-hydroxy-17β-estradiol |
| ABL | Abelson tyrosine kinase |
| ADT | androgen deprivation therapy |
| AKT | protein kinase B |
| ALT | alantolactone |
| AR | androgen receptor |
| ATP | adenosine triphosphate |
| Bcl | B-cell lymphoma |
| Bcl-xl | B-cell lymphoma-extra large |
| BCR | biochemical recurrence |
| BIRC5 | baculoviral inhibitor of apoptosis repeat-containing 5 |
| BPH | benign prostate hyperplasia |
| Capz | capsazepine |
| CCL | chemokine (C-C motif) ligand |
| CCR | C-C chemokine receptor type |
| ccRCC | clear cell renal cell carcinoma |
| CD | cluster of differentiation |
| CDK | cyclin-dependent kinase |
| CK | compound K |
| COX-2 | cyclooxygenase-2 |
| cPLA2 | cytosolic phospholipase A2 |
| CRPC | castration resistant prostate cancer |
| CSCs | cancer stem cells |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| CXCL | chemokine (C-X-C motif) ligand |
| DNMTB3 | DNA (cytosine-5)-methyltransferase 3 beta |
| EA | ethacrynic acid |
| EGF | epidermal growth factor |
| EMT | epithelial-mesenchymal transition |
| ENO2 | enolase 2 |
| ENZ | enzalutamide |
| EP4/2 | prostaglandin E2 receptor 4 |
| ESR1 | estrogen receptor 1 gene |
| EZH2 | enhancer of zeste homolog 2 |
| FBP1 | fructose-1,6-bisphosphatase |
| FGFR3 | fibroblast growth factor receptor 3 |
| G3BP1 | Ras GTPase-activating protein-binding protein 1 |
| GLN | glutamine |
| GLUT-1 | glucose transporter 1 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GS | Gleason Score |
| HAVCR/KIM1 | hepatitis A virus cellular receptor gene/kidney injury molecule 1 |
| HDAC1 | histone deacetylase 1 |
| HepaCAM | hepatocyte cell adhesion molecule |
| HIF-1α | hypoxia-inducible factor 1α |
| HK2 | hexokinase 2 |
| HOXA10 | homeobox protein Hox-A10 |
| HPV | human papillomavirus |
| ICD | immunogenic cell death |
| IDO1 | indoleamine-pyrrole 2,3-dioxygenase |
| IFN-α | interferon α |
| IGF | insulin-like growth factor |
| IGF1R | insulin-like growth factor receptor |
| IGFBP3 | insulin-like growth factor-binding protein 3 |
| IL | interleukin |
| JAK | Janus kinase |
| Ki-67 | protein encoded by MKI67 gene |
| KLF5 | Krüpple-like transcription factor |
| LDHA | lactate dehydrogenase A |
| lncRNA | long non-coding RNA |
| LPS | lipopolysaccharide |
| LTF | lactoferrin |
| MALAT1 | metastasis associated lung adenocarcinoma transcript 1 |
| Mcl-1 | induced myeloid leukemia cell differentiation protein 1 gene |
| MDA-9 | melanoma differentiation associated protein-9 |
| MDSCs | myeloid-derived suppressor cells |
| MEG2 | protein tyrosine phosphatase encoded by ptpn9 gene |
| MEK | mitogen-activated protein kinase kinase |
| MIBC | muscle invasive bladder cancer |
| MMP | matrix metalloproteinase |
| MSCs | mesenchymal stem cells |
| mTKI | multiple tyrosine kinases inhibitor |
| MYC | myelocytomatosis (family of regulator genes) |
| NDRG1 | N-myc downstream-regulated gene 1 |
| NDV | Newcastle disease virus |
| NED | neuroendocrine differentiation |
| NEPCs | neuroendocrine prostate cancer cells |
| NF-κB | nuclear factor κ0light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| NKG2D | natural killer group 2D |
| NMIBC | non-muscle invasive bladder cancer |
| PA | palmitic acid |
| pAKT | phosphorylated protein kinase B |
| PCa | prostate cancer |
| PCSCs | prostate cancer stem cells |
| PD-L1 | programmed death-ligand 1 |
| PeCa | penile cancer |
| PGE-2 | prostanglandin E2 |
| PIAS | protein inhibitors of activated STATs |
| pJAK | phosphorylated Janus kinase |
| PLCε | phospholipase C ε |
| PLZF | promyelocytic leukemia zinc finger |
| pSTAT3 | phosphorylated STAT3 |
| PTMs | post-translational modifications |
| PTP | protein tyrosine phosphatase |
| RACGAP1 | Rac GTPase-activating protein 1 |
| RCC | renal cell carcinoma |
| RORC | RAR-related orphan receptor C |
| ROS | reactive oxygen species |
| SAM | S-adenosylmethionine |
| SH2 | SRC2 homology |
| SHCBP1 | SHC SH2 domain-binding protein 1 gene |
| SHP | SRC homology region 2 domain-containing phosphatase |
| SLUG | zinc finger protein SNAI2 |
| SNAIL | zinc finger protein SNAI1 |
| SOCS | suppressors of cytokine signaling |
| STAT | signal transducer and activator of transcription |
| STAT3Ser727 | STAT3 phosphorylated at Ser727 |
| STAT3Tyr705 | STAT3 phosphorylated at Tyr705 |
| TAMs | tumor-associated macrophages |
| TDO | tryptophan 2,3-dioxygenase |
| TGCT | testicular germ cell tumor |
| TGF-β1 | transforming growth factor β1 |
| TLR9 | Toll-like receptor 9 |
| TME | tumor microenvironment |
| tSTAT3 | total STAT3 |
| TYK2 | tyrosine kinase 2 |
| UTUC | upper tract urothelial carcinoma |
| VDR | vitamin D receptors |
| VEGF | vascular endothelial growth factor |
