Illustration of Gut–Thyroid Axis in Alcohol Use Disorder: Interplay of Gut Dysfunction, Pro-Inflammatory Responses, and Thyroid Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Eligibility Criteria and Randