The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury
Abstract
:1. Spinal Cord Injury-Induced Neuropathic Pain
2. Included Rodent Models of SCI-NP
2.1. Characteristics of Included Experimental Models
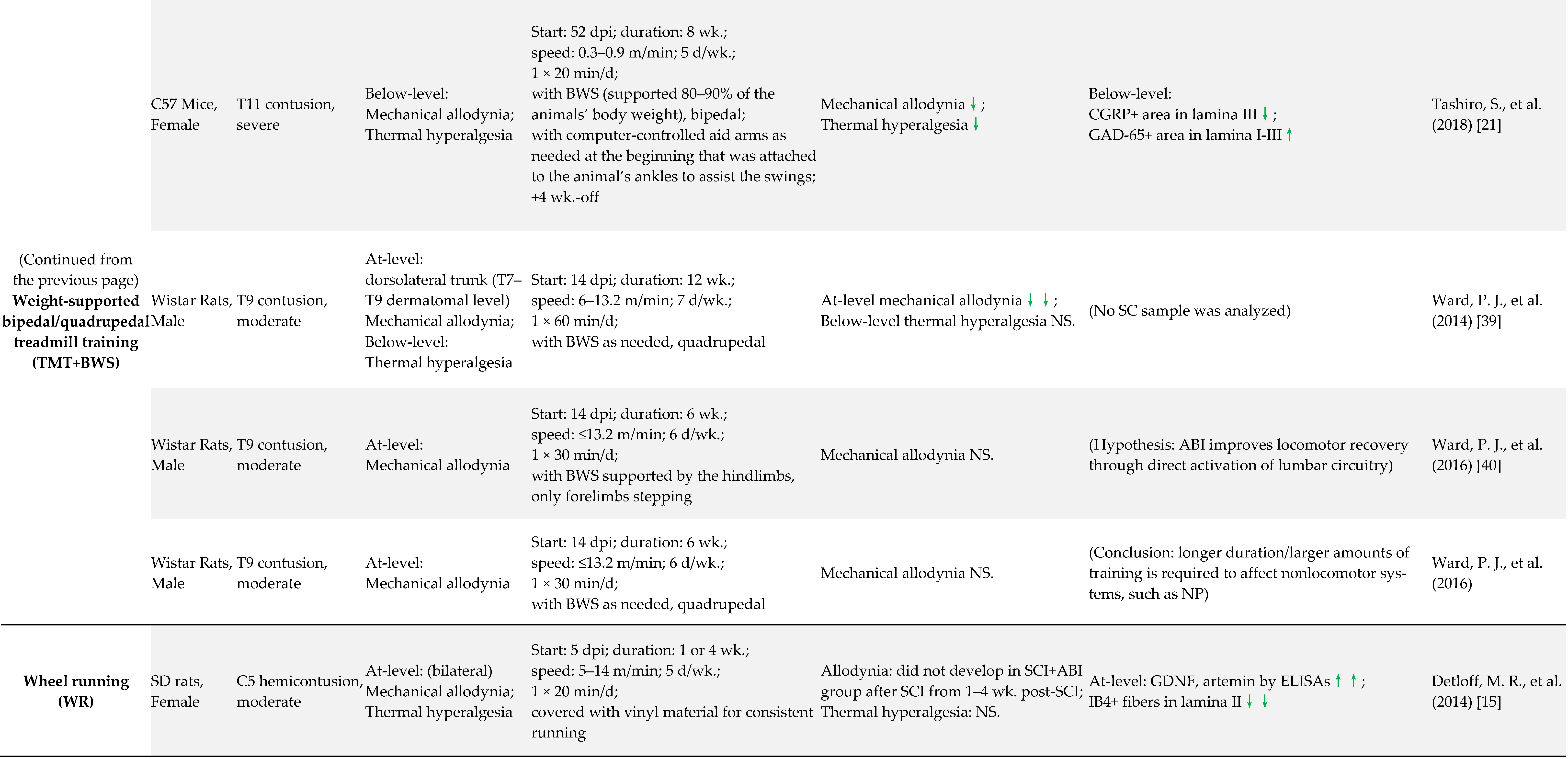
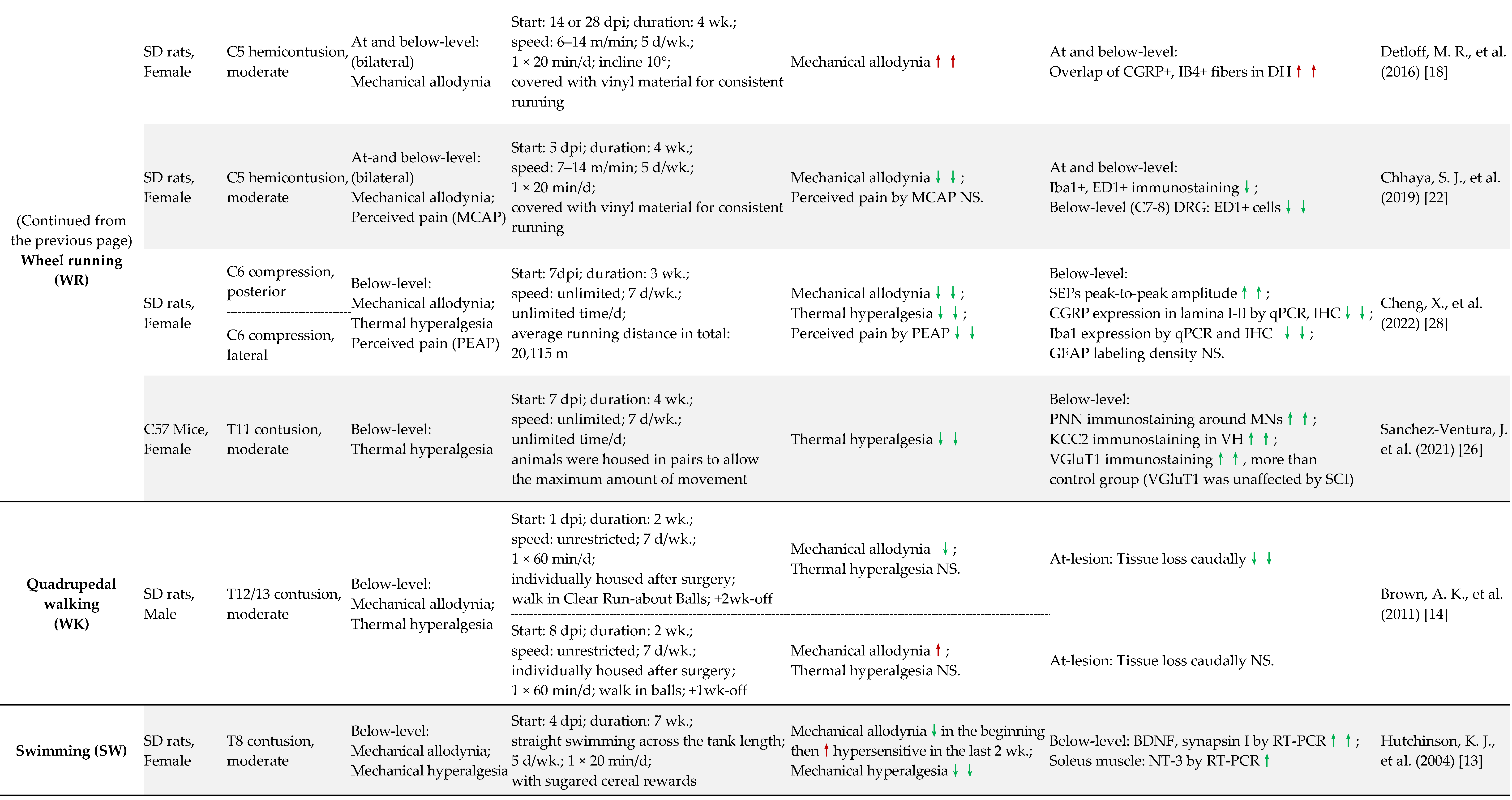
2.2. SCI-NP Measurements of Experimental Models
3. Mechanisms of SCI-Induced NP
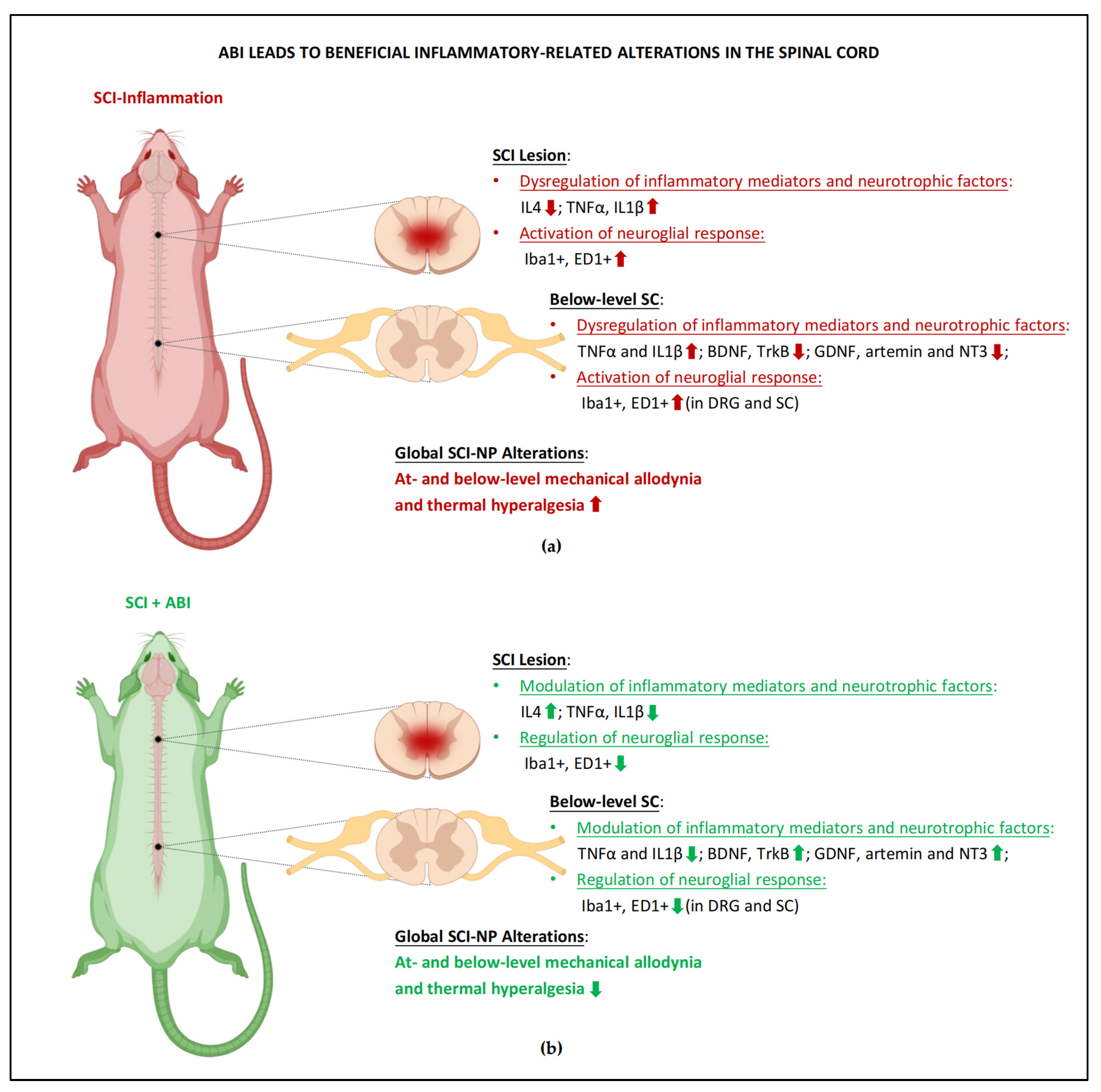
3.1. Maladaptive Changes of Immune System and Neurotrophic Mediators
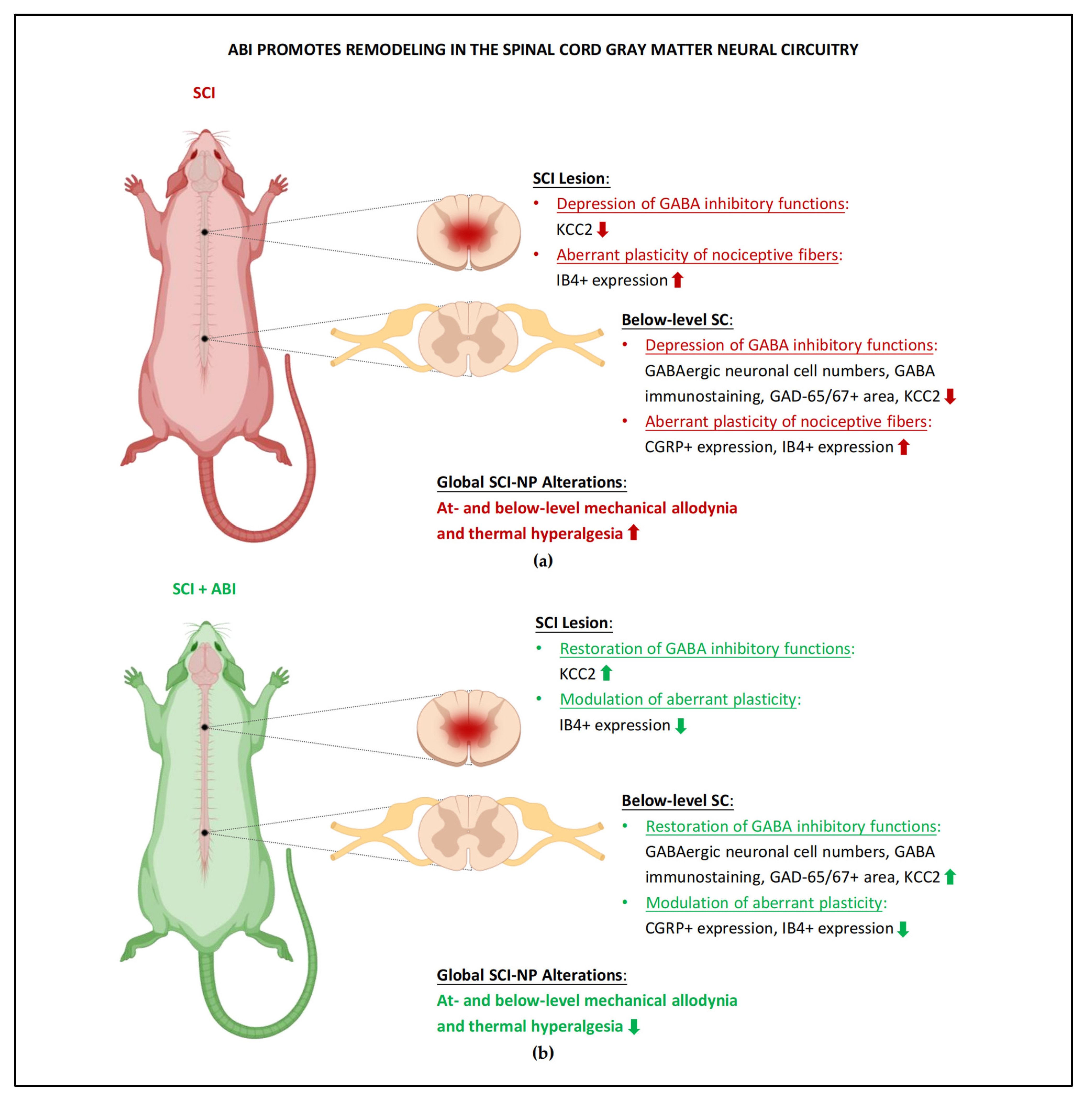
3.2. Spinal Cord Gray Matter Neural Circuitry Impairments
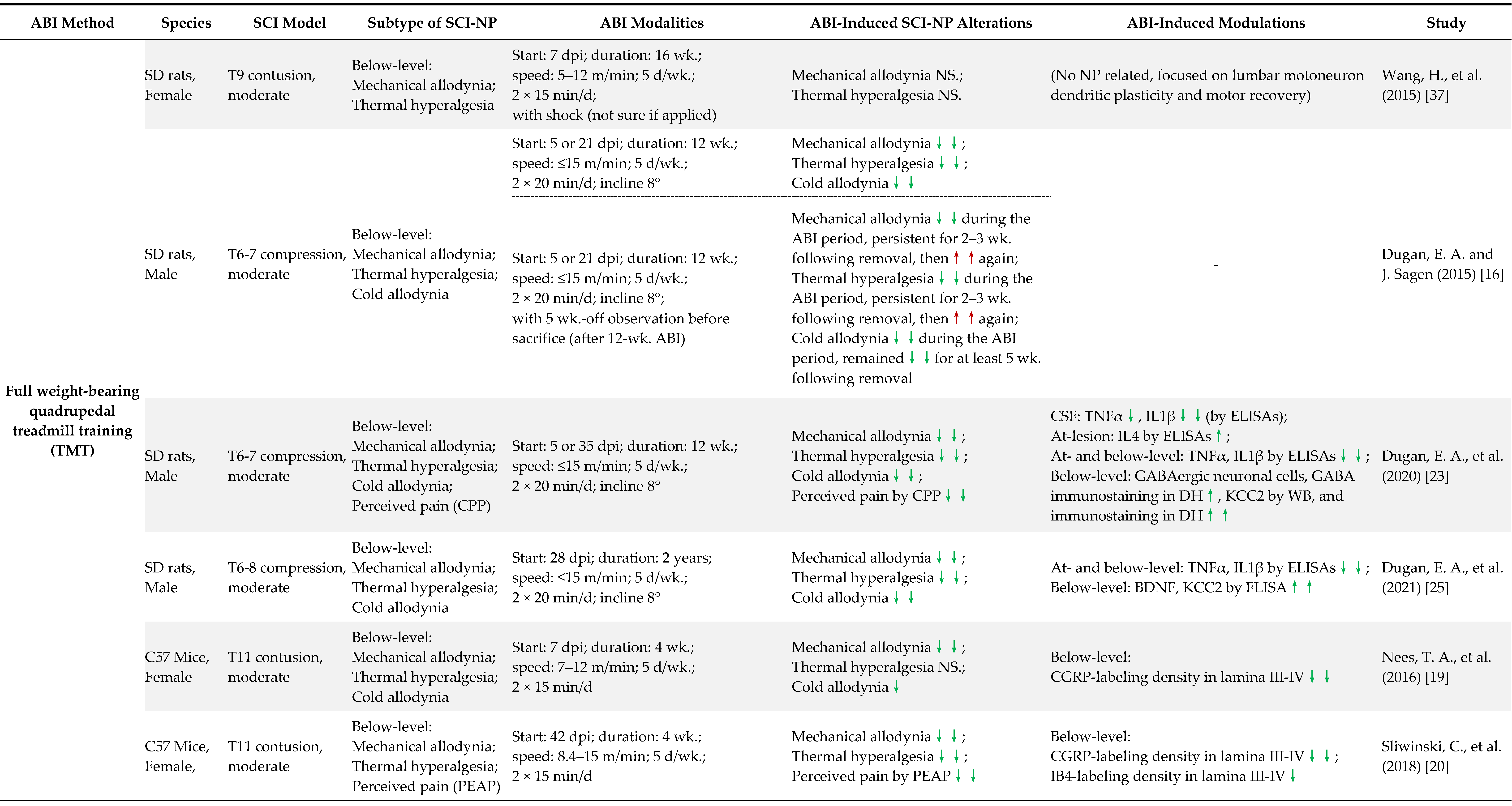
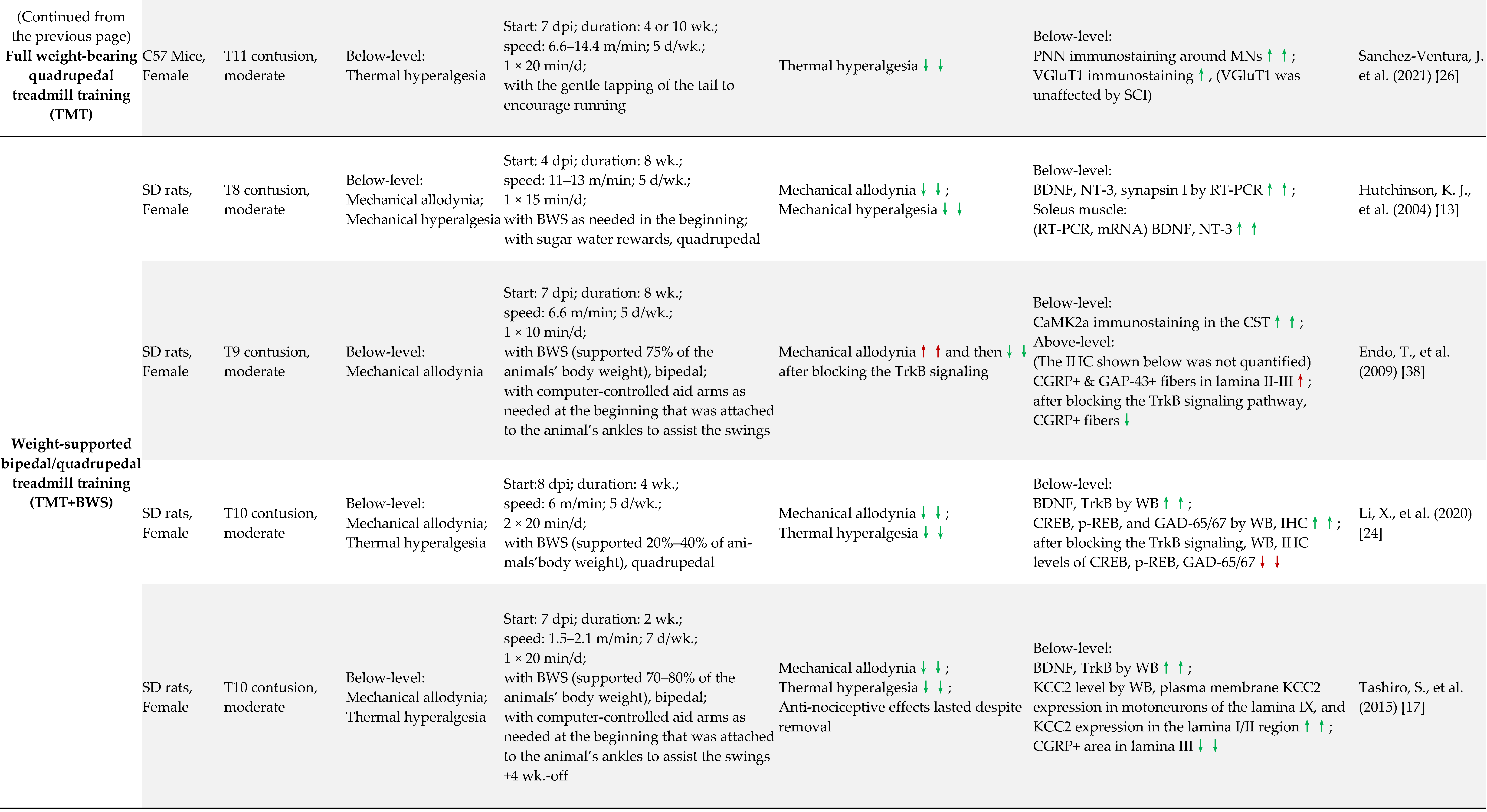
4. Preclinical Evidence of ABI in the Treatment of SCI-Induced NP
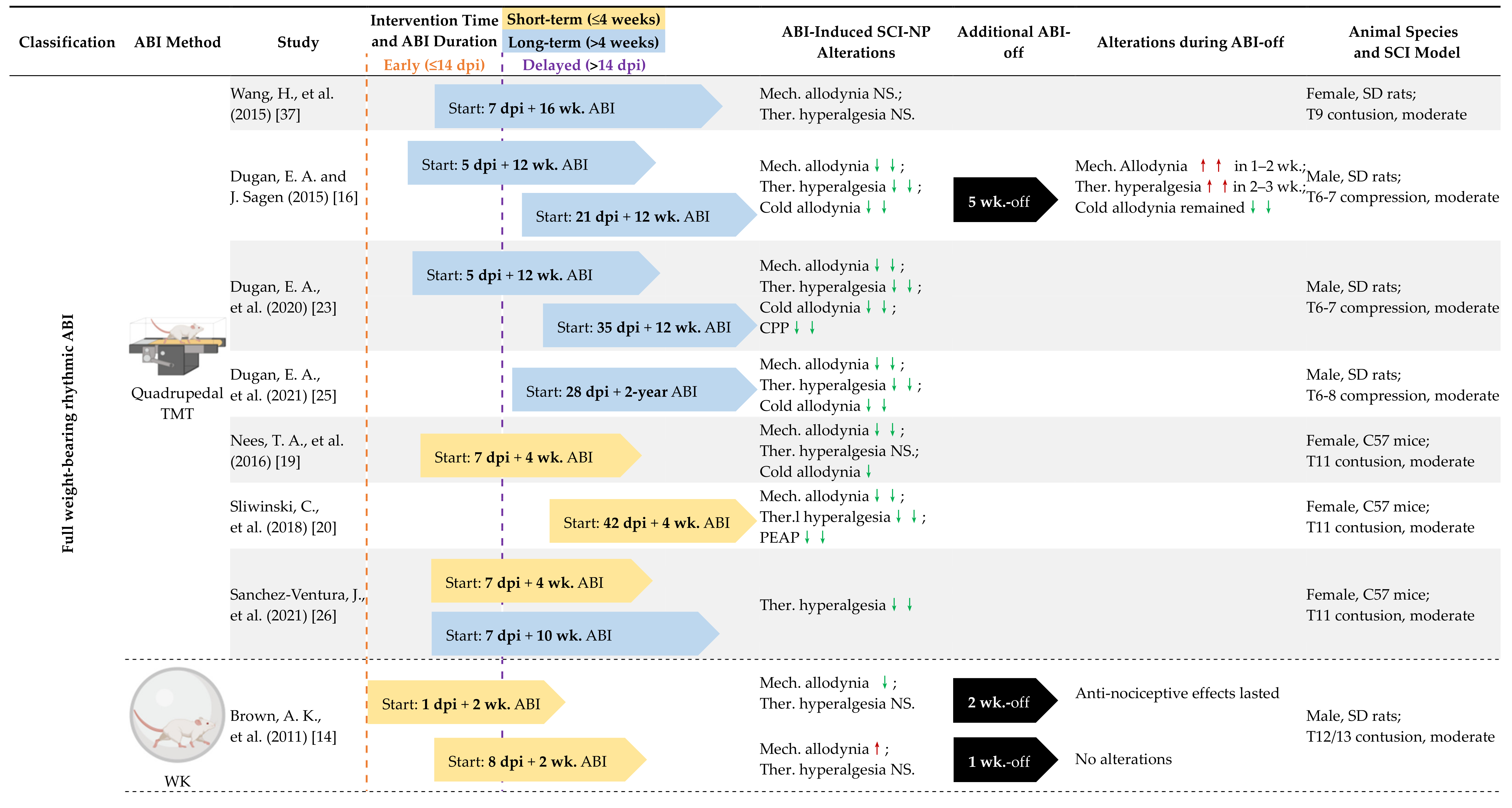
4.1. Early vs. Delayed Intervention
4.2. Long-Term vs. Short-Term ABI
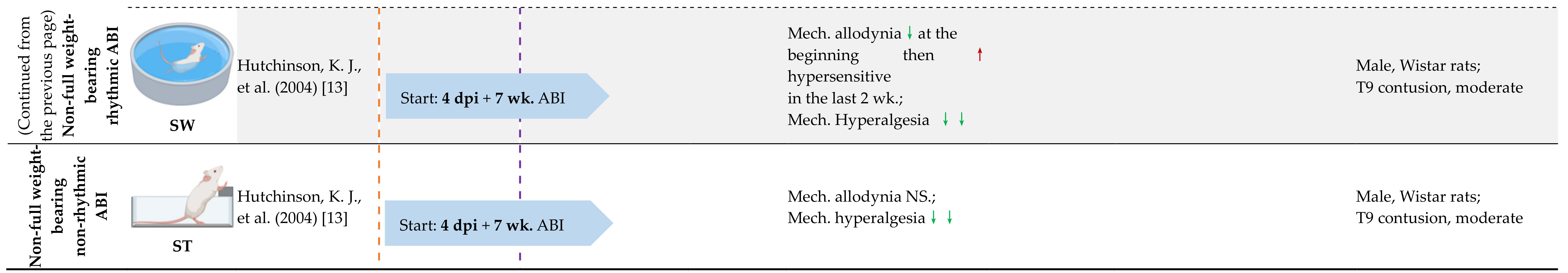
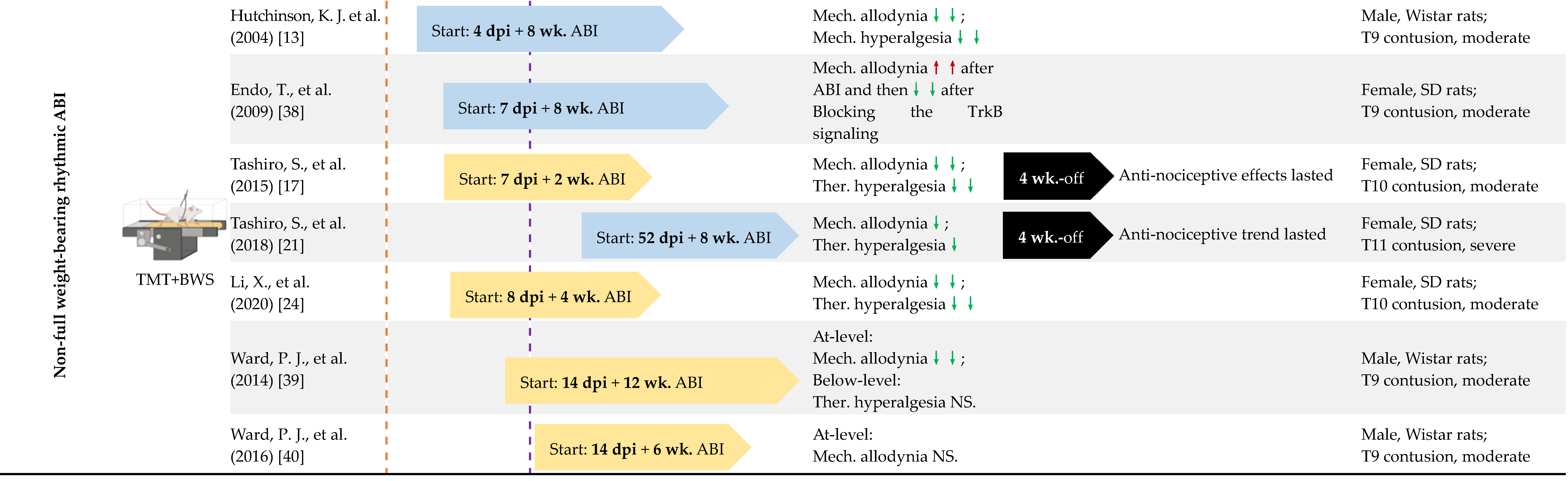
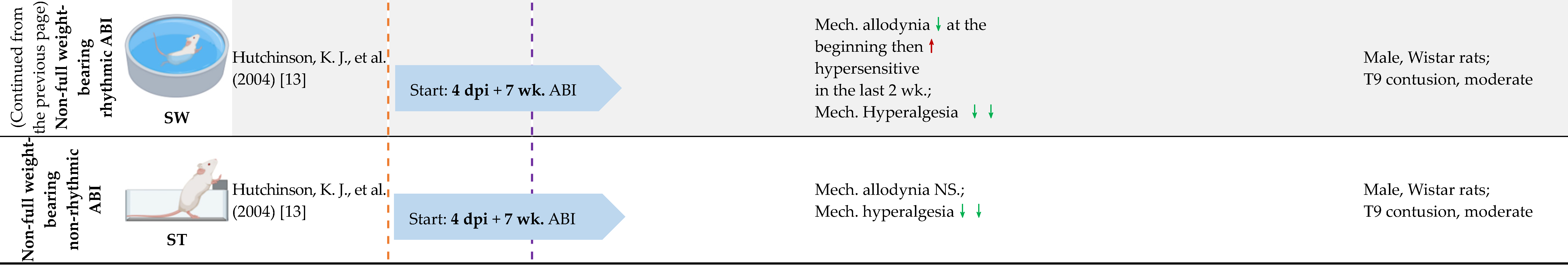
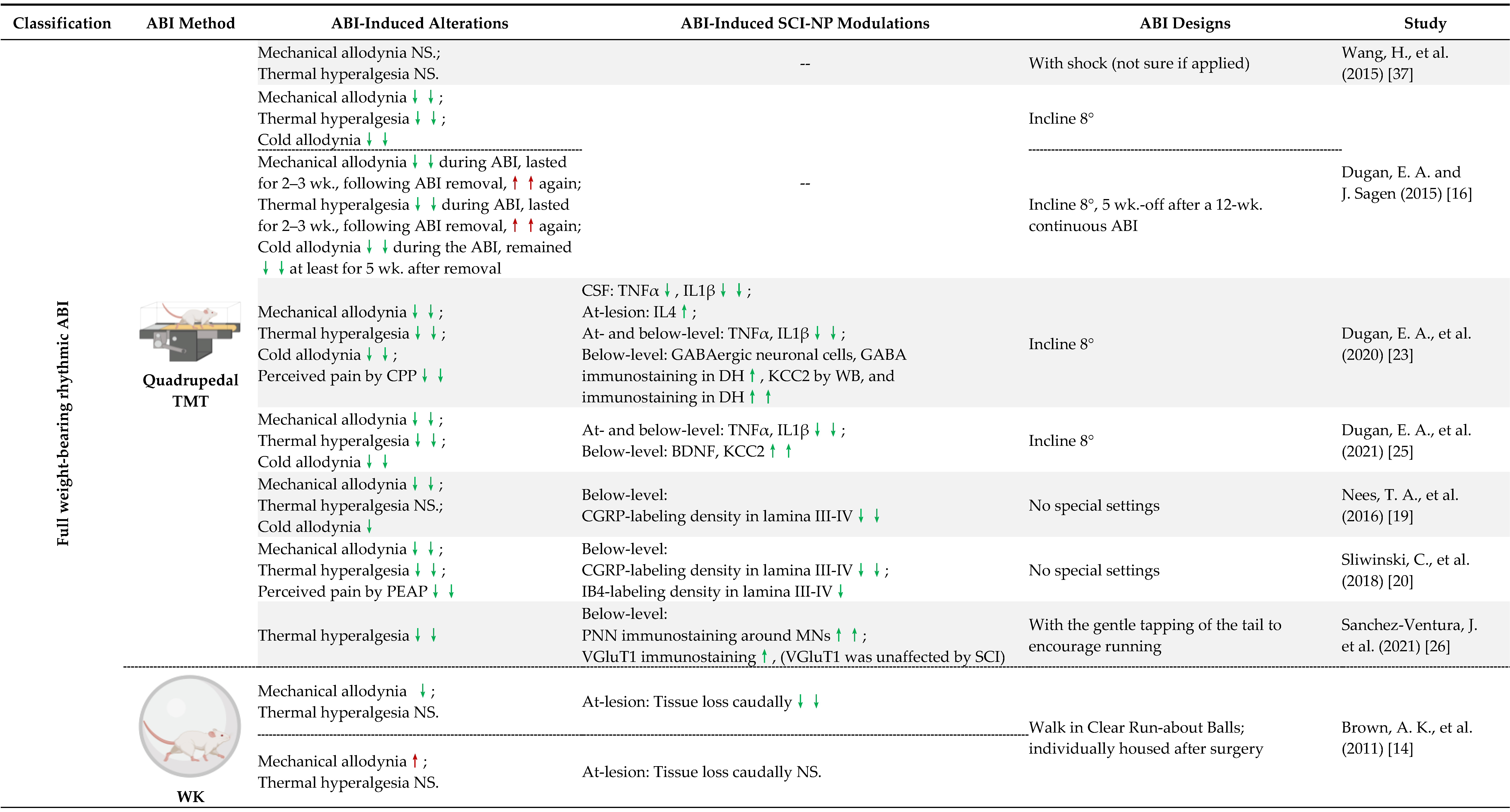
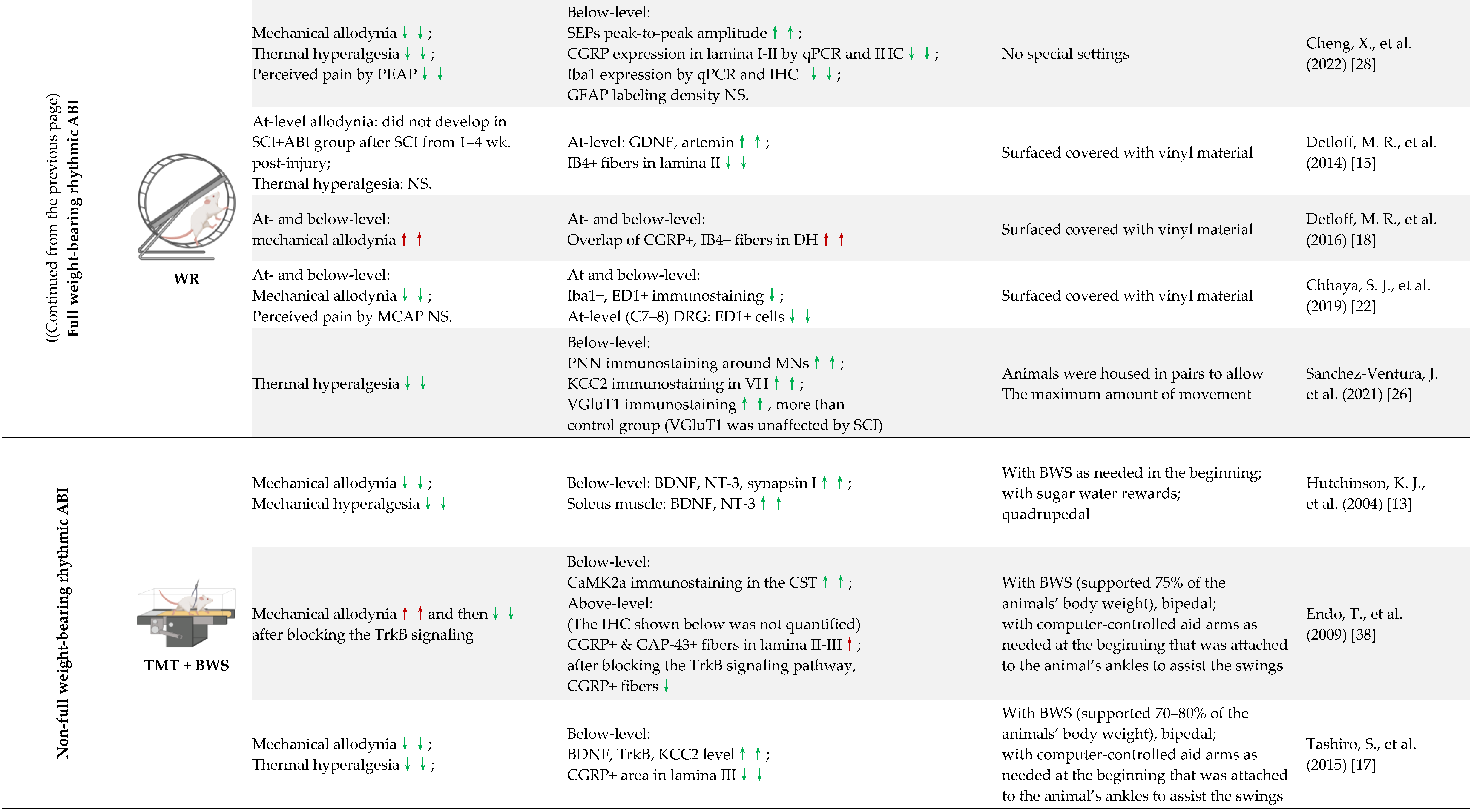
4.3. Full Weight-Bearing vs. Non-Full Weight-Bearing Rhythmic ABI
4.4. Other Factors May Contribute to the NP Outcome
5. Mechanisms of ABI to Ameliorate SCI-Induced NP
5.1. ABI-Induced Modulations of Inflammatory and Neurotrophic Mediators
5.2. Remodeling of Spinal Cord Gray Matter Neural Circuitry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Activity-Based Intervention(s); |
| BDNF | Brain-Derived Neurotrophic Factor; |
| BWS | Body Weight Support; |
| CGRP | Calcitonin Gene-Related Peptide; |
| CPP | Conditioned Place Preference; |
| CSF | Cerebrospinal Fluid; |
| DH | (Spinal Cord) Dorsal Horn; |
| dpi | Day(s) Post Injury; |
| DRG | Dorsal Root Ganglia; |
| GAD-65/67 | Glutamic Acid Decarboxylase-65/67; |
| GDNF | Glial Cell-Derived Neurotrophic Factor; |
| GFL | GDNF Family of Ligands; |
| IB4 | Isolectin B4; |
| KCC2 | The Neuron-Specific K+ Cl− Cotransporter; |
| MCAP | Mechanical Conflict-Avoidance Paradigm; |
| Mech. | Mechanical; |
| MN (s) | Motoneuron(s); |
| NP | Neuropathic Pain; |
| PEAP | Place Escape/Avoidance Paradigm; |
| PNN | Perineuronal Net(s); |
| SCI | Spinal Cord Injury; |
| SCI-NP | Spinal Cord Injury-Induced Neuropathic Pain; |
| SD rats | Sprague–Dawley rats; |
| ST | Standing; |
| SW | Swimming; |
| Ther. | Thermal; |
