Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Anesthesia, and Euthanasia
2.2. Study Design and Study Groups
2.3. Ovariectomy Procedure
2.4. Optic Nerve Crush Procedure
2.5. Electroretinography
2.6. Immunohistochemistry
2.7. Cell Count
2.8. Western Blot
2.9. Statistics
3. Results
3.1. Two Weeks of Surgical Menopause Induced by Ovariectomy Led to the Loss of Retinal Interneurons but Not RGCs in the Rat Model
3.2. Two Weeks of Surgical Menopause Evoked Cellular Translocation of ERβ and Increased Retinal Neuroinflammation
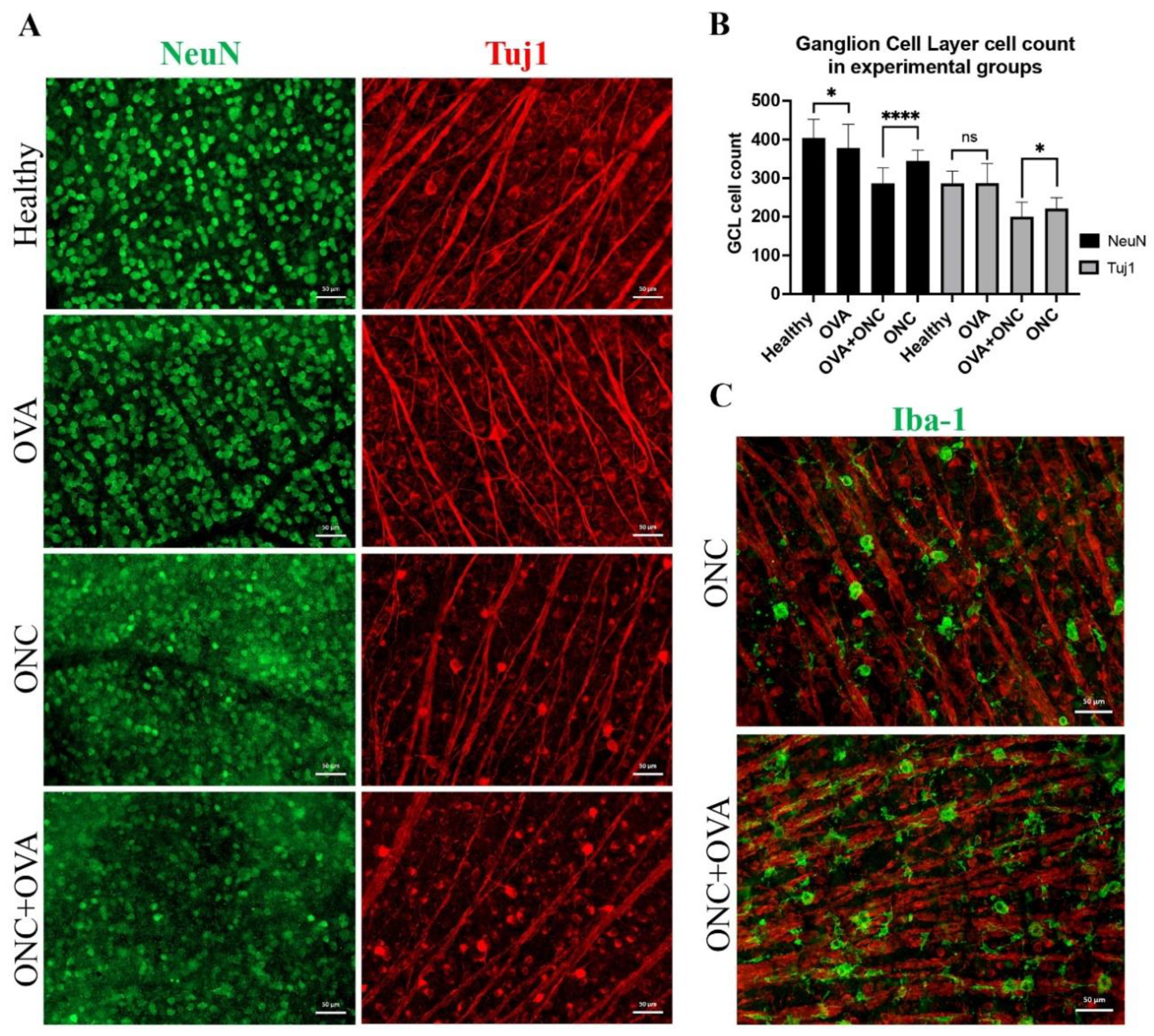
3.3. Six Weeks of Surgical Menopause Affected Retinal Interneuron Counts and Exacerbated RGC Neurodegeneration in Acute Optic Neuropathy
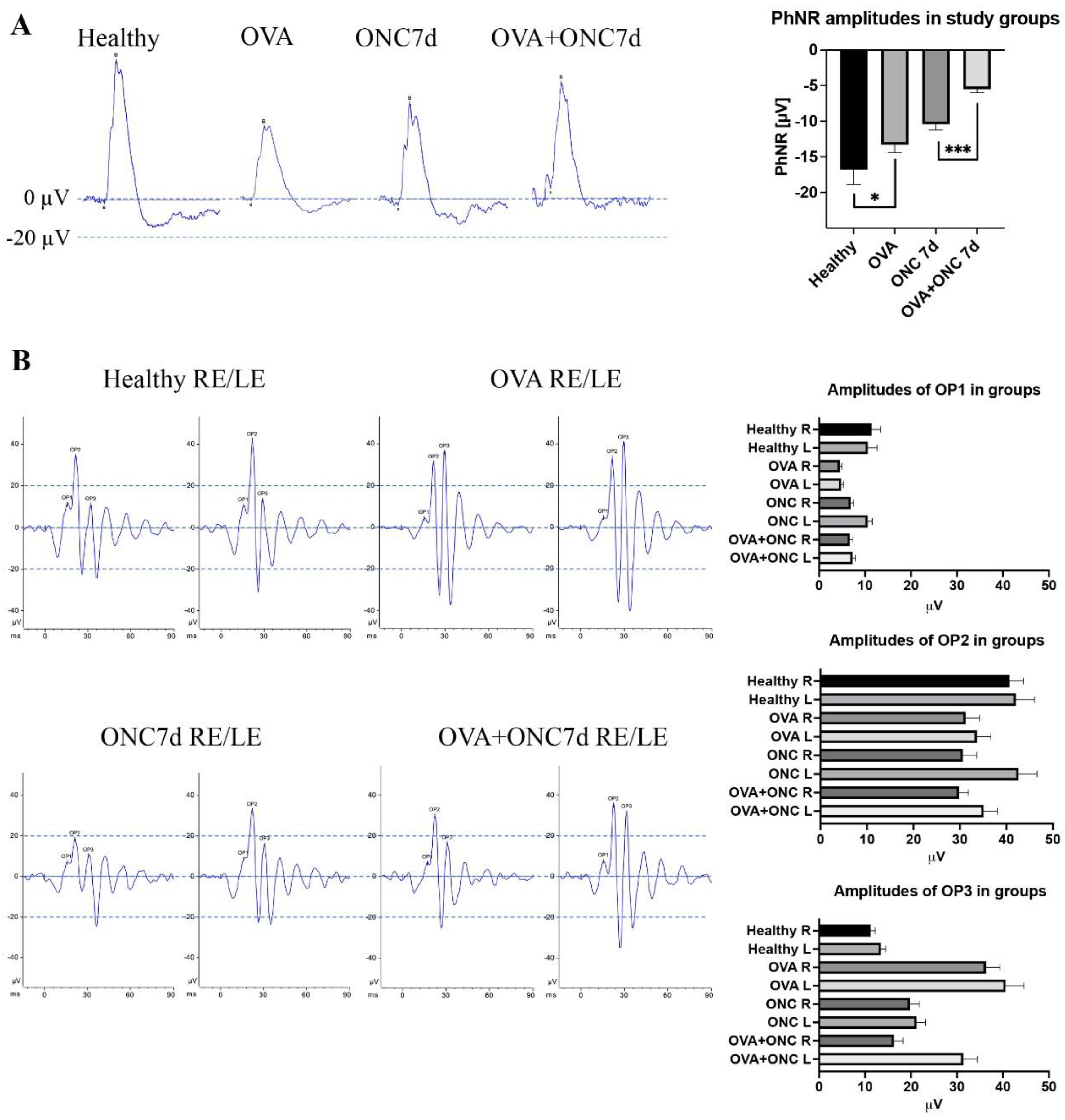
3.4. The Electrical Function of Different Populations of Retinal Neurons Was Compromised by Both Ovariectomy and Optic Nerve Crush
3.5. Human Glaucomatous Retinas Revealed Alterations in Estrogen Receptor Expression and Cellular Localization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Segura, L.M.; Azcoitia, I.; DonCarlos, L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001, 63, 29–60. [Google Scholar] [CrossRef]
- Christiansen, C.; Riis, B.J. 17β-Estradiol and Continuous Norethisterone: A Unique Treatment for Established Osteoporosis in Elderly Women. J. Clin. Endocrinol. Metab. 1990, 71, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.; Riis, B.J. Five years with continuous combined oestrogen/ progestogen therapy. Effects on calcium metabolism, lipoproteins, and bleeding pattern. BJOG: Int. J. Obstet. Gynaecol. 1990, 97, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Gurvich, C.; Le, J.; Thomas, N.; Thomas, E.H.X.; Kulkarni, J. Sex Hormones and Cognition in Aging. Vitam. Horm. 2021, 115, 511–533. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Maglione, A.; Rolla, S.; De Mercanti, S.F.; Cutrupi, S.; Clerico, M. The Adaptive Immune System in Multiple Sclerosis: An Estrogen-Mediated Point of View. Cells 2019, 8, 1280. [Google Scholar] [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2014, 16, 17–29. [Google Scholar] [CrossRef]
- Kövesdi, E.; Szabó-Meleg, E.; Abrahám, I.M. The Role of Estradiol in Traumatic Brain Injury: Mechanism and Treatment Potential. Int. J. Mol. Sci. 2020, 22, 11. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Medica. 2014, 24, 329–342. [Google Scholar] [CrossRef]