| VHL | Von Hippel-Lindau tumor suppressor gene |
| Wnt | portmanteau of Wingless and Int-1. |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Nielsen, M.E.; Filippou, P. Epidemiology of Prostate and Testicular Cancer. Semin. Interv. Radiol. 2016, 33, 182–185. [Google Scholar] [CrossRef]
- Farling, K.B. Bladder cancer. Nurse Practice 2017, 42, 26–33. [Google Scholar] [CrossRef]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar] [PubMed]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.-M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernandez, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2016, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.-P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch. Ärzteblatt Int. 2018, 115, 646–652. [Google Scholar] [CrossRef]
- De Araujo, E.D.; Orlova, A.; Neubauer, H.A.; Bajusz, D.; Seo, H.-S.; Dhe-Paganon, S.; Keserű, G.M.; Moriggl, R.; Gunning, P.T. Structural Implications of STAT3 and STAT5 SH2 Domain Mutations. Cancers 2019, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Guanizo, A.C.; Fernando, C.D.; Garama, D.J.; Gough, D.J. STAT3: A multifaceted oncoprotein. Growth Factors 2018, 36, 1–14. [Google Scholar] [CrossRef]
- Avalle, L.; Camporeale, A.; Camperi, A.; Poli, V. STAT3 in cancer: A double edged sword. Cytokine 2017, 98, 42–50. [Google Scholar] [CrossRef]
- Turkson, J.; Jove, R. STAT proteins: Novel molecular targets for cancer drug discovery. Oncogene 2000, 19, 6613–6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanaei, M.; Taheri, F.; Heshmati, M.; Bashash, D.; Nazmabadi, R.; Mohammad-Alibeigi, F.; Nahid-Samiei, M.; Shirzad, H.; Bagheri, N. Comparing the frequency of CD33 + pSTAT3 + myeloid-derived suppressor cells and IL-17 + lymphocytes in patients with prostate cancer and benign prostatic hyperplasia. Cell Biol. Int. 2021, 45, 2086–2095. [Google Scholar] [CrossRef]
- Cao, H.; Wang, D.; Gao, R.; Feng, Y.; Chen, L. Qi Ling Decreases Paclitaxel Resistance in the Human Prostate Cancer by Reversing Tumor-Associated Macrophages Function. Aging 2022, 14, 1812–1821. [Google Scholar] [CrossRef]
- McGuire, J.J.; Frieling, J.S.; Lo, C.H.; Li, T.; Muhammad, A.; Lawrence, H.R.; Lawrence, N.J.; Cook, L.M.; Lynch, C.C. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Pencik, J.; Schlederer, M.; Gruber, W.; Unger, C.; Walker, S.M.; Chalaris, A.; Marié, I.J.; Hassler, M.R.; Javaheri, T.; Aksoy, O.; et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat. Commun. 2015, 6, 7736. [Google Scholar] [CrossRef]
- Shuai, K. The STAT family of proteins in cytokine signaling. Prog. Biophys. Mol. Biol. 1999, 71, 405–422. [Google Scholar] [CrossRef]
- Sgrignani, J.; Garofalo, M.; Matkovic, M.; Merulla, J.; Catapano, C.V.; Cavalli, A. Structural Biology of STAT3 and Its Implications for Anticancer Therapies Development. Int. J. Mol. Sci. 2018, 19, 1591. [Google Scholar] [CrossRef]
- El-Tanani, M.; Al Khatib, A.O.; Aladwan, S.M.; Abuelhana, A.; McCarron, P.A.; Tambuwala, M.M. Importance of STAT3 signalling in cancer, metastasis and therapeutic interventions. Cell. Signal. 2022, 92, 110275. [Google Scholar] [CrossRef]
- Tošić, I.; Frank, D.A. STAT3 as a mediator of oncogenic cellular metabolism: Pathogenic and therapeutic implications. Neoplasia 2021, 23, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Man, Q.; Huo, F.; Gao, X.; Lin, H.; Li, S.; Wang, J.; Su, F.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef]
- Lee, H.-J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.-J.; Settleman, J. Drug Resistance via Feedback Activation of Stat3 in Oncogene-Addicted Cancer Cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in Cancer Metastasis and Translational Advances. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L. The Etiology of Prostate Cancer; Bott, S.R.J., Ng, K.L., Eds.; Exon Publications: Brisbane, Australia, 2021; ISBN 978-0-6450017-5-4. [Google Scholar]
- Ma, J.-B.; Bai, J.-Y.; Zhang, H.-B.; Jia, J.; Shi, Q.; Yang, C.; Wang, X.; He, D.; Guo, P. KLF5 inhibits STAT3 activity and tumor metastasis in prostate cancer by suppressing IGF1 transcription cooperatively with HDAC1. Cell Death Dis. 2020, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tong, D.; Liu, G.; Xu, J.; Do, K.; Geary, K.; Zhang, D.; Zhang, J.; Zhang, Y.; Li, Y.; et al. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β1/STAT3 axis-regulated EMT. Cell Death Dis. 2017, 8, e3007. [Google Scholar] [CrossRef] [PubMed]