| TMT | Treadmill Training; |
| TrkB | Tropomyosin-Related Kinase B; |
| VH | (Spinal Cord) Ventral Horn; |
| WB | West. Blot; |
| wk. | week(s) |
| WK | Walking; |
| WR | Wheel Running |
References
- Backonja, M.M. Defining neuropathic pain. Anesth. Analg. 2003, 97, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Finnerup, N.B.; Blesch, A.; Weidner, N. Neuropathic pain after spinal cord injury: The impact of sensorimotor activity. Pain 2017, 158, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.P. Pain following spinal cord injury: Pathophysiology and central mechanisms. Prog. Brain Res. 2000, 129, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Johannesen, I.L.; Sindrup, S.H.; Bach, F.W.; Jensen, T.S. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal. Cord. 2001, 39, 256–262. [Google Scholar] [CrossRef]
- Turner, J.A.; Cardenas, D.D.; Warms, C.A.; McClellan, C.B. Chronic pain associated with spinal cord injuries: A community survey. Arch. Phys. Med. Rehabil. 2001, 82, 501–509. [Google Scholar] [CrossRef]
- Siddall, P.J. Management of neuropathic pain following spinal cord injury: Now and in the future. Spinal. Cord. 2009, 47, 352–359. [Google Scholar] [CrossRef]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A.; et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003, 60, 1524–1534. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Norrbrink, C.; Trok, K.; Piehl, F.; Johannesen, I.L.; Sorensen, J.C.; Jensen, T.S.; Werhagen, L. Phenotypes and predictors of pain following traumatic spinal cord injury: A prospective study. J. Pain 2014, 15, 40–48. [Google Scholar] [CrossRef]
- Bryce, T.N.; Biering-Sorensen, F.; Finnerup, N.B.; Cardenas, D.D.; Defrin, R.; Lundeberg, T.; Norrbrink, C.; Richards, J.S.; Siddall, P.; Stripling, T.; et al. International spinal cord injury pain classification: Part I. Background and description. Spinal. Cord. 2012, 50, 413–417. [Google Scholar] [CrossRef]
- Siddall, P.J.; Middleton, J.W. Spinal cord injury-induced pain: Mechanisms and treatments. Pain Manag. 2015, 5, 493–507. [Google Scholar] [CrossRef]
- Hutchinson, K.J.; Gomez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef]
- Carlton, S.M.; Du, J.; Tan, H.Y.; Nesic, O.; Hargett, G.L.; Bopp, A.C.; Yamani, A.; Lin, Q.; Willis, W.D.; Hulsebosch, C.E. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009, 147, 265–276. [Google Scholar] [CrossRef]
