Structural Basis of β2 Integrin Inside—Out Activation
Abstract
:1. Introduction
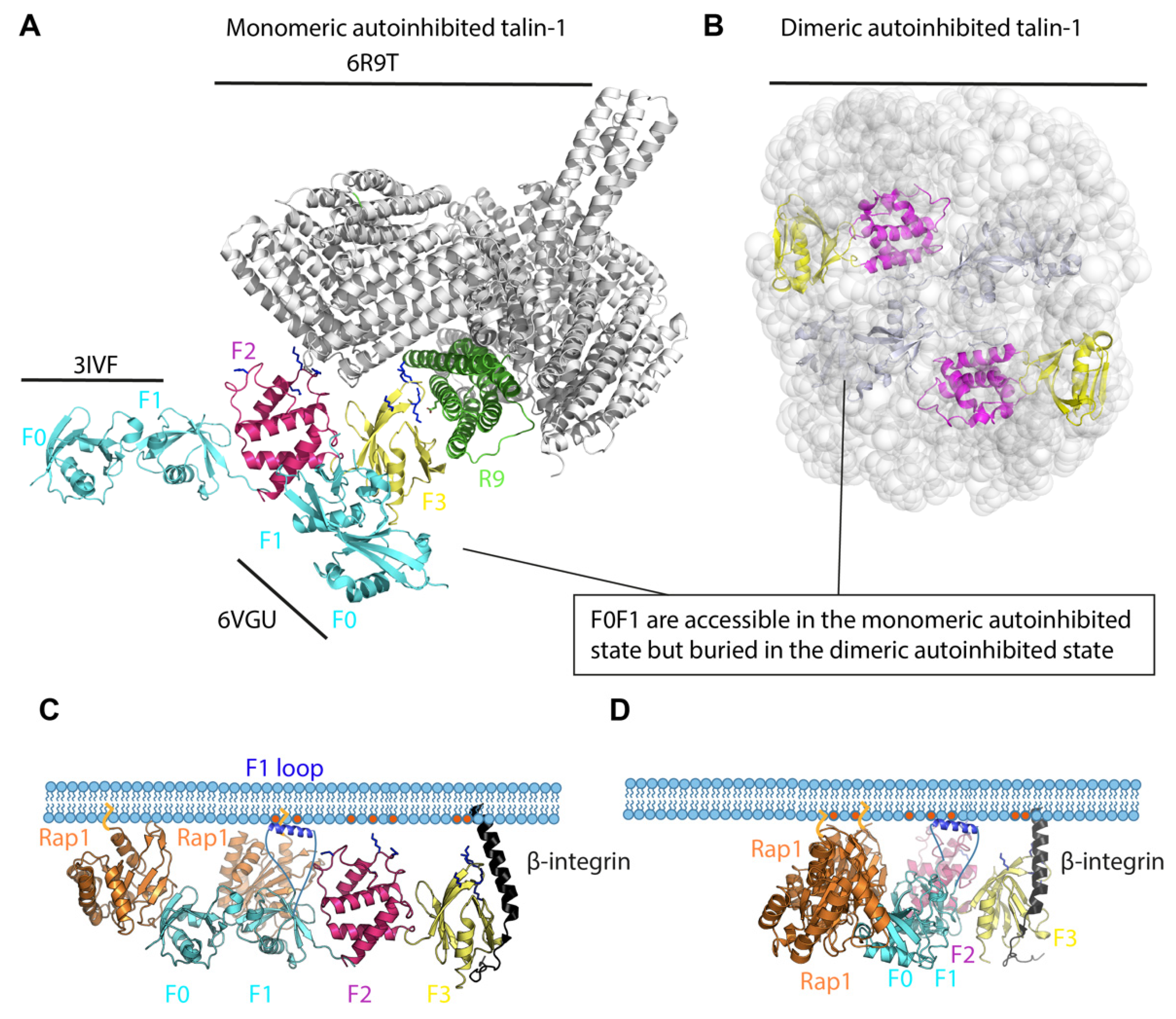
2. Talin-1 Activation
The Talin Head—A Rigid Clover-Leaf or a Versatile Modulatable Structure?