- Gorrab, A.; Pagano, A.; Ayed, K.; Chebil, M.; Derouiche, A.; Kovacic, H.; Gati, A. Leptin Promotes Prostate Cancer Proliferation and Migration by Stimulating STAT3 Pathway. Nutr. Cancer 2020, 73, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Liu, B.; Huang, C.; Fatima, S.; Su, T.; Zhao, X.; Ho, A.H.M.; Han, Q.; Hu, X.; Gong, R.-H.; et al. Signal transducer and activator of transcription-3 drives the high-fat diet-associated prostate cancer growth. Cell Death Dis. 2019, 10, 637. [Google Scholar] [CrossRef]
- Kang, T.-S.; Wang, W.; Zhong, H.-J.; Dong, Z.-Z.; Huang, Q.; Mok, S.W.F.; Leung, C.-H.; Wong, V.K.W.; Ma, D.-L. An anti-prostate cancer benzofuran-conjugated iridium(III) complex as a dual inhibitor of STAT3 and NF-κB. Cancer Lett. 2017, 396, 76–84. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Ko, J.; Um, J.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J. Cell. Physiol. 2020, 236, 5253–5264. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Chen, X.; Fan, Y.; Yang, Y.; Yang, J.; Tan, L. STAT3 phosphorylation is required for the HepaCAM-mediated inhibition of castration-resistant prostate cancer cell viability and metastasis. Prostate 2021, 81, 603–611. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, D.-Y.; Lee, H.-J.; Im, E.; Sim, D.-Y.; Park, J.-E.; Park, W.-Y.; Shim, B.-S.; Kim, S.-H. Inhibition of STAT3/PD-L1 and Activation of miR193a-5p Are Critically Involved in Apoptotic Effect of Compound K in Prostate Cancer Cells. Cells 2021, 10, 2151. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Zhao, Y.-X.; Li, S.-Y.; Qiang, J.-W.; Ji, Y.-Z. Anti-Tumor Effects of Astaxanthin by Inhibition of the Expression of STAT3 in Prostate Cancer. Mar. Drugs 2020, 18, 415. [Google Scholar] [CrossRef]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Liu, Q.; Liu, G.; Xu, J.; Lan, W.; Jiang, Y.; Xiao, H.; Zhang, D.; Jiang, J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2016, 389, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Pan, H.-F.; Shao, S.-L.; Xu, X.-M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, X.; Shen, M.; Yang, D.-R.; Fang, L.; Weng, G.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Inhibition of IL-6-JAK/Stat3 signaling in castration-resistant prostate cancer cells enhances the NK cell-mediated cytotoxicity via alteration of PD-L1/NKG2D ligand levels. Mol. Oncol. 2017, 12, 269–286. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chen, C.-Y.; An, J.-H.; Wu, J.-B.; Shen, H. Normal Basal Epithelial Cells Stimulate the Migration and Invasion of Prostate Cancer Cell RM-1 by TGF-β1/STAT3 Axis in vitro. Cancer Manag. Res. 2021, 13, 3685–3697. [Google Scholar] [CrossRef]
- Thulin, M.H.; Määttä, J.; Linder, A.; Sterbova, S.; Ohlsson, C.; Damber, J.; Widmark, A.; Persson, E. Inhibition of STAT3 prevents bone metastatic progression of prostate cancer in vivo. Prostate 2021, 81, 452–462. [Google Scholar] [CrossRef]
- Schmidt, T. S-Adenosylmethionine affects ERK1/2 and STAT3 pathway in androgen-independent prostate cancer cells. Mol. Biol. Rep. 2022, 49, 4805–4817. [Google Scholar] [CrossRef]
- Noh, K.H.; Jeong, A.J.; Lee, H.; Lee, S.-H.; Yi, E.; Chang, P.-S.; Kwak, C.; Ye, S.-K. Crosstalk between Prostate Cancer Cells and Tumor-Associated Fibroblasts Enhances the Malignancy by Inhibiting the Tumor Suppressor PLZF. Cancers 2020, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cheng, Y.; Xiong, Y. LTF Regulates the Immune Microenvironment of Prostate Cancer Through JAK/STAT3 Pathway. Front. Oncol. 2021, 11, 692117. [Google Scholar] [CrossRef]
- Solís-Martínez, R.; Marentes, M.E.C.; Hernandez-Flores, G.; Ortiz-Lazareno, P.; Mandujano-Álvarez, G.; Cruz-Gálvez, C.; Sierra-Díaz, E.; Rodríguez-Padilla, C.; Jave-Suárez, L.; Aguilar-Lemarroy, A.; et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol. Lett. 2018, 196, 140–148. [Google Scholar] [CrossRef]
- Zheng, T.; Ma, G.; Tang, M.; Li, Z.; Xu, R. IL-8 Secreted from M2 Macrophages Promoted Prostate Tumorigenesis via STAT3/MALAT1 Pathway. Int. J. Mol. Sci. 2018, 20, 98. [Google Scholar] [CrossRef]
- Hellsten, R.; Lilljebjörn, L.; Johansson, M.; Leandersson, K.; Bjartell, A. The STAT3 inhibitor galiellalactone inhibits the generation of MDSC-like monocytes by prostate cancer cells and decreases immunosuppressive and tumorigenic factors. Prostate 2019, 79, 1611–1621. [Google Scholar] [CrossRef]
