Endothelial Nogo-B Suppresses Cancer Cell Proliferation via a Paracrine TGF-β/Smad Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Specimens
2.2. Laser Capture Microdissection (LCM)
2.3. Quantitative Real-Time PCR
2.4. Immunohistochemistry and Microarray Analysis
2.5. Lentivirus and Cell Lines
2.6. Cell Proliferation Analysis
2.7. In Vitro Co-Culture Assays
2.8. In Vivo Co-Culture Assays
2.9. Antibody Arrays
2.10. Luciferase Reporter Assays and ELISA
2.11. Western Blot Assays
2.12. Matrigel Tube Formation Assays
2.13. Scratch Wound-Healing Assay
2.14. Transwell Migration Assays
2.15. Statistical Analysis
3. Results
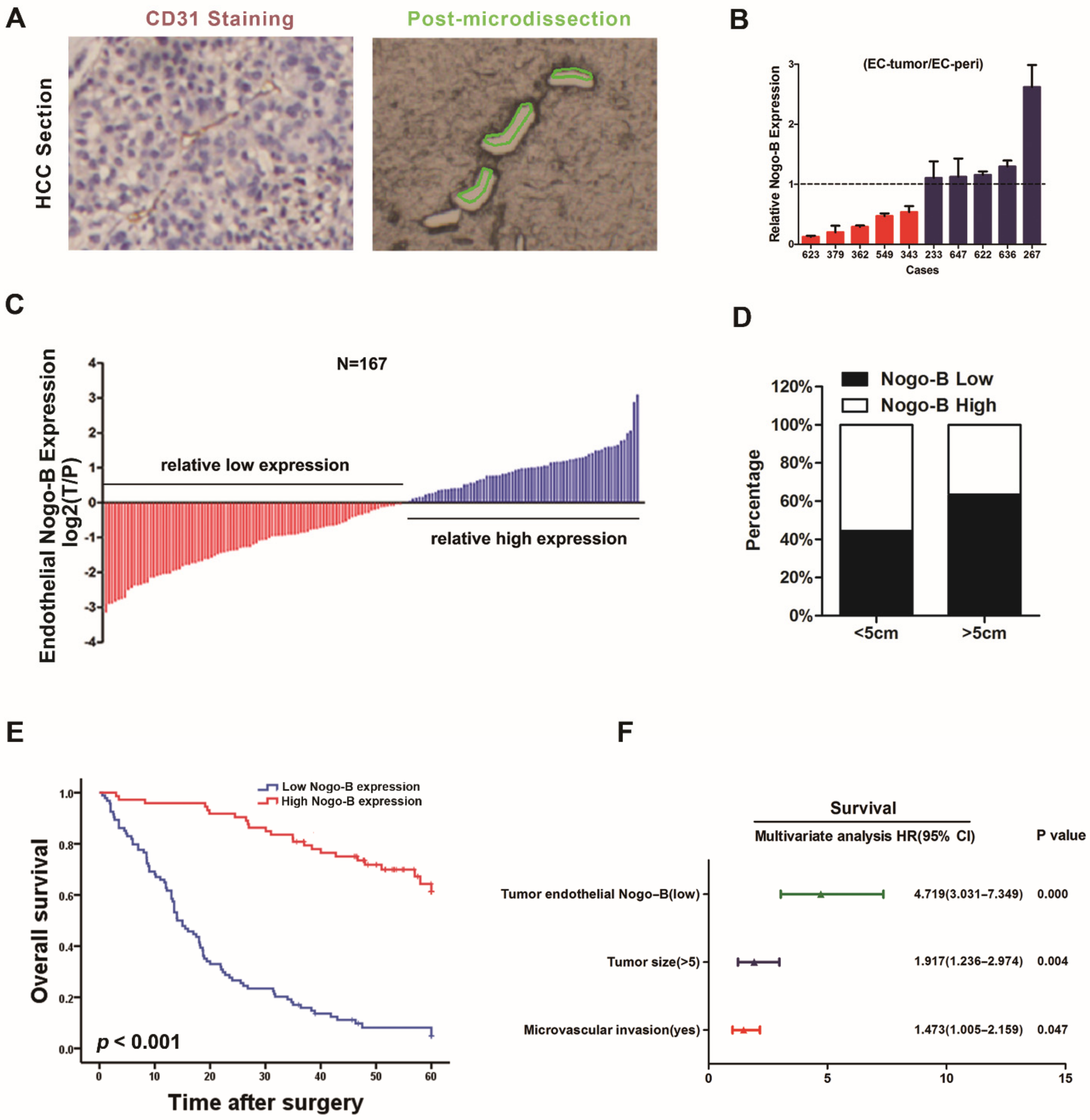
3.1. Reduction of Tumor Endothelial Nogo-B Expression Predicts a Poor Prognosis for Patients
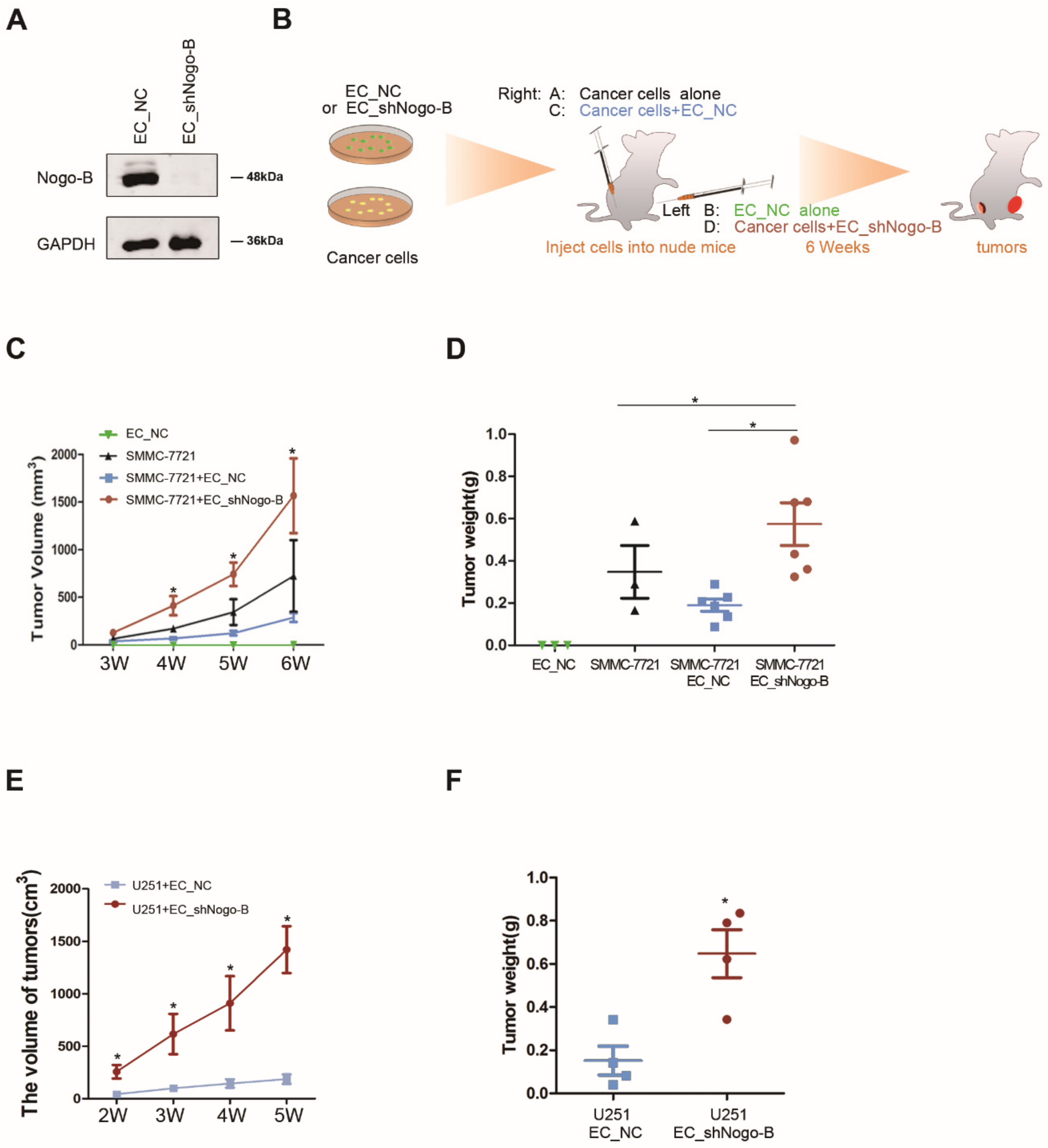
3.2. Down-Regulation of Endothelial Nogo-B Expression Facilitates Cancer Growth
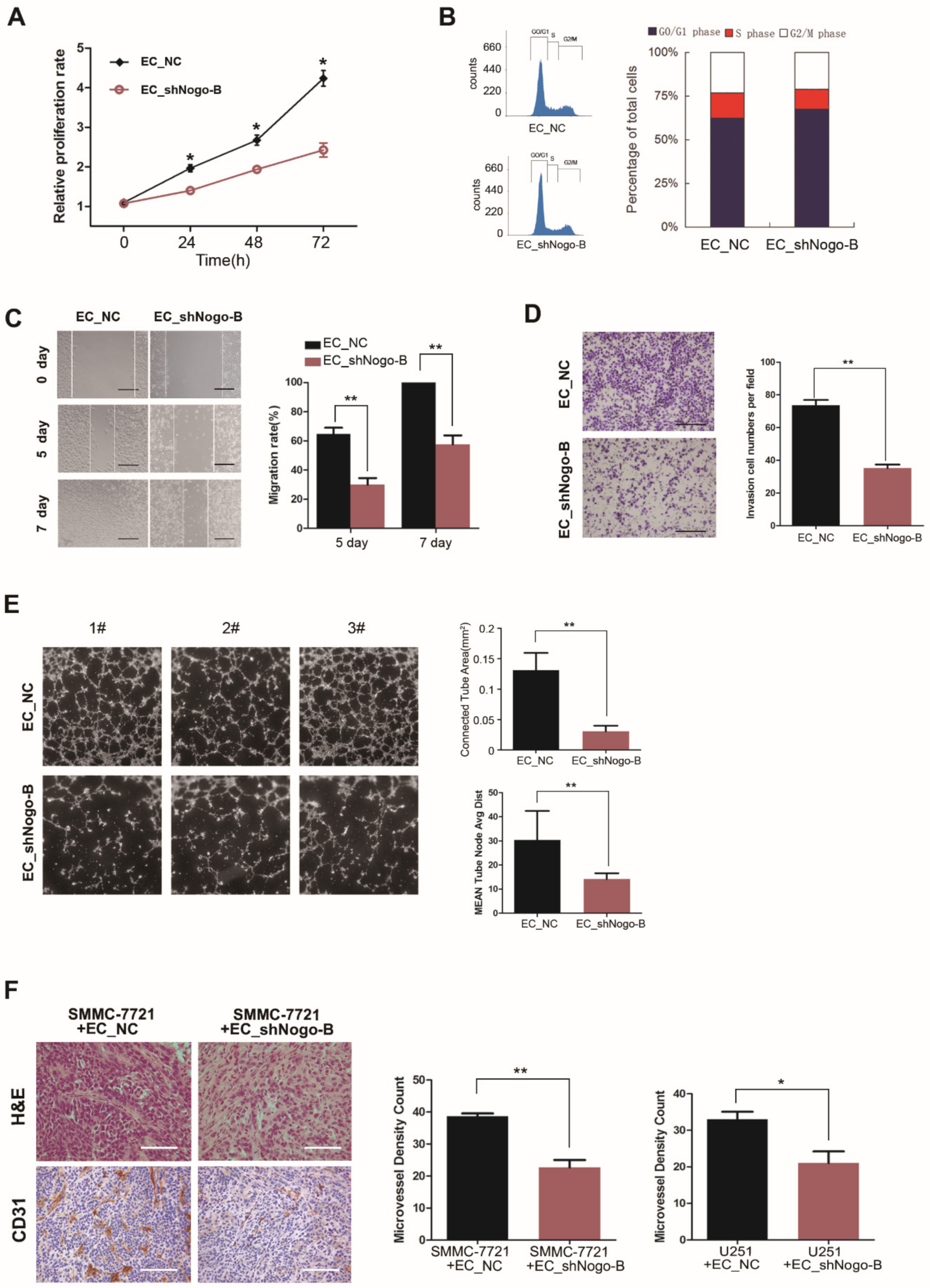
3.3. Endothelial Nogo-B Knockdown Impairs Angiogenesis In Vitro and In Vivo
3.4. Reduction of Endothelial Nogo-B Expression Enhances the Proliferation of Cancer Cells
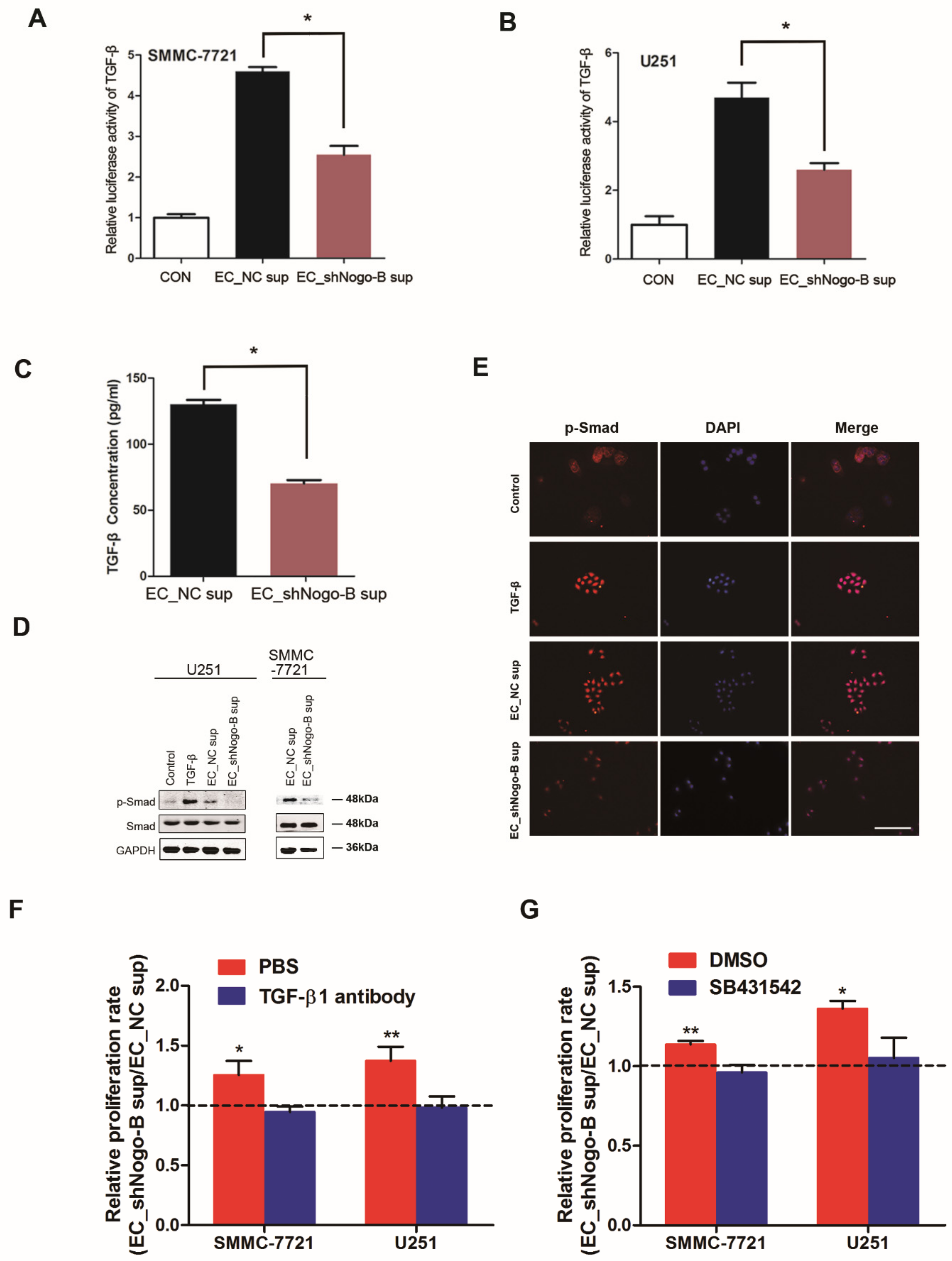
3.5. Endothelial Nogo-B Promotes TGF-β Secretion to Inhibit Cancer Cell Proliferation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J. Clin. Diagn. Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Molecular heterogeneity of tumor endothelium. Cell Tissue Res. 2009, 335, 271–281. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes expressed in human tumor endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Klagsbrun, M. A new perspective on tumor endothelial cells: Unexpected chromosome and centrosome abnormalities. Cancer Res. 2005, 65, 2507–2510. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Sakurai, Y.; Hida, Y.; Harashima, H. Heterogeneity of tumor endothelial cells and drug delivery. Adv. Drug Deliv. Rev. 2016, 99, 140–147. [Google Scholar] [CrossRef]
- Franses, J.W.; Drosu, N.C.; Gibson, W.J.; Chitalia, V.C.; Edelman, E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer 2013, 133, 1334–1344. [Google Scholar] [CrossRef]
- Franses, J.W.; Baker, A.B.; Chitalia, V.C.; Edelman, E.R. Stromal endothelial cells directly influence cancer progression. Sci. Transl. Med. 2011, 3, 66ra5. [Google Scholar] [CrossRef]
- Yu, J.; Fernandez-Hernando, C.; Suarez, Y.; Schleicher, M.; Hao, Z.; Wright, P.L.; DiLorenzo, A.; Kyriakides, T.R.; Sessa, W.C. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17511–17516. [Google Scholar] [CrossRef]
- Acevedo, L.; Yu, J.; Erdjument-Bromage, H.; Miao, R.Q.; Kim, J.E.; Fulton, D.; Tempst, P.; Strittmatter, S.M.; Sessa, W.C. A new role for Nogo as a regulator of vascular remodeling. Nat. Med. 2004, 10, 382–388. [Google Scholar] [CrossRef]