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The Estrogenic Retina: The Potential Contribution to Healthy Aging and Age-Related Neurodegenerative Diseases of the Retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Gonadal Hormones and Retinal Disorders: A Review. Front. Endocrinol. 2018, 9, 66. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Talwar, N.; Nan, B.; Musch, D.; Pasquale, L.R.; Stein, J.D. The Potential Association Between Postmenopausal Hormone Use and Primary Open-Angle Glaucoma. JAMA Ophthalmol. 2014, 132, 298–303. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-Estradiol Eye Drops Protect the Retinal Ganglion Cell Layer and Preserve Visual Function in an in Vivo Model of Glaucoma. Mol. Pharm. 2013, 10, 3253–3261. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Gender Differences in Ocular Blood Flow. Curr. Eye Res. 2014, 40, 201–212. [Google Scholar] [CrossRef]
- Davis, B.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Nuschke, A.C.; Farrell, S.; Levesque, J.M.; Chauhan, B.C. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss. Exp. Eye Res. 2015, 141, 111–124. [Google Scholar] [CrossRef]
- Malik, R.; Swanson, W.; Garway-Heath, D. ‘Structure-function relationship’ in glaucoma: Past thinking and current concepts. Clin. Exp. Ophthalmol. 2012, 40, 369–380. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Retinal Ganglion Cell Apoptotic Pathway in Glaucoma: Initiating and Downstream Mechanisms. Prog. Brain Res. 2015, 220, 37–57. [Google Scholar] [CrossRef]
- Doozandeh, A.; Yazdani, S. Neuroprotection in Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2016, 52, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.C.; Liu, J. Neurodegeneration and Neuroprotection in Glaucoma. Yale J. Biol. Med. 2016, 89, 73–79. [Google Scholar] [PubMed]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Huang, P.; Zhang, C. Neuroprotective therapies for glaucoma. Drug Des. Dev. Ther. 2015, 9, 1469–1479. [Google Scholar] [CrossRef]
- Dikopf, M.S.; Vajaranant, T.S.; Edward, D.P. Topical treatment of glaucoma: Established and emerging pharmacology. Expert. Opin. Pharmacother. 2017, 18, 885–898. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- Sena, D.F.; Lindsley, K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2017, 2017, CD006539. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Munemasa, Y.; Hayashi, Y.; Kuribayashi, J.; Koseki, N.; Kojima, K.; Kumai, T.; Ueno, S. Axonal Protection by 17β-Estradiol through Thioredoxin-1 in Tumor Necrosis Factor-Induced Optic Neuropathy. Endocrinology 2011, 152, 2775–2785. [Google Scholar] [CrossRef][Green Version]