- Moreira, D.; Adamus, T.; Zhao, X.; Su, Y.-L.; Zhang, Z.; White, S.V.; Swiderski, P.; Lu, X.; DePinho, R.A.; Pal, S.K.; et al. STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers. Clin. Cancer Res. 2018, 24, 5948–5962. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.; Evans-Axelsson, S.; Lundqvist, A.; Johansson, M.; Bjartell, A.; Hellsten, R. Inhibition of STAT3 augments antitumor efficacy of anti-CTLA-4 treatment against prostate cancer. Cancer Immunol. Immunother. 2021, 70, 3155–3166. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Capradossi, F.; Pelliccia, A.; Briganti, S.; Bruni, E.; Traversa, E.; Torino, F.; Reichle, A.; Ghibelli, L. Apoptosis as Driver of Therapy-Induced Cancer Repopulation and Acquired Cell-Resistance (CRAC): A Simple In Vitro Model of Phoenix Rising in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, Y.; Zhang, J.; Jin, X.; Wu, H.; Huang, H. Fructose-1,6-bisphosphatase loss modulates STAT3-dependent expression of PD-L1 and cancer immunity. Theranostics 2020, 10, 1033–1045. [Google Scholar] [CrossRef]
- Lin, Q.; Cao, J.; Du, X.; Yang, K.; Yang, X.; Liang, Z.; Shi, J.; Zhang, J. CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation. Cell Commun. Signal. 2022, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Ansari, M.H.K.; Aziz, S.G.-G.; Bazl, M.R. Alantolactone inhibits stem-like cell phenotype, chemoresistance and metastasis in PC3 cells through STAT3 signaling pathway. Res. Pharm. Sci. 2020, 15, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhao, Y.; Yu, Y.; Fang, J.-M.; Cui, R.; Liu, Z.-Q.; Guo, X.-L.; Xu, Q. Docetaxel-mediated autophagy promotes chemoresistance in castration-resistant prostate cancer cells by inhibiting STAT3. Cancer Lett. 2018, 416, 24–30. [Google Scholar] [CrossRef]
- Canesin, G.; Maggio, V.; Palominos, M.; Stiehm, A.; Contreras, H.R.; Castellón, E.A.; Morote, J.; Paciucci, R.; Maitland, N.J.; Bjartell, A.; et al. STAT3 inhibition with galiellalactone effectively targets the prostate cancer stem-like cell population. Sci. Rep. 2020, 10, 13958. [Google Scholar] [CrossRef] [PubMed]
- Thaper, D.; Vahid, S.; Kaur, R.; Kumar, S.; Nouruzi, S.; Bishop, J.L.; Johansson, M.; Zoubeidi, A. Galiellalactone inhibits the STAT3/AR signaling axis and suppresses Enzalutamide-resistant Prostate Cancer. Sci. Rep. 2018, 8, 17307. [Google Scholar] [CrossRef]
- Luo, J.; Wang, K.; Yeh, S.; Sun, Y.; Liang, L.; Xiao, Y.; Xu, W.; Niu, Y.; Cheng, L.; Maity, S.N.; et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, X.-M.; Wei, S.-Z.; Cui, D.-S.; Deng, K.-L.; Liang, G.; Luo, Y.; Luo, B.; Liang, X.-J. STAT3 as a target for sensitizing prostate cancer cells to irradiation. J. Radiat. Res. 2021, 63, 174–182. [Google Scholar] [CrossRef]
- Guo, Y.; Zang, Y.; Lv, L.; Cai, F.; Qian, T.; Zhang, G.; Feng, Q. IL-8 promotes proliferation and inhibition of apoptosis via STAT3/AKT/NF-κB pathway in prostate cancer. Mol. Med. Rep. 2017, 16, 9035–9042. [Google Scholar] [CrossRef]
- Lim, S.C.; Geleta, B.; Maleki, S.; Richardson, D.R.; Kovačević, Z. The metastasis suppressor NDRG1 directly regulates androgen receptor signaling in prostate cancer. J. Biol. Chem. 2021, 297, 101414. [Google Scholar] [CrossRef]
- Wei, X.; Hou, Y.; Zhang, Y.; Zhang, H.; Sun, Z.; Meng, X.; Wang, Z. Long non-coding RNA MAGI2-AS3 inactivates STAT3 pathway to inhibit prostate cancer cell proliferation via acting as a microRNA-424-5p sponge. J. Cancer 2022, 13, 343–353. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J. miR-583 inhibits the proliferation and invasion of prostate cancer cells by targeting JAK1. Mol. Med. Rep. 2021, 23, 199. [Google Scholar] [CrossRef]
- Jiang, H.; Deng, W.; Zhu, K.; Zeng, Z.; Hu, B.; Zhou, Z.; Xie, A.; Zhang, C.; Fu, B.; Zhou, X.; et al. LINC00467 Promotes Prostate Cancer Progression via M2 Macrophage Polarization and the miR-494-3p/STAT3 Axis. Front. Oncol. 2021, 11, 661431. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.; Liu, Z.; Zhang, C.; Meng, M.; Bai, Z. The long non-coding RNA LINC00473 contributes to cell proliferation via JAK-STAT3 signaling pathway by regulating miR-195-5p/SEPT2 axis in prostate cancer. Biosci. Rep. 2020, 40, BSR20191850. [Google Scholar] [CrossRef]
- Dambal, S.; Alfaqih, M.; Sanders, S.; Maravilla, E.; Ramirez-Torres, A.; Galvan, G.C.; Reis-Sobreiro, M.; Rotinen, M.; Driver, L.M.; Behrove, M.S.; et al. 27-Hydroxycholesterol Impairs Plasma Membrane Lipid Raft Signaling as Evidenced by Inhibition of IL6–JAK–STAT3 Signaling in Prostate Cancer Cells. Mol. Cancer Res. 2020, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.-Y.; Zhang, Z.-H.; Xu, S.; Hong, Q.; Tian, Q.-X.; Ye, Q.-L.; Wang, H.; Yu, D.-X.; Xu, D.-X.; Xie, D.-D. Calcitriol inhibits lipopolysaccharide-induced proliferation, migration and invasion of prostate cancer cells through suppressing STAT3 signal activation. Int. Immunopharmacol. 2020, 82, 106346. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.-X.; Zhang, Z.-H.; Ye, Q.-L.; Xu, S.; Hong, Q.; Xing, W.-Y.; Chen, L.; Yu, D.-X.; Xu, D.-X.; Xie, D.-D. Melatonin Inhibits Migration and Invasion in LPS-Stimulated and -Unstimulated Prostate Cancer Cells Through Blocking Multiple EMT-Relative Pathways. J. Inflamm. Res. 2021, 14, 2253–2265. [Google Scholar] [CrossRef]