3. Kindlin-3 Activation
4. Talin-1 and Kindlin-3 Binding to β2 Integrin
5. A Model of Integrin Activation
6. Integrating the Known Steps of Integrin Activation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, L.; Moser, M.; Ley, K. Molecular mechanisms of leukocyte beta2 integrin activation. Blood 2022, 139, 3480–3492. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.J.; Handel, T.M.; Proudfoot, A.E. Leukocyte Adhesion: Reconceptualizing Chemokine Presentation by Glycosaminoglycans. Trends Immunol. 2019, 40, 472–481. [Google Scholar] [CrossRef]
- Zarbock, A.; Lowell, C.A.; Ley, K. Spleen Tyrosine Kinase Syk Is Necessary for E-Selectin-Induced αLβ2 Integrin-Mediated Rolling on Intercellular Adhesion Molecule-1. Immunity 2007, 26, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Lefort, C.T.; Rossaint, J.; Moser, M.; Petrich, B.G.; Zarbock, A.; Monkley, S.J.; Critchley, D.R.; Ginsberg, M.H.; Fässler, R.; Ley, K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 2012, 119, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Marki, A.; Wang, Z.; Orecchioni, M.; Makings, J.; Billitti, M.; Wang, E.; Suthahar, S.S.; Kim, K.; Kiosses, W.B.; et al. A humanized β2 integrin knockin mouse reveals localized intra- and extravascular neutrophil integrin activation in vivo. Cell Rep. 2022, 39, 110876. [Google Scholar] [CrossRef]
- Shimaoka, M.; Xiao, T.; Liu, J.-H.; Yang, Y.; Dong, Y.; Jun, C.-D.; McCormack, A.; Zhang, R.; Joachimiak, A.; Takagi, J.; et al. Structures of the αL I Domain and Its Complex with ICAM-1 Reveal a Shape-Shifting Pathway for Integrin Regulation. Cell 2003, 112, 99–111. [Google Scholar] [CrossRef]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Fan, Z.; Ley, K. Leukocyte arrest: Biomechanics and molecular mechanisms of β 2 integrin activation. Biorheology 2016, 52, 353–377. [Google Scholar] [CrossRef]
- Gingras, A.R.; Lagarrigue, F.; Cuevas, M.N.; Valadez, A.J.; Zorovich, M.; McLaughlin, W.; Lopez-Ramirez, M.A.; Seban, N.; Ley, K.; Kiosses, W.B.; et al. Rap1 binding and a lipid-dependent helix in talin F1 domain promote integrin activation in tandem. J. Cell Biol. 2019, 218, 1799–1809. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Yang, J.; Bromberger, T.; Holly, A.; Lu, F.; Liu, H.; Sun, K.; Klapproth, S.; Hirbawi, J.; Byzova, T.; et al. Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun. 2017, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, F.; Kim, C.; Ginsberg, M.H. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 2016, 128, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Coló, G.P.; Lafuente, E.M.; Teixidó, J. The MRL proteins: Adapting cell adhesion, migration and growth. Eur. J. Cell Biol. 2012, 91, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; McArdle, S.; Mark, G.; Mikulski, Z.; Gutierrez, E.; Engelhardt, B.; Deutsch, U.; Ginsberg, M.; Groisman, A.; Ley, K. Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nat. Commun. 2016, 7, 12658. [Google Scholar] [CrossRef] [PubMed]
- Bolomini-Vittori, M.; Montresor, A.; Giagulli, C.; Staunton, D.; Rossi, B.; Martinello, M.; Constantin, G.; Laudanna, C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat. Immunol. 2009, 10, 185–194. [Google Scholar] [CrossRef]
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylänne, J.; Calderwood, D.A. The Molecular Basis of Filamin Binding to Integrins and Competition with Talin. Mol. Cell 2006, 21, 337–347. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Fujioka, Y.; de Pereda, J.M.; García-Alvarez, B.; Nakamoto, T.; Margolis, B.; McGlade, C.J.; Liddington, R.C.; Ginsberg, M.H. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 2272–2277. [Google Scholar] [CrossRef]