- Nakazawa, T.; Takahashi, H.; Shimura, M. Estrogen has a neuroprotective effect on axotomized RGCs through ERK signal transduction pathway. Brain Res. 2006, 1093, 141–149. [Google Scholar] [CrossRef]
- Bernstein, S.L.; Mehrabyan, Z.; Guo, Y.; Moianie, N. Estrogen Is Not Neuroprotective in a Rodent Model of Optic Nerve Stroke. Mol. Vis. 2007, 13, 1920–1925. [Google Scholar]
- Nonaka, A.; Kiryu, J.; Tsujikawa, A.; Yamashiro, K.; Miyamoto, K.; Nishiwaki, H.; Mandai, M.; Honda, Y.; Ogura, Y. Administration of 17beta-Estradiol Attenuates Retinal Ischemia-Reperfusion Injury in Rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2689–2696. [Google Scholar]
- Giordano, C.; Montopoli, M.; Perli, E.; Orlandi, M.; Fantin, M.; Ross-Cisneros, F.N.; Caparrotta, L.; Martinuzzi, A.; Ragazzi, E.; Ghelli, A.; et al. Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy. Brain 2010, 134, 220–234. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Zhu, C.; Feng, Y.; Wang, S.; Shahzad, M.; Hu, C.; Mo, M.; Du, F.; Yu, X. 17β-Estradiol Impedes Bax-Involved Mitochondrial Apoptosis of Retinal Nerve Cells Induced by Oxidative Damage via the Phosphatidylinositol 3-Kinase/Akt Signal Pathway. J. Mol. Neurosci. 2013, 50, 482–493. [Google Scholar] [CrossRef]
- Russo, R.; Cavaliere, F.; Watanabe, C.; Nucci, C.; Bagetta, G.; Corasaniti, M.T.; Sakurada, S.; Morrone, L.A. 17Beta-Estradiol Prevents Retinal Ganglion Cell Loss Induced by Acute Rise of Intraocular Pressure in Rat. Prog. Brain Res. 2008, 173, 583–590. [Google Scholar] [CrossRef]
- Vajaranant, T.S.; Pasquale, L.R. Estrogen deficiency accelerates aging of the optic nerve. Menopause 2012, 19, 942–947. [Google Scholar] [CrossRef]
- Hutchinson, C.V.; A Walker, J.; Davidson, C. Oestrogen, ocular function and low-level vision: A review. J. Endocrinol. 2014, 223, R9–R18. [Google Scholar] [CrossRef]
- Tan, H.-B.; Shen, X.; Cheng, Y.; Jiao, Q.; Yang, Z.-J.; Zhong, Y.-S. Evaluation of a partial optic nerve crush model in rats. Exp. Ther. Med. 2012, 4, 401–404. [Google Scholar] [CrossRef][Green Version]