- Das, S.K.; Pradhan, A.K.; Bhoopathi, P.; Talukdar, S.; Shen, X.-N.; Sarkar, D.; Emdad, L.; Fisher, P.B. The MDA-9/Syntenin/IGF1R/STAT3 Axis Directs Prostate Cancer Invasion. Cancer Res. 2018, 78, 2852–2863. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Song, H.; Yoon, Y.J.; Park, S.-J.; Kim, S.-Y.; Han, D.C.; Kwon, B.-M. Ethacrynic acid inhibits STAT3 activity through the modulation of SHP2 and PTP1B tyrosine phosphatases in DU145 prostate carcinoma cells. Biochem. Pharmacol. 2020, 175, 113920. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.-H.; Lee, S.G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2016, 8, 17700–17711. [Google Scholar] [CrossRef]
- Cocchiola, R.; Rubini, E.; Altieri, F.; Chichiarelli, S.; Paglia, G.; Romaniello, D.; Carissimi, S.; Giorgi, A.; Giamogante, F.; Macone, A.; et al. STAT3 Post-Translational Modifications Drive Cellular Signaling Pathways in Prostate Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1815. [Google Scholar] [CrossRef]
- Lin, W.; Luo, J.; Sun, Y.; Lin, C.; Li, G.; Niu, Y.; Chang, C. ASC-J9 ® suppresses prostate cancer cell invasion via altering the sumoylation-phosphorylation of STAT3. Cancer Lett. 2018, 425, 21–30. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Gu, L.; Jiang, K.; Wang, S.; Chen, J.; Fang, J.; Guo, X.; Yuan, M.; Shi, J.; et al. Targeting STAT3 enhances NDV-induced immunogenic cell death in prostate cancer cells. J. Cell. Mol. Med. 2020, 24, 4286–4297. [Google Scholar] [CrossRef]
- Peng, H.H.; Wang, J.N.; Xiao, L.F.; Yan, M.; Chen, S.P.; Wang, L.; Yang, K. Elevated Serum FGG Levels Prognosticate and Promote the Disease Progression in Prostate Cancer. Front. Genet. 2021, 12, 651647. [Google Scholar] [CrossRef]
- Wang, J.; Nasser, M.I.; Adlat, S.; Jiang, M.M.; Jiang, N.; Gao, L. Atractylenolide II Induces Apoptosis of Prostate Cancer Cells through Regulation of AR and JAK2/STAT3 Signaling Pathways. Molecules 2018, 23, 3298. [Google Scholar] [CrossRef]
- He, Y.; Khan, M.; Yang, J.; Yao, M.; Yu, S.; Gao, H. Proscillaridin A induces apoptosis, inhibits STAT3 activation and augments doxorubicin toxicity in prostate cancer cells. Int. J. Med. Sci. 2018, 15, 832–839. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, S.-K.; Xu, Q.-Q.; Yuan, H.-Q.; Guo, Y.-X.; Wang, Q.; Kong, F.; Lin, Z.-M.; Sun, D.-Q.; Wang, R.-M.; et al. Acetyl-11-keto-β-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacol. Sin. 2018, 40, 689–698. [Google Scholar] [CrossRef]
- Heidarian, E.; Keloushadi, M. Antiproliferative and anti-invasion effects of carvacrol on PC3 human prostate cancer cells through reducing pSTAT3, pAKT, and pERK1/2 signaling proteins. Int. J. Prev. Med. 2019, 10, 156. [Google Scholar] [CrossRef]
- Yun, S.; Lee, Y.-J.; Choi, J.; Kim, N.D.; Han, D.C.; Kwon, B.-M. Acacetin Inhibits the Growth of STAT3-Activated DU145 Prostate Cancer Cells by Directly Binding to Signal Transducer and Activator of Transcription 3 (STAT3). Molecules 2021, 26, 6204. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, Y.-H.; Lee, Y.-J.; Choi, J.; Kim, C.-H.; Han, D.C.; Kwon, B.-M. 2′-Hydroxycinnamaldehyde inhibits proliferation and induces apoptosis via signal transducer and activator of transcription 3 inactivation and reactive oxygen species generation. Cancer Sci. 2018, 110, 366–378. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yoon, Y.J.; Lee, Y.-J.; Kim, C.-H.; Lee, S.; Choung, D.H.; Han, D.C.; Kwon, B.-M. Piperlongumine derivative, CG-06, inhibits STAT3 activity by direct binding to STAT3 and regulating the reactive oxygen species in DU145 prostate carcinoma cells. Bioorganic Med. Chem. Lett. 2018, 28, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Y.; Jiang, S.; Dang, Y.; Yang, Y.; Zuo, W.; Zhu, Q.; Liu, P.; Gao, Y.; Lu, S. Traditional Chinese medicine CFF-1 exerts a potent anti-tumor immunity to hinder tumor growth and metastasis in prostate cancer through EGFR/JAK1/STAT3 pathway to inhibit PD-1/PD-L1 checkpoint signaling. Phytomedicine 2022, 99, 153939. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.H.; Park, J.Y.; Lee, Y.-J.; Oh, H.-M.; Choi, S.-K.; Han, D.C.; Kwon, B.-M. Methyllucidone inhibits STAT3 activity by regulating the expression of the protein tyrosine phosphatase MEG2 in DU145 prostate carcinoma cells. Bioorganic Med. Chem. Lett. 2018, 28, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, X.; Patel, N.; Xu, X.; Wu, P.; Liu, W.; Zhang, K.; Goodin, S.; Li, D.; Zheng, X. Nobiletin, a citrus polymethoxyflavone, enhances the effects of bicalutamide on prostate cancer cells via down regulation of NF-κB, STAT3, and ERK activation. RSC Adv. 2020, 10, 10254–10262. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Azeem, W.; Shen, Y.; Zhang, S.; Olsen, J.R.; Øyan, A.M.; Ke, X.; Zhang, W.; Kalland, K. Dual androgen receptor (AR) and STAT 3 inhibition by a compound targeting the AR amino-terminal domain. Pharmacol. Res. Perspect. 2018, 6, e00437. [Google Scholar] [CrossRef]