- Bonet, R.; Vakonakis, I.; Campbell, I.D. Characterization of 14-3-3-ζ Interactions with Integrin Tails. J. Mol. Biol. 2013, 425, 3060–3072. [Google Scholar] [CrossRef]
- Chang, D.D.; Wong, C.; Smith, H.; Liu, J. ICAP-1, a Novel β1 Integrin Cytoplasmic Domain–associated Protein, Binds to a Conserved and Functionally Important NPXY Sequence Motif of β1 Integrin. J. Cell Biol. 1997, 138, 1149–1157. [Google Scholar] [CrossRef]
- Brunner, M.; Millon-Frémillon, A.; Chevalier, G.; Nakchbandi, I.A.; Mosher, D.; Block, M.R.; Albigès-Rizo, C.; Bouvard, D. Osteoblast mineralization requires β1 integrin/ICAP-1–dependent fibronectin deposition. J. Cell Biol. 2011, 194, 307–322. [Google Scholar] [CrossRef] [Green Version]
- A Otey, C.; Pavalko, F.M.; Burridge, K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J. Cell Biol. 1990, 111, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, D.; Pouwels, J.; De Franceschi, N.; Ivaska, J. Integrin inactivators: Balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 2013, 14, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Grönholm, M. How integrin phosphorylations regulate cell adhesion and signaling. Trends Biochem. Sci. 2021, 47, 265–278. [Google Scholar] [CrossRef]
- Fagerholm, S.; Morrice, N.; Gahmberg, C.G.; Cohen, P. Phosphorylation of the Cytoplasmic Domain of the Integrin CD18 Chain by Protein Kinase C Isoforms in Leukocytes. J. Biol. Chem. 2002, 277, 1728–1738. [Google Scholar] [CrossRef]
- Jahan, F.; Madhavan, S.; Rolova, T.; Viazmina, L.; Grönholm, M.; Gahmberg, C.G. Phosphorylation of the α-chain in the integrin LFA-1 enables β2-chain phosphorylation and α-actinin binding required for cell adhesion. J. Biol. Chem. 2018, 293, 12318–12330. [Google Scholar] [CrossRef]
- Goult, B.T.; Zacharchenko, T.; Bate, N.; Tsang, R.; Hey, F.; Gingras, A.R.; Elliott, P.R.; Roberts, G.C.K.; Ballestrem, C.; Critchley, D.R.; et al. RIAM and Vinculin Binding to Talin Are Mutually Exclusive and Regulate Adhesion Assembly and Turnover. J. Biol. Chem. 2013, 288, 8238–8249. [Google Scholar] [CrossRef]
- Gingras, A.R.; Bate, N.; Goult, B.T.; Hazelwood, L.; Canestrelli, I.; Grossmann, J.G.; Liu, H.; Putz, N.S.M.; Roberts, G.C.K.; Volkmann, N.; et al. The structure of the C-terminal actin-binding domain of talin. EMBO J. 2007, 27, 458–469. [Google Scholar] [CrossRef]
- Yao, M.; Goult, B.T.; Klapholz, B.; Hu, X.; Toseland, C.P.; Guo, Y.; Cong, P.; Sheetz, M.P.; Yan, J. The mechanical response of talin. Nat. Commun. 2016, 7, 11966. [Google Scholar] [CrossRef] [Green Version]
- Goult, B.T.; Yan, J.; Schwartz, M.A. Talin as a mechanosensitive signaling hub. J. Cell Biol. 2018, 217, 3776–3784. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Brown, N.H.; Schwartz, M.A. Talin in mechanotransduction and mechanomemory at a glance. J. Cell Sci. 2021, 134, jcs258749. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.M.; Brahme, N.N.; Calderwood, D.A. Integrin Cytoplasmic Tail Interactions. Biochemistry 2014, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin Binding to Integrin β Tails: A Final Common Step in Integrin Activation. Science 2003, 302, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Bouaouina, M.; Lad, Y.; Calderwood, D.A. The N-terminal Domains of Talin Cooperate with the Phosphotyrosine Binding-like Domain to Activate β1 and β3 Integrins. J. Biol. Chem. 2008, 283, 6118–6125. [Google Scholar] [CrossRef]
- Hemmings, L.; Rees, D.; Ohanian, V.; Bolton, S.; Gilmore, A.; Patel, B.; Priddle, H.; Trevithick, J.; Hynes, R.; Critchley, D. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J. Cell Sci. 1996, 109, 2715–2726. [Google Scholar] [CrossRef]