- Brown, A.K.; Woller, S.A.; Moreno, G.; Grau, J.W.; Hook, M.A. Exercise therapy and recovery after SCI: Evidence that shows early intervention improves recovery of function. Spinal. Cord. 2011, 49, 623–628. [Google Scholar] [CrossRef]
- Detloff, M.R.; Smith, E.J.; Quiros Molina, D.; Ganzer, P.D.; Houle, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef]
- Dugan, E.A.; Sagen, J. An Intensive Locomotor Training Paradigm Improves Neuropathic Pain following Spinal Cord Compression Injury in Rats. J. Neurotrauma. 2015, 32, 622–632. [Google Scholar] [CrossRef]
- Tashiro, S.; Shinozaki, M.; Mukaino, M.; Renault-Mihara, F.; Toyama, Y.; Liu, M.; Nakamura, M.; Okano, H. BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabil. Neural. Repair 2015, 29, 677–689. [Google Scholar] [CrossRef]
- Detloff, M.R.; Quiros-Molina, D.; Javia, A.S.; Daggubati, L.; Nehlsen, A.D.; Naqvi, A.; Ninan, V.; Vannix, K.N.; McMullen, M.K.; Amin, S.; et al. Delayed Exercise Is Ineffective at Reversing Aberrant Nociceptive Afferent Plasticity or Neuropathic Pain After Spinal Cord Injury in Rats. Neurorehabil. Neural. Repair 2016, 30, 685–700. [Google Scholar] [CrossRef]
- Nees, T.A.; Tappe-Theodor, A.; Sliwinski, C.; Motsch, M.; Rupp, R.; Kuner, R.; Weidner, N.; Blesch, A. Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 2016, 157, 687–697. [Google Scholar] [CrossRef]
- Sliwinski, C.; Nees, T.A.; Puttagunta, R.; Weidner, N.; Blesch, A. Sensorimotor Activity Partially Ameliorates Pain and Reduces Nociceptive Fiber Density in the Chronically Injured Spinal Cord. J. Neurotrauma 2018, 35, 2222–2238. [Google Scholar] [CrossRef]
- Tashiro, S.; Nishimura, S.; Shinozaki, M.; Takano, M.; Konomi, T.; Tsuji, O.; Nagoshi, N.; Toyama, Y.; Liu, M.; Okano, H.; et al. The Amelioration of Pain-Related Behavior in Mice with Chronic Spinal Cord Injury Treated with Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training. J. Neurotrauma. 2018, 35, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, S.J.; Quiros-Molina, D.; Tamashiro-Orrego, A.D.; Houle, J.D.; Detloff, M.R. Exercise-Induced Changes to the Macrophage Response in the Dorsal Root Ganglia Prevent Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma. 2019, 36, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Ding, J.; Wang, S.; Dong, C.; Wu, Q. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol. Pain 2020, 16, 1744806920924511. [Google Scholar] [CrossRef]