- Marginean, F.E.; Hellsten, R.; Krzyzanowska, A.; Bjartell, A. Nuclear expression of pSTAT3Tyr705 and pSTAT3Ser727 in the stromal compartment of localized hormone-naïve prostate cancer. Pathol. Res. Practice 2022, 232, 153811. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, A.; Don-Doncow, N.; Marginean, F.E.; Gaber, A.; Watson, R.W.; Hellsten, R.; Bjartell, A. Expression of tSTAT3, pSTAT3 727, and pSTAT3 705 in the epithelial cells of hormone-naïve prostate cancer. Prostate 2019, 79, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2019, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Cancer 2020, 21, 104–121. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA: A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Chen, C.-L.; Cen, L.; Kohout, J.; Hutzen, B.; Chan, C.; Hsieh, F.-C.; Loy, A.; Huang, V.; Cheng, G.; Lin, J. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol. Cancer 2008, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Hindupur, S.V.; Schmid, S.C.; Koch, J.A.; Youssef, A.; Baur, E.-M.; Wang, D.; Horn, T.; Slotta-Huspenina, J.; Gschwend, J.E.; Holm, P.S.; et al. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 1106. [Google Scholar] [CrossRef]
- Gatta, L.B.; Melocchi, L.; Bugatti, M.; Missale, F.; Lonardi, S.; Zanetti, B.; Cristinelli, L.; Belotti, S.; Simeone, C.; Ronca, R.; et al. Hyper-Activation of STAT3 Sustains Progression of Non-Papillary Basal-Type Bladder Cancer via FOSL1 Regulome. Cancers 2019, 11, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboushousha, T.; Hammam, O.; Aref, A.; Kamel, A.; Badawy, M.; Hamid, A.A. Tissue Profile of CDK4 and STAT3 as Possible Innovative Therapeutic Targets in Urinary Bladder Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 547–554. [Google Scholar] [CrossRef]
- Tsujita, Y.; Horiguchi, A.; Tasaki, S.; Isono, M.; Asano, T.; Ito, K.; Asano, T.; Mayumi, Y.; Kushibiki, T. STAT3 inhibition by WP1066 suppresses the growth and invasiveness of bladder cancer cells. Oncol. Rep. 2017, 38, 2197–2204. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Chang, S.-F.; Liao, K.-F.; Chiu, S.-C. Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. Int. J. Mol. Sci. 2017, 18, 1616. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Zhang, Z.; Guo, Y.; Wu, Y.; Wang, R.; Wang, L.; Mao, S.; Yao, X. Overexpression of Indoleamine 2,3-Dioxygenase 1 Promotes Epithelial-Mesenchymal Transition by Activation of the IL-6/STAT3/PD-L1 Pathway in Bladder Cancer. Transl. Oncol. 2018, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Dong, N.; Wu, S.; Gui, D.; Ye, Z.; Wu, H.; Zhong, X. miR-4500 suppresses cell proliferation and migration in bladder cancer via inhibition of STAT3/CCR7 pathway. J. Cell. Biochem. 2019, 121, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, X.; Zhang, X.; Xie, Y.; Ji, Z. LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways. Transl. Androl. Urol. 2020, 9, 2251–2261. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; He, J.; Xian, S.; Yang, P.; Mai, Z.; Li, M.; Liu, Y.; Zhang, X. Occludin facilitates tumour angiogenesis in bladder cancer by regulating IL8/STAT3 through STAT4. J. Cell. Mol. Med. 2022, 26, 2363–2376. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, Y.; Gao, Y.; He, Y.; Luo, C.; Sun, W.; Yuan, M.; Wu, X. PLCε promotes urinary bladder cancer cells proliferation through STAT3/LDHA pathway-mediated glycolysis. Oncol. Rep. 2019, 41, 2844–2854. [Google Scholar] [CrossRef]
- He, H.; Yi, L.; Zhang, B.; Yan, B.; Xiao, M.; Ren, J.; Zi, D.; Zhu, L.; Zhong, Z.; Zhao, X.; et al. USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int. J. Biol. Sci. 2021, 17, 2417–2429. [Google Scholar] [CrossRef]
- Sun, N.; Liang, Y.; Chen, Y.; Wang, L.; Li, D.; Liang, Z.; Sun, L.; Wang, Y.; Niu, H. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int. J. Mol. Med. 2019, 44, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Qi, Z.; Pang, Y.; Li, H.; Xie, H.; Wu, J.; Huang, Y.; Zhu, Y.; Shen, Y.; Zhu, Y.; et al. Retinoic Acid–Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019, 79, 2604–2618. [Google Scholar] [CrossRef]
- Ge, Q.; Lu, M.; Ju, L.; Qian, K.; Wang, G.; Wu, C.-L.; Liu, X.; Xiao, Y.; Wang, X. miR-4324-RACGAP1-STAT3-ESR1 feedback loop inhibits proliferation and metastasis of bladder cancer. Int. J. Cancer 2018, 144, 3043–3055. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, X.; Mao, G.; Zhang, X.; Kong, Z. STAT3 enhances radiation-induced tumor migration, invasion and stem-like properties of bladder cancer. Mol. Med. Rep. 2020, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Wei, H. Interleukin 6 signaling maintains the stem-like properties of bladder cancer stem cells. Transl. Cancer Res. 2019, 8, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Petros, F.G. Epidemiology, clinical presentation, and evaluation of upper-tract urothelial carcinoma. Transl. Androl. Urol. 2020, 9, 1794–1798. [Google Scholar] [CrossRef]
- Califano, G.; Ouzaid, I.; Laine-Caroff, P.; Peyrottes, A.; Ruvolo, C.C.; Pradère, B.; Elalouf, V.; Misrai, V.; Hermieu, J.-F.; Shariat, S.F.; et al. Current Advances in Immune Checkpoint Inhibition and Clinical Genomics in Upper Tract Urothelial Carcinoma: State of the Art. Curr. Oncol. 2022, 29, 687–697. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fujita, K.; Hayashi, Y.; Matsushita, M.; Nojima, S.; Jingushi, K.; Kato, T.; Kawashima, A.; Ujike, T.; Nagahara, A.; et al. STAT3 expression is a prognostic marker in upper urinary tract urothelial carcinoma. PLoS ONE 2018, 13, e0201256. [Google Scholar] [CrossRef]
- Huang, W.-T.; Yang, S.-F.; Wu, C.-C.; Chen, W.-T.; Huang, Y.-C.; Su, Y.-C.; Chai, C.-Y. Expression of Signal Transducer and Activator of Transcription 3 and Suppressor of Cytokine Signaling 3 in Urothelial Carcinoma. Kaohsiung J. Med. Sci. 2009, 25, 640–646. [Google Scholar] [CrossRef]
- Li, W.-M.; Huang, C.-N.; Lee, Y.-C.; Chen, S.-H.; Lin, H.-H.; Wu, W.-J.; Li, C.-C.; Yeh, H.-C.; Chang, L.-L.; Hsu, W.-C.; et al. Over-expression of Activated Signal Transducer and Activator of Transcription 3 Predicts Poor Prognosis in Upper Tract Urothelial Carcinoma. Int. J. Med. Sci. 2017, 14, 1360–1367. [Google Scholar] [CrossRef] [Green Version]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Oya, M.; Marumo, K.; Murai, M. STAT3, but not ERKs, mediates the IL-6–induced proliferation of renal cancer cells, ACHN and 769P. Kidney Int. 2002, 61, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.; Sharma, A.; Bai, S.; Heneidi, S.; Lee, T.J.; Kodeboyina, S.K.; Patel, N.; Sharma, S. Comparative STAT3-Regulated Gene Expression Profile in Renal Cell Carcinoma Subtypes. Front. Oncol. 2019, 9, 72. [Google Scholar] [CrossRef]
- Horiguchi, A.; Oya, M.; Shimada, T.; Uchida, A.; Marumo, K.; Murai, M. Activation of Signal Transducer and Activator of Transcription 3 in Renal Cell Carcinoma: A Study of Incidence and Its Association With Pathological Features and Clinical Outcome. J. Urol. 2002, 168, 762–765. [Google Scholar] [CrossRef]
- Jung, J.E.; Lee, H.-G.; Cho, I.-H.; Chung, D.H.; Yoon, S.-H.; Yang, Y.M.; Lee, J.W.; Choi, S.; Park, J.-W.; Ye, S.-K.; et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005, 19, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Asano, T.; Kuroda, K.; Sato, A.; Asakuma, J.; Ito, K.; Hayakawa, M.; Sumitomo, M. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br. J. Cancer 2010, 102, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, Q.; Liu, J.; Wang, S.; Zhang, N.; Yang, Y. The compound AST-003 could effectively promote apoptosis of renal cell carcinoma cells in vitro. Transl. Cancer Res. 2021, 10, 2120–2133. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Luo, Q.; Fu, D.; Li, H.; Li, H.; Zhang, P.; Chong, T. EZH2 enhances the invasive capability of renal cell carcinoma cells via activation of STAT3. Mol. Med. Rep. 2017, 17, 3621–3626. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, D.; Chen, Y.; Su, J.; Wang, Y.; Li, X.; Zhai, W.; Niu, Y.; Yue, D.; Geng, H. G3BP1 promotes tumor progression and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal cell carcinomas. Cell Death Dis. 2018, 9, 501. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.H.; Fu, L.; Song, J.; Xie, D.D.; Yu, D.X.; Xu, D.X.; Sun, G.P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and β-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020, 111, 59–71. [Google Scholar] [CrossRef]
- Chen, L.-B.; Zhu, S.-P.; Liu, T.-P.; Zhao, H.; Chen, P.-F.; Duan, Y.-J.; Hu, R. Cancer Associated Fibroblasts Promote Renal Cancer Progression Through a TDO/Kyn/AhR Dependent Signaling Pathway. Front. Oncol. 2021, 11, 628821. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xu, R.; Song, G.; Lu, H.; Xue, D.; He, X.; Xia, Y. GATA3 suppresses human fibroblasts-induced metastasis of clear cell renal cell carcinoma via an anti-IL6/STAT3 mechanism. Cancer Gene Ther. 2019, 27, 726–738. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Lv, P.; Gao, J.; Wang, M.; Wang, Y. IL-6 is involved in malignancy and doxorubicin sensitivity of renal carcinoma cells. Cell Adhes. Migr. 2017, 12, 28–36. [Google Scholar] [CrossRef]