- Atherton, P.; Stutchbury, B.; Wang, D.-Y.; Jethwa, D.; Tsang, R.; Meiler-Rodriguez, E.; Wang, P.; Bate, N.; Zent, R.; Barsukov, I.L.; et al. Vinculin controls talin engagement with the actomyosin machinery. Nat. Commun. 2015, 6, 10038. [Google Scholar] [CrossRef]
- Azizi, L.; Varela, L.; Turkki, P.; Mykuliak, V.V.; Korpela, S.; O Ihalainen, T.; Church, J.; Hytönen, V.P.; Goult, B.T. Talin variant P229S compromises integrin activation and associates with multifaceted clinical symptoms. Hum. Mol. Genet. 2022. [Google Scholar] [CrossRef]
- Khan, R.; Goult, B. Adhesions Assemble!—Autoinhibition as a Major Regulatory Mechanism of Integrin-Mediated Adhesion. Front. Mol. Biosci. 2019, 6, 144. [Google Scholar] [CrossRef]
- Goksoy, E.; Ma, Y.-Q.; Wang, X.; Kong, X.; Perera, D.; Plow, E.F.; Qin, J. Structural Basis for the Autoinhibition of Talin in Regulating Integrin Activation. Mol. Cell 2008, 31, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Goult, B.T.; Bate, N.; Anthis, N.J.; Wegener, K.L.; Gingras, A.R.; Patel, B.; Barsukov, I.L.; Campbell, I.D.; Roberts, G.C.K.; Critchley, D.R. The Structure of an Interdomain Complex That Regulates Talin Activity. J. Biol. Chem. 2009, 284, 15097–15106. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, J.; Hirbawi, J.; Ye, S.; Perera, H.D.; Goksoy, E.; Dwivedi, P.; Plow, E.F.; Zhang, R.; Qin, J. A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion. Cell Res. 2012, 22, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Dedden, D.; Schumacher, S.; Kelley, C.F.; Zacharias, M.; Biertümpfel, C.; Fässler, R.; Mizuno, N. The Architecture of Talin1 Reveals an Autoinhibition Mechanism. Cell 2019, 179, 120–131.e13. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Goult, B.; Kopp, P.M.; Bate, N.; Grossmann, J.G.; Roberts, G.; Critchley, D.R.; Barsukov, I.L. The Structure of the Talin Head Reveals a Novel Extended Conformation of the FERM Domain. Structure 2010, 18, 1289–1299. [Google Scholar] [CrossRef]
- Zhang, P.; Azizi, L.; Kukkurainen, S.; Gao, T.; Baikoghli, M.; Jacquier, M.-C.; Sun, Y.; Määttä, J.A.E.; Cheng, R.H.; Wehrle-Haller, B.; et al. Crystal structure of the FERM-folded talin head reveals the determinants for integrin binding. Proc. Natl. Acad. Sci. USA 2020, 117, 32402–32412. [Google Scholar] [CrossRef]
- Goult, B.T.; Xu, X.-P.; Gingras, A.R.; Swift, M.; Patel, B.; Bate, N.; Kopp, P.M.; Barsukov, I.L.; Critchley, D.R.; Volkmann, N.; et al. Structural studies on full-length talin1 reveal a compact auto-inhibited dimer: Implications for talin activation. J. Struct. Biol. 2013, 184, 21–32. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Rangarajan, E.S.; Izard, T. The interaction of talin with the cell membrane is essential for integrin activation and focal adhesion formation. Proc. Natl. Acad. Sci. USA 2018, 115, 10339–10344. [Google Scholar] [CrossRef]
- Rangarajan, E.S.; Primi, M.C.; Colgan, L.A.; Chinthalapudi, K.; Yasuda, R.; Izard, T. A distinct talin2 structure directs isoform specificity in cell adhesion. J. Biol. Chem. 2020, 295, 12885–12899. [Google Scholar] [CrossRef]
- Goult, B.; Bouaouina, M.; Elliott, P.; Bate, N.; Patel, B.; Gingras, A.; Grossmann, J.G.; Roberts, G.; Calderwood, D.; Critchley, D.R.; et al. Structure of a double ubiquitin-like domain in the talin head: A role in integrin activation. EMBO J. 2010, 29, 1069–1080. [Google Scholar] [CrossRef]
- Lau, T.-L.; Kim, C.; Ginsberg, M.H.; Ulmer, T.S. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 2009, 28, 1351–1361. [Google Scholar] [CrossRef]