- Dugan, E.A.; Schachner, B.; Jergova, S.; Sagen, J. Intensive Locomotor Training Provides Sustained Alleviation of Chronic Spinal Cord Injury-Associated Neuropathic Pain: A Two-Year Pre-Clinical Study. J. Neurotrauma. 2021, 38, 789–802. [Google Scholar] [CrossRef]
- Sanchez-Ventura, J.; Gimenez-Llort, L.; Penas, C.; Udina, E. Voluntary wheel running preserves lumbar perineuronal nets, enhances motor functions and prevents hyperreflexia after spinal cord injury. Exp. Neurol. 2021, 336, 113533. [Google Scholar] [CrossRef]
- Vierck, C. Mechanisms of Below-Level Pain Following Spinal Cord Injury (SCI). J. Pain 2020, 21, 262–280. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, Z.; Hu, W.; Chen, J.; Chen, W.; Wang, L.; Li, X.; Zhang, W.; Chen, J.; Zou, X.; et al. Voluntary exercise ameliorates neuropathic pain by suppressing calcitonin gene-related peptide and ionized calcium-binding adapter molecule 1 overexpression in the lumbar dorsal horns in response to injury to the cervical spinal cord. Exp. Neurol. 2022, 354, 114105. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Aldington, D.; Cole, P.; Rice, A.S.; Lunn, M.P.; Hamunen, K.; Haanpaa, M.; Kalso, E.A. Antiepileptic drugs for neuropathic pain and fibromyalgia—An overview of Cochrane reviews. Cochrane. Database Syst. Rev. 2013, CD010567. [Google Scholar] [CrossRef]
- Tasker, R.R.; DeCarvalho, G.T.; Dolan, E.J. Intractable pain of spinal cord origin: Clinical features and implications for surgery. J. Neurosurg. 1992, 77, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Lenggenhager, B.; Pazzaglia, M.; Scivoletto, G.; Molinari, M.; Aglioti, S.M. The sense of the body in individuals with spinal cord injury. PLoS ONE 2012, 7, e50757. [Google Scholar] [CrossRef]
- Crossman, M.W. Sensory deprivation in spinal cord injury--an essay. Spinal. Cord. 1996, 34, 573–577. [Google Scholar] [CrossRef]
- Martinez, M.; Brezun, J.M.; Zennou-Azogui, Y.; Baril, N.; Xerri, C. Sensorimotor training promotes functional recovery and somatosensory cortical map reactivation following cervical spinal cord injury. Eur. J. Neurosci. 2009, 30, 2356–2367. [Google Scholar] [CrossRef]