- Oguro, T.; Ishibashi, K.; Sugino, T.; Hashimoto, K.; Tomita, S.; Takahashi, N.; Yanagida, T.; Haga, N.; Aikawa, K.; Suzutani, T.; et al. Humanised antihuman IL-6R antibody with interferon inhibits renal cell carcinoma cell growth in vitro and in vivo through suppressed SOCS3 expression. Eur. J. Cancer 2013, 49, 1715–1724. [Google Scholar] [CrossRef]
- Tian, P.; Wei, J.; Li, J.; Ren, J.; Yang, J. LncRNA SNHG1 regulates immune escape of renal cell carcinoma by targeting miR-129-3p to activate STAT3 and PD-L1. Cell Biol. Int. 2021, 45, 1546–1560. [Google Scholar] [CrossRef]
- Cuadros, T.; Trilla, E.; Sarro, E.; Vila, M.R.; Vilardell, J.; de Torres, I.; Salcedo, M.; Lopez-Hellin, J.; Sanchez, A.; Cajal, S.R.Y.; et al. Molecular and Cellular Pathobiology HAVCR/KIM-1 Activates the IL-6/STAT-3 Pathway in Clear Cell Renal Cell Carcinoma and Determines Tumor Progression and Patient Outcome. Cancer Res. 2014, 74, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Kamai, T.; Abe, H.; Arai, K.; Yoshida, K.-I. Is Stat3 and/or p53 mRNA expression a prognostic marker for renal cell carcinoma? Biomed. Res. 2009, 30, 171–176. [Google Scholar] [CrossRef]
- Watanabe, A.; Yamamoto, K.; Ioroi, T.; Hirata, S.; Harada, K.; Miyake, H.; Fujisawa, M.; Nakagawa, T.; Yano, I.; Hirai, M. Association of Single Nucleotide Polymorphisms in STAT3, ABCB1, and ABCG2 with Stomatitis in Patients with Metastatic Renal Cell Carcinoma Treated with Sunitinib: A Retrospective Analysis in Japanese Patients. Biol. Pharm. Bull. 2017, 40, 458–464. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shinomiya, K.; Ioroi, T.; Hirata, S.; Harada, K.; Suno, M.; Nishioka, T.; Kume, M.; Makimoto, H.; Nakagawa, T.; et al. Association of Single Nucleotide Polymorphisms in STAT3 with Hand-Foot Skin Reactions in Patients with Metastatic Renal Cell Carcinoma Treated with Multiple Tyrosine Kinase Inhibitors: A Retrospective Analysis in Japanese Patients. Target. Oncol. 2015, 11, 93–99. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ioroi, T.; Kanaya, K.; Shinomiya, K.; Komoto, S.; Hirata, S.; Harada, K.; Watanabe, A.; Suno, M.; Nishioka, T.; et al. STAT3 polymorphism rs4796793 may be a predictive factor of tumor response to multiple tyrosine kinase inhibitors in metastatic renal cell carcinoma in Japanese population. Med. Oncol. 2016, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Torbrand, C.; Wigertz, A.; Drevin, L.; Folkvaljon, Y.; Lambe, M.; Håkansson, U.; Kirrander, P. Socioeconomic factors and penile cancer risk and mortality; a population-based study. Br. J. Urol. 2016, 119, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Tong, S.; Huang, W.; Cai, Y.; Zu, X.; Hu, X. High serum CCL20 is associated with tumor progression in penile cancer. J. Cancer 2020, 11, 6812–6822. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Tong, S.; Li, T.; Zu, X.; Hu, X. Serum CXCL13 Level is Associated with Tumor Progression and Unfavorable Prognosis in Penile Cancer. OncoTargets Ther. 2020, 13, 8757–8769. [Google Scholar] [CrossRef]
- Mo, M.; Li, Y.; Hu, X. Serum CXCL5 level is associated with tumor progression in penile cancer. Biosci. Rep. 2021, 41, BSR20202133. [Google Scholar] [CrossRef]
- Mo, M.; Tong, S.; Yin, H.; Jin, Z.; Zu, X.; Hu, X. SHCBP1 Regulates STAT3/c-Myc Signaling Activation to Promote Tumor Progression in Penile Cancer. Am. J. Cancer Res. 2020, 10, 3138. [Google Scholar]
- Chen, R.; Li, H.; Li, Y.; Fazli, L.; Gleave, M.; Nappi, L.; Dong, X. Loss of Nuclear Functions of HOXA10 Is Associated With Testicular Cancer Proliferation. Front. Oncol. 2018, 8, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golus, M.; Bugajski, P.; Chorbińska, J.; Krajewski, W.; Lemiński, A.; Saczko, J.; Kulbacka, J.; Szydełko, T.; Małkiewicz, B. STAT3 and Its Pathways’ Dysregulation—Underestimated Role in Urological Tumors. Cells 2022, 11, 3024. https://doi.org/10.3390/cells11193024
Golus M, Bugajski P, Chorbińska J, Krajewski W, Lemiński A, Saczko J, Kulbacka J, Szydełko T, Małkiewicz B. STAT3 and Its Pathways’ Dysregulation—Underestimated Role in Urological Tumors. Cells. 2022; 11(19):3024. https://doi.org/10.3390/cells11193024
Chicago/Turabian StyleGolus, Maciej, Piotr Bugajski, Joanna Chorbińska, Wojciech Krajewski, Artur Lemiński, Jolanta Saczko, Julita Kulbacka, Tomasz Szydełko, and Bartosz Małkiewicz. 2022. "STAT3 and Its Pathways’ Dysregulation—Underestimated Role in Urological Tumors" Cells 11, no. 19: 3024. https://doi.org/10.3390/cells11193024