- Maeda, K.; Sawada, A.; Matsubara, M.; Nakai, Y.; Hara, A.; Yamamoto, T. A Novel Neuroprotectant against Retinal Ganglion Cell Damage in a Glaucoma Model and an Optic Nerve Crush Model in the Rat. Investig. Opthalmol. Vis. Sci. 2004, 45, 851–856. [Google Scholar] [CrossRef][Green Version]
- Cameron, E.G.; Xia, X.; Galvao, J.; Ashouri, M.; Kapiloff, M.S.; Goldberg, J.L. Optic Nerve Crush in Mice to Study Retinal Ganglion Cell Survival and Regeneration. Bio-Protoc. 2020, 10, e3559. [Google Scholar] [CrossRef]
- Gellrich, N.-C.; Schimming, R.; Zerfowski, M.; Eysel, U. Quantification of histological changes after calibrated crush of the intraorbital optic nerve in rats. Br. J. Ophthalmol. 2002, 86, 233–237. [Google Scholar] [CrossRef][Green Version]
- Smedowski, A.; Liu, X.; Pietrucha-Dutczak, M.; Matuszek, I.; Varjosalo, M.; Lewin-Kowalik, J. Predegenerated Schwann cells–a novel prospect for cell therapy for glaucoma: Neuroprotection, neuroregeneration and neuroplasticity. Sci. Rep. 2016, 6, 23187. [Google Scholar] [CrossRef] [PubMed]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling Menopause: The Utility of Rodents in Translational Behavioral Endocrinology Research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.S.; Douglass, A.; Vo, H.; Feola, A.J. Ovariectomy worsens visual function after mild optic nerve crush in rodents. Exp. Eye Res. 2020, 202, 108333. [Google Scholar] [CrossRef] [PubMed]
- Feola, A.J.; Fu, J.; Allen, R.; Yang, V.; Campbell, I.C.; Ottensmeyer, A.; Ethier, C.R.; Pardue, M. Menopause exacerbates visual dysfunction in experimental glaucoma. Exp. Eye Res. 2019, 186, 107706. [Google Scholar] [CrossRef]
- Feola, A.J.; Sherwood, J.M.; Pardue, M.T.; Overby, D.R.; Ethier, C.R. Age and Menopause Effects on Ocular Compliance and Aqueous Outflow. Investig. Opthalmol. Vis. Sci. 2020, 61, 16. [Google Scholar] [CrossRef]
- Sheldahl, L.; Shapiro, R.; Bryant, D.; Koerner, I.; Dorsa, D. Estrogen induces rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience 2008, 153, 751–761. [Google Scholar] [CrossRef]
- Cascio, C.; Russo, D.; Drago, G.; Galizzi, G.; Passantino, R.; Guarneri, R. 17β-Estradiol synthesis in the adult male rat retina. Exp. Eye Res. 2007, 85, 166–172. [Google Scholar] [CrossRef]
- Giddabasappa, A.; Bauler, M.; Yepuru, M.; Chaum, E.; Dalton, J.T.; Eswaraka, J. 17-β Estradiol Protects ARPE-19 Cells from Oxidative Stress through Estrogen Receptor-β. Investig. Opthalmol. Vis. Sci. 2010, 51, 5278–5287. [Google Scholar] [CrossRef]