- Walchli, T.; Ulmann-Schuler, A.; Hintermuller, C.; Meyer, E.; Stampanoni, M.; Carmeliet, P.; Emmert, M.Y.; Bozinov, O.; Regli, L.; Schwab, M.E.; et al. Nogo-A regulates vascular network architecture in the postnatal brain. J. Cereb. Blood Flow Metab. 2017, 37, 614–631. [Google Scholar] [CrossRef]
- Raines, E.W. Nogo puts the brake on vascular lesions. Nat. Med. 2004, 10, 348–349. [Google Scholar] [CrossRef]
- Cantalupo, A.; Zhang, Y.; Kothiya, M.; Galvani, S.; Obinata, H.; Bucci, M.; Giordano, F.J.; Jiang, X.C.; Hla, T.; Di Lorenzo, A. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat. Med. 2015, 21, 1028–1037. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, C.; Huang, M.; Liu, Y.; Qi, F.; Liu, L.; Wen, J.; Liu, J.; Xie, K.; Ma, H.; et al. A genetic variant in pseudogene E2F3P1 contributes to prognosis of hepatocellular carcinoma. J. Biomed. Res. 2014, 28, 194–200. [Google Scholar]
- Men, R.; Wen, M.; Dan, X.; Zhu, Y.; Wang, W.; Li, J.; Wu, W.; Liu, X.; Yang, L. Nogo-B: A potential indicator for hepatic cirrhosis and regulator in hepatic stellate cell activation. Hepatol. Res. 2015, 45, 113–122. [Google Scholar] [CrossRef]
- Quaegebeur, A.; Lange, C.; Carmeliet, P. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron 2011, 71, 406–424. [Google Scholar] [CrossRef]