- Maier, I.C.; Ichiyama, R.M.; Courtine, G.; Schnell, L.; Lavrov, I.; Edgerton, V.R.; Schwab, M.E. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain 2009, 132, 1426–1440. [Google Scholar] [CrossRef]
- Chen, K.; Marsh, B.C.; Cowan, M.; Al’Joboori, Y.D.; Gigout, S.; Smith, C.C.; Messenger, N.; Gamper, N.; Schwab, M.E.; Ichiyama, R.M. Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp. Neurol. 2017, 292, 135–144. [Google Scholar] [CrossRef]
- Kiss Bimbova, K.; Bacova, M.; Kisucka, A.; Galik, J.; Zavacky, P.; Lukacova, N. Activation of Three Major Signaling Pathways After Endurance Training and Spinal Cord Injury. Mol. Neurobiol. 2022, 59, 950–967. [Google Scholar] [CrossRef]
- Wang, H.; Liu, N.K.; Zhang, Y.P.; Deng, L.; Lu, Q.B.; Shields, C.B.; Walker, M.J.; Li, J.; Xu, X.M. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp. Neurol. 2015, 271, 368–378. [Google Scholar] [CrossRef]
- Endo, T.; Ajiki, T.; Inoue, H.; Kikuchi, M.; Yashiro, T.; Nakama, S.; Hoshino, Y.; Murakami, T.; Kobayashi, E. Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem. Biophys. Res. Commun. 2009, 381, 339–344. [Google Scholar] [CrossRef]
- Ward, P.J.; Herrity, A.N.; Smith, R.R.; Willhite, A.; Harrison, B.J.; Petruska, J.C.; Harkema, S.J.; Hubscher, C.H. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J. Neurotrauma. 2014, 31, 819–833. [Google Scholar] [CrossRef]
- Ward, P.J.; Herrity, A.N.; Harkema, S.J.; Hubscher, C.H. Training-Induced Functional Gains following SCI. Neural. Plast. 2016, 2016, 4307694. [Google Scholar] [CrossRef] [Green Version]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Safdarian, M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; et al. Animal models of spinal cord injury: A systematic review. Spinal. Cord. 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Palandi, J.; Bobinski, F.; de Oliveira, G.M.; Ilha, J. Neuropathic pain after spinal cord injury and physical exercise in animal models: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 108, 781–795. [Google Scholar] [CrossRef]
- Sroga, J.M.; Jones, T.B.; Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003, 462, 223–240. [Google Scholar] [CrossRef]