- Poola, I.; Abraham, J.; Baldwin, K.; Saunders, A.; Bhatnagar, R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERβ isoforms. Endocrine 2005, 27, 227–268. [Google Scholar] [CrossRef]
- Petersen, D.N.; Tkalcevic, G.T.; Koza-Taylor, P.H.; Turi, T.G.; Brown, T.A. Identification of Estrogen Receptor 2. A Functional Variant of Estrogen Receptor Expressed in Normal Rat Tissues. Endocrinology 1998, 139, 1082–1092. [Google Scholar] [CrossRef]
- Peng, B.; Lu, B.; Leygue, E.; Murphy, L.C. Putative functional characteristics of human estrogen receptor-beta isoforms. J. Mol. Endocrinol. 2003, 30, 13–29. [Google Scholar] [CrossRef]
- Lavoie, H.A.; DeSimone, D.C.; Gillio-Meina, C.; Hui, Y.Y. Cloning and Characterization of Porcine Ovarian Estrogen Receptor β Isoforms1. Biol. Reprod. 2002, 66, 616–623. [Google Scholar] [CrossRef]
- Mukhara, D.; Oh, U.; Neigh, G.N. Neuroinflammation. Handb. Clin. Neurol. 2020, 175, 235–259. [Google Scholar] [CrossRef]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef]
- Streit, W.J.; Conde, J.R.; Fendrick, S.E.; Flanary, B.E.; Mariani, C.L. Role of microglia in the central nervous system’s immune response. Neurol. Res. 2005, 27, 685–691. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.-S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2018, 97, 70–76. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer′s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Honig, M.G.; Del Mar, N.A.; Henderson, D.L.; O’Neal, D.; Doty, J.B.; Cox, R.; Li, C.; Perry, A.M.; Moore, B.M.; Reiner, A. Raloxifene Modulates Microglia and Rescues Visual Deficits and Pathology After Impact Traumatic Brain Injury. Front. Neurosci. 2021, 15, 701317. [Google Scholar] [CrossRef]
- Hulsman, C.A.A.; Westendorp, I.C.D.; Ramrattan, R.S.; Wolfs, R.C.W.; Witteman, J.C.M.; Vingerling, J.R.; Hofman, A.; De Jong, P.T.V.M. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am. J. Epidemiol. 2001, 154, 138–144. [Google Scholar] [CrossRef]