- Walchli, T.; Wacker, A.; Frei, K.; Regli, L.; Schwab, M.E.; Hoerstrup, S.P.; Gerhardt, H.; Engelhardt, B. Wiring the Vascular Network with Neural Cues: A CNS Perspective. Neuron 2015, 87, 271–296. [Google Scholar] [CrossRef]
- Sun, W.; Ding, J.; Wu, K.; Ning, B.F.; Wen, W.; Sun, H.Y.; Han, T.; Huang, L.; Dong, L.W.; Yang, W.; et al. Gankyrin-mediated dedifferentiation facilitates the tumorigenicity of rat hepatocytes and hepatoma cells. Hepatology 2011, 54, 1259–1272. [Google Scholar] [CrossRef]
- Xiang, D.M.; Cheng, Z.; Liu, H.; Wang, X.; Han, T.; Sun, W.; Li, X.F.; Yang, W.; Chen, C.; Xia, M.Y.; et al. Shp2 promotes liver cancer stem cell expansion by augmenting beta-catenin signaling and predicts chemotherapeutic response of patients. Hepatology 2017, 65, 1566–1580. [Google Scholar] [CrossRef]
- Han, T.; Zheng, H.; Zhang, J.; Yang, P.; Li, H.; Cheng, Z.; Xiang, D.; Wang, R. Downregulation of MUC15 by miR-183-5p.1 promotes liver tumor-initiating cells properties and tumorigenesis via regulating c-MET/PI3K/AKT/SOX2 axis. Cell Death Dis. 2022, 13, 200. [Google Scholar] [CrossRef]
- Li, H.; Yang, P.; Wang, J.; Zhang, J.; Ma, Q.; Jiang, Y.; Wu, Y.; Han, T.; Xiang, D. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J. Hematol. Oncol. 2022, 15, 2. [Google Scholar] [CrossRef]