- Carpenter, R.S.; Kigerl, K.A.; Marbourg, J.M.; Gaudet, A.D.; Huey, D.; Niewiesk, S.; Popovich, P.G. Traumatic spinal cord injury in mice with human immune systems. Exp. Neurol. 2015, 271, 432–444. [Google Scholar] [CrossRef]
- Kerr, B.J.; David, S. Pain behaviors after spinal cord contusion injury in two commonly used mouse strains. Exp. Neurol. 2007, 206, 240–247. [Google Scholar] [CrossRef]
- Bleul, T.; Zhuang, X.; Hildebrand, A.; Lange, C.; Bohringer, D.; Schlunck, G.; Reinhard, T.; Lapp, T. Different Innate Immune Responses in BALB/c and C57BL/6 Strains following Corneal Transplantation. J. Innate Immun. 2021, 13, 49–59. [Google Scholar] [CrossRef]
- Heinla, I.; Ahlgren, J.; Vasar, E.; Voikar, V. Behavioural characterization of C57BL/6N and BALB/c female mice in social home cage—Effect of mixed housing in complex environment. Physiol. Behav. 2018, 188, 32–41. [Google Scholar] [CrossRef]
- Bouhassira, D.; Lanteri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef]
- Thompson, C.; Mutch, J.; Parent, S.; Mac-Thiong, J.M. The changing demographics of traumatic spinal cord injury: An 11-year study of 831 patients. J. Spinal. Cord. Med. 2015, 38, 214–223. [Google Scholar] [CrossRef]
- Shiao, R.; Lee-Kubli, C.A. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics 2018, 15, 635–653. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Rua, A.J.; Cannon, R.L.; Yezierski, R.P.; Vierck, C.J. Sex differences in effects of excitotoxic spinal injury on below-level pain sensitivity. Brain Res. 2011, 1419, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Ayala, M.T.; Schleicher, W.E.; Smith, E.J.; Bateman, E.M.; Maier, S.F.; Watkins, L.R. Exploring acute-to-chronic neuropathic pain in rats after contusion spinal cord injury. Exp. Neurol. 2017, 295, 46–54. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, K.; Otto, T.E.; Bailey, W.M.; Veldhorst, A.K.; Donahue, R.R.; Taylor, B.K.; Gensel, J.C. Effect of Sex on Motor Function, Lesion Size, and Neuropathic Pain after Contusion Spinal Cord Injury in Mice. J. Neurotrauma. 2020, 37, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.L.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.; Liu, L.J.; Ramer, M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2017, 95, 1295–1306. [Google Scholar] [CrossRef]