- Bivona, T.G.; Wiener, H.H.; Ahearn, I.; Silletti, J.; Chiu, V.K.; Philips, M.R. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J. Cell Biol. 2004, 164, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, L.; Zhang, H.; Hirbawi, J.; Fukuda, K.; Dwivedi, P.; Liu, J.; Byzova, T.; Plow, E.F.; Wu, J.; et al. Conformational activation of talin by RIAM triggers integrin-mediated cell adhesion. Nat. Commun. 2014, 5, 5880. [Google Scholar] [CrossRef]
- Sun, H.; Lagarrigue, F.; Ginsberg, M.H. The Connection Between Rap1 and Talin1 in the Activation of Integrins in Blood Cells. Front. Cell Dev. Biol. 2022, 10, 908622. [Google Scholar] [CrossRef]
- Plak, K.; Pots, H.; Van Haastert, P.J.M.; Kortholt, A. Direct Interaction between TalinB and Rap1 is necessary for adhesion of Dictyostelium cells. BMC Cell Biol. 2016, 17, 1. [Google Scholar] [CrossRef]
- Camp, D.; Haage, A.; Solianova, V.; Castle, W.M.; Xu, Q.A.; Lostchuck, E.; Goult, B.T.; Tanentzapf, G. Direct binding of Talin to Rap1 is required for cell-ECM adhesion in Drosophila. J. Cell Sci. 2018, 131, jcs225144. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, T.; Klapproth, S.; Rohwedder, I.; Zhu, L.; Mittmann, L.; Reichel, C.A.; Sperandio, M.; Qin, J.; Moser, M. Direct Rap1/Talin1 interaction regulates platelet and neutrophil integrin activity in mice. Blood 2018, 132, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, F.; Gingras, A.R.; Paul, D.S.; Valadez, A.J.; Cuevas, M.N.; Sun, H.; Lopez-Ramirez, M.A.; Goult, B.T.; Shattil, S.J.; Bergmeier, W.; et al. Rap1 binding to the talin 1 F0 domain makes a minimal contribution to murine platelet GPIIb-IIIa activation. Blood Adv. 2018, 2, 2358–2368. [Google Scholar] [CrossRef]
- Bromberger, T.; Zhu, L.; Klapproth, S.; Qin, J.; Moser, M. Rap1 and membrane lipids cooperatively recruit talin to trigger integrin activation. J. Cell Sci. 2019, 132, jcs235531. [Google Scholar] [CrossRef]
- Kalli, A.; Wegener, K.L.; Goult, B.; Anthis, N.; Campbell, I.D.; Sansom, M.S. The Structure of the Talin/Integrin Complex at a Lipid Bilayer: An NMR and MD Simulation Study. Structure 2010, 18, 1280–1288. [Google Scholar] [CrossRef]
- Anthis, N.J.; Wegener, K.L.; Ye, F.; Kim, C.; Goult, B.T.; Lowe, E.D.; Vakonakis, I.; Bate, N.; Critchley, D.R.; Ginsberg, M.H.; et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009, 28, 3623–3632. [Google Scholar] [CrossRef] [Green Version]
- Saltel, F.; Mortier, E.; Hytönen, V.P.; Jacquier, M.-C.; Zimmermann, P.; Vogel, V.; Liu, W.; Wehrle-Haller, B. New PI(4,5)P2- and membrane proximal integrin–binding motifs in the talin head control β3-integrin clustering. J. Cell Biol. 2009, 187, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Malinin, N.L.; Zhang, L.; Choi, J.; Ciocea, A.; Razorenova, O.; Ma, Y.-Q.; A Podrez, E.; Tosi, M.; Lennon, D.P.; I Caplan, A.; et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat. Med. 2009, 15, 313–318. [Google Scholar] [CrossRef]
- Svensson, L.; Howarth, K.; McDowall, A.; Patzak, I.; Evans, R.; Ussar, S.; Moser, M.; Metin, A.; Fried, M.; Tomlinson, I.; et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat. Med. 2009, 15, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.W.; van de Vijver, E.; Weterman, M.A.J.; de Boer, M.; Tool, A.T.J.; Berg, T.K.V.D.; Moser, M.; Jakobs, M.E.; Seeger, K.; Sanal, O.; et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 2009, 113, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Bouaouina, M.; Harburger, D.S.; Bate, N.; Patel, B.; Anthis, N.J.; Campbell, I.D.; Calderwood, D.A.; Barsukov, I.L.; Roberts, G.C.; et al. The Structure of the N-Terminus of Kindlin-1: A Domain Important for αIIbβ3 Integrin Activation. J. Mol. Biol. 2009, 394, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.-L.; Tan, S.