- Li, Y.; Qu, X.; Cao, B.; Yang, T.; Bao, Q.; Yue, H.; Zhang, L.; Zhang, G.; Wang, L.; Qiu, P.; et al. Selectively Suppressing Tumor Angiogenesis for Targeted Breast Cancer Therapy by Genetically Engineered Phage. Adv. Mater. 2020, 32, 2001260. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Wen, M.; Men, R.; Yang, Z.; Dan, X.; Wu, W.; Liu, X.; Yang, L. The Value of Circulating Nogo-B for Evaluating Hepatic Functional Reserve in Patients with Cirrhosis. Dis. Markers 2015, 2015, 419124. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ziebarth, A.J.; Nowsheen, S.; Steg, A.D.; Shah, M.M.; Katre, A.A.; Dobbin, Z.C.; Han, H.D.; Lopez-Berestein, G.; Sood, A.K.; Conner, M.; et al. Endoglin (CD105) contributes to platinum resistance and is a target for tumor-specific therapy in epithelial ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Zhang, Y.; Snyder, J.; Kalinichenko, V.V.; Kalin, T.V. Endothelial cell-specific deletion of transcription factor FoxM1 increases urethane-induced lung carcinogenesis. Cancer Res. 2011, 71, 40–50. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A Positive Feedback Loop between Mesenchymal-like Cancer Cells and Macrophages Is Essential to Breast Cancer Metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFbeta in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef]
- Krishnan, S.; Szabo, E.; Burghardt, I.; Frei, K.; Tabatabai, G.; Weller, M. Modulation of cerebral endothelial cell function by TGF-beta in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget 2015, 6, 22480–22495. [Google Scholar] [CrossRef]