- Krzyzanowska, A.; Avendano, C. Behavioral testing in rodent models of orofacial neuropathic and inflammatory pain. Brain Behav. 2012, 2, 678–697. [Google Scholar] [CrossRef]
- Rosner, J.; Lutolf, R.; Hostettler, P.; Villiger, M.; Clijsen, R.; Hohenauer, E.; Barbero, M.; Curt, A.; Hubli, M. Assessment of neuropathic pain after spinal cord injury using quantitative pain drawings. Spinal. Cord. 2021, 59, 529–537. [Google Scholar] [CrossRef]
- Bryce, T.N.; Richards, J.S.; Bombardier, C.H.; Dijkers, M.P.; Fann, J.R.; Brooks, L.; Chiodo, A.; Tate, D.G.; Forchheimer, M. Screening for neuropathic pain after spinal cord injury with the spinal cord injury pain instrument (SCIPI): A preliminary validation study. Spinal. Cord. 2014, 52, 407–412. [Google Scholar] [CrossRef]
- Tuttle, A.H.; Molinaro, M.J.; Jethwa, J.F.; Sotocinal, S.G.; Prieto, J.C.; Styner, M.A.; Mogil, J.S.; Zylka, M.J. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 2018, 14, 1744806918763658. [Google Scholar] [CrossRef]
- Sperry, M.M.; Yu, Y.H.; Welch, R.L.; Granquist, E.J.; Winkelstein, B.A. Grading facial expression is a sensitive means to detect grimace differences in orofacial pain in a rat model. Sci. Rep. 2018, 8, 13894. [Google Scholar] [CrossRef]
- Larson, C.M.; Wilcox, G.L.; Fairbanks, C.A. The Study of Pain in Rats and Mice. Comp. Med. 2019, 69, 555–570. [Google Scholar] [CrossRef]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 2014, 34, 6316–6322. [Google Scholar] [CrossRef]
- DeLeo, J.A.; Yezierski, R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001, 90, 1–6. [Google Scholar] [CrossRef]
- Hulsebosch, C.E. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp. Neurol. 2008, 214, 6–9. [Google Scholar] [CrossRef]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Peng, X.M.; Zhou, Z.G.; Glorioso, J.C.; Fink, D.J.; Mata, M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann. Neurol. 2006, 59, 843–851. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef]
- Hains, B.C.; Klein, J.P.; Saab, C.Y.; Craner, M.J.; Black, J.A.; Waxman, S.G. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003, 23, 8881–8892. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Baastrup, C. Spinal cord injury pain: Mechanisms and management. Curr. Pain Headache Rep. 2012, 16, 207–216. [Google Scholar] [CrossRef]