-M.; Bhattacharjya, S. NMR Characterization and Membrane Interactions of the Loop Region of Kindlin-3 F1 Subdomain. PLoS ONE 2016, 11, e0153501. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Y.; Sun, K.; Yang, H.; Liu, J.; Wang, M.; Zhang, Z.; Lin, J.; Wu, C.; Wei, Z.; et al. Structural basis of kindlin-mediated integrin recognition and activation. Proc. Natl. Acad. Sci. USA 2017, 114, 9349–9354. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, D.; Ni, Y.; Zhang, T.; Cao, Z.; Xu, Z.; Nguyen, H.; Zhang, J.; White, G.C.; Ding, J.; et al. Structure basis of the FERM domain of kindlin-3 in supporting integrin αIIbβ3 activation in platelets. Blood Adv. 2020, 4, 3128–3135. [Google Scholar] [CrossRef]
- Ni, T.; Kalli, A.C.; Naughton, F.B.; Yates, L.A.; Naneh, O.; Kozorog, M.; Anderluh, G.; Sansom, M.S.; Gilbert, R.J. Structure and lipid-binding properties of the kindlin-3 pleckstrin homology domain. Biochem. J. 2017, 474, 539–556. [Google Scholar] [CrossRef]
- Bu, W.; Levitskaya, Z.; Loh, Z.Y.; Jin, S.; Basu, S.; Ero, R.; Yan, X.; Wang, M.; Ngan, S.F.C.; Sze, S.K.; et al. Structural basis of human full-length kindlin-3 homotrimer in an auto-inhibited state. PLoS Biol. 2020, 18, e3000755. [Google Scholar] [CrossRef]
- Hart, R.; Stanley, P.; Chakravarty, P.; Hogg, N. The Kindlin 3 Pleckstrin Homology Domain Has an Essential Role in Lymphocyte Function-associated Antigen 1 (LFA-1) Integrin-mediated B Cell Adhesion and Migration. J. Biol. Chem. 2013, 288, 14852–14862. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Marki, A.; Roy, P.; McArdle, S.; Sun, H.; Fan, Z.; Gingras, A.R.; Ginsberg, M.H.; Ley, K. Kindlin-3 recruitment to the plasma membrane precedes high-affinity β2-integrin and neutrophil arrest from rolling. Blood 2021, 137, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The Tail of Integrins, Talin, and Kindlins. Science 2009, 324, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Kahner, B.N.; Kato, H.; Banno, A.; Ginsberg, M.H.; Shattil, S.J.; Ye, F. Kindlins, Integrin Activation and the Regulation of Talin Recruitment to αIIbβ3. PLoS ONE 2012, 7, e34056. [Google Scholar] [CrossRef]
- Morrison, V.; MacPherson, M.; Savinko, T.; Lek, H.S.; Prescott, A.; Fagerholm, S.C. The β2 integrin–kindlin-3 interaction is essential for T-cell homing but dispensable for T-cell activation in vivo. Blood 2013, 122, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Ueda, Y.; Kinashi, T. Kindlin-3 disrupts an intersubunit association in the integrin LFA1 to trigger positive feedback activation by Rap1 and talin1. Sci. Signal. 2021, 14, eabf2184. [Google Scholar] [CrossRef]
- Bachir, A.I.; Zareno, J.; Moissoglu, K.; Plow, E.F.; Gratton, E.; Horwitz, A.R. Integrin-Associated Complexes Form Hierarchically with Variable Stoichiometry in Nascent Adhesions. Curr. Biol. 2014, 24, 1845–1853. [Google Scholar] [CrossRef]
- Kim, M.; Carman, C.V.; Springer, T.A. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science 2003, 301, 1720–1725. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Lyu, Q.; Ley, K.; Goult, B.T. Structural Basis of β2 Integrin Inside—Out Activation. Cells 2022, 11, 3039. https://doi.org/10.3390/cells11193039
Wen L, Lyu Q, Ley K, Goult BT. Structural Basis of β2 Integrin Inside—Out Activation. Cells. 2022; 11(19):3039. https://doi.org/10.3390/cells11193039
Chicago/Turabian StyleWen, Lai, Qingkang Lyu, Klaus Ley, and Benjamin T. Goult. 2022. "Structural Basis of β2 Integrin Inside—Out Activation" Cells 11, no. 19: 3039. https://doi.org/10.3390/cells11193039
APA StyleWen, L., Lyu, Q., Ley, K., & Goult, B. T. (2022). Structural Basis of β2 Integrin Inside—Out Activation. Cells, 11(19), 3039. https://doi.org/10.3390/cells11193039