- Rämö, O.; Kumar, D.; Gucciardo, E.; Joensuu, M.; Saarekas, M.; Vihinen, H.; Belevich, I.; Smolander, O.P.; Qian, K.; Auvinen, P.; et al. NOGO-A/RTN4A and NOGO-B/RTN4B are simultaneously expressed in epithelial, fibroblast and neuronal cells and maintain ER morphology. Sci. Rep. 2016, 6, 35969. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cheng, Z.; Yang, P.; Huang, W.; Li, X.; Xiang, D.; Wu, X. Endothelial Nogo-B Suppresses Cancer Cell Proliferation via a Paracrine TGF-β/Smad Signaling. Cells 2022, 11, 3084. https://doi.org/10.3390/cells11193084
Li H, Cheng Z, Yang P, Huang W, Li X, Xiang D, Wu X. Endothelial Nogo-B Suppresses Cancer Cell Proliferation via a Paracrine TGF-β/Smad Signaling. Cells. 2022; 11(19):3084. https://doi.org/10.3390/cells11193084
Chicago/Turabian StyleLi, Hengyu, Zhuo Cheng, Pinghua Yang, Wei Huang, Xizhou Li, Daimin Xiang, and Xiaojun Wu. 2022. "Endothelial Nogo-B Suppresses Cancer Cell Proliferation via a Paracrine TGF-β/Smad Signaling" Cells 11, no. 19: 3084. https://doi.org/10.3390/cells11193084
APA StyleLi, H., Cheng, Z., Yang, P., Huang, W., Li, X., Xiang, D., & Wu, X. (2022). Endothelial Nogo-B Suppresses Cancer Cell Proliferation via a Paracrine TGF-β/Smad Signaling. Cells, 11(19), 3084. https://doi.org/10.3390/cells11193084