- Vajaranant, T.S.; Grossardt, B.R.; Maki, P.M.; Pasquale, L.R.; Sit, A.J.; Shuster, L.T.; Rocca, W.A. Risk of glaucoma after early bilateral oophorectomy. Menopause 2014, 21, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Shao, M.; Li, Y.; Song, Y.; Sun, X.; Cao, W. Association Between 17-β-Estradiol and Interleukin-8 and Visual Field Progression in Postmenopausal Women with Primary Angle Closure Glaucoma. Am. J. Ophthalmol. 2020, 217, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.; Agey, P.; Hare, W.A. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis. Neurosci. 2004, 21, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, M.; Dorfman, A.L.; Trang, N.; Gauthier, M.; Little, J.M.; Lina, J.-M.; Lachapelle, P. Assessing the Contribution of the Oscillatory Potentials to the Genesis of the Photopic ERG with the Discrete Wavelet Transform. BioMed Res. Int. 2016, 2016, 2790194. [Google Scholar] [CrossRef]
- Turner, R.J.; Kerber, I.J. Eu-estrogenemia and Retinal Blood Flow. Investig. Opthalmol. Vis. Sci. 2010, 51, 6901–6902. [Google Scholar] [CrossRef][Green Version]
- Deschênes, M.C.; Descovich, D.; Moreau, M.; Granger, L.; Kuchel, G.; Mikkola, T.; Fick, G.H.; Chemtob, S.; Vaucher, E.; Lesk, M.R. Postmenopausal Hormone Therapy Increases Retinal Blood Flow and Protects the Retinal Nerve Fiber Layer. Investig. Opthalmol. Vis. Sci. 2010, 51, 2587–2600. [Google Scholar] [CrossRef]
- Plotnikova, T.; Shulgau, Z.; Aliev, O.; Borovskaya, T. Hyperviscosity syndrome in ovariectomized rats. Bull. Exp. Biol. Med. 2008, 146, 92–95. [Google Scholar] [CrossRef]
- Dewundara, S.S.; Wiggs, J.L.; Sullivan, D.A.; Pasquale, L.R. Is Estrogen a Therapeutic Target for Glaucoma? Semin. Ophthalmol. 2016, 31, 140–146. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olakowska, E.; Rodak, P.; Pacwa, A.; Machowicz, J.; Machna, B.; Lewin-Kowalik, J.; Smedowski, A. Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy. Cells 2022, 11, 3062. https://doi.org/10.3390/cells11193062
Olakowska E, Rodak P, Pacwa A, Machowicz J, Machna B, Lewin-Kowalik J, Smedowski A. Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy. Cells. 2022; 11(19):3062. https://doi.org/10.3390/cells11193062
Chicago/Turabian StyleOlakowska, Edyta, Piotr Rodak, Anna Pacwa, Joanna Machowicz, Bartosz Machna, Joanna Lewin-Kowalik, and Adrian Smedowski. 2022. "Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy" Cells 11, no. 19: 3062. https://doi.org/10.3390/cells11193062
APA StyleOlakowska, E., Rodak, P., Pacwa, A., Machowicz, J., Machna, B., Lewin-Kowalik, J., & Smedowski, A. (2022). Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy. Cells, 11(19), 3062. https://doi.org/10.3390/cells11193062