- Saeed, A.W.; Ribeiro-da-Silva, A. Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord. Mol. Pain 2012, 8, 64. [Google Scholar] [CrossRef]
- Sun, R.Q.; Tu, Y.J.; Lawand, N.B.; Yan, J.Y.; Lin, Q.; Willis, W.D. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J. Neurophysiol. 2004, 92, 2859–2866. [Google Scholar] [CrossRef]
- Akay, T.; Murray, A.J. Relative Contribution of Proprioceptive and Vestibular Sensory Systems to Locomotion: Opportunities for Discovery in the Age of Molecular Science. Int. J. Mol. Sci. 2021, 22, 1467. [Google Scholar] [CrossRef]
- Jirkof, P. Effects of experimental housing conditions on recovery of laboratory mice. Lab. Anim. 2015, 44, 65–70. [Google Scholar] [CrossRef]
- Manouze, H.; Ghestem, A.; Poillerat, V.; Bennis, M.; Ba-M’hamed, S.; Benoliel, J.J.; Becker, C.; Bernard, C. Effects of Single Cage Housing on Stress, Cognitive, and Seizure Parameters in the Rat and Mouse Pilocarpine Models of Epilepsy. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Jirkof, P.; Cesarovic, N.; Rettich, A.; Fleischmann, T.; Arras, M. Individual housing of female mice: Influence on postsurgical behaviour and recovery. Lab. Anim. 2012, 46, 325–334. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Br. J. Psychiatry 2013, 35 (Suppl. 2), S101–S111. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, S.H.; Kwon, J.; Lee, H.W.; Kim, H. Mice subjected to uncontrollable electric shocks show depression-like behaviors irrespective of their state of helplessness. Behav. Brain Res. 2017, 322, 138–144. [Google Scholar] [CrossRef]
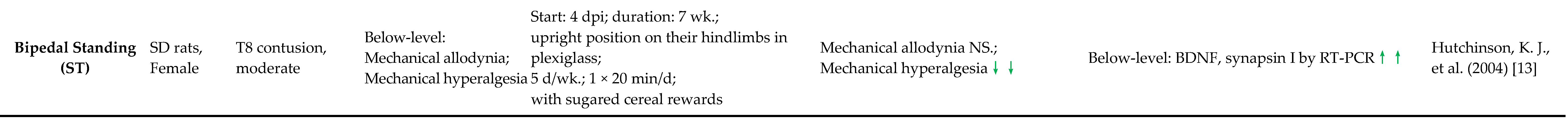


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Weidner, N.; Puttagunta, R. The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury. Cells 2022, 11, 3087. https://doi.org/10.3390/cells11193087
Chen J, Weidner N, Puttagunta R. The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury. Cells. 2022; 11(19):3087. https://doi.org/10.3390/cells11193087
Chicago/Turabian StyleChen, Jing, Norbert Weidner, and Radhika Puttagunta. 2022. "The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury" Cells 11, no. 19: 3087. https://doi.org/10.3390/cells11193087
APA StyleChen, J., Weidner, N., & Puttagunta, R. (2022). The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury. Cells, 11(19), 3087. https://doi.org/10.3390/cells11193087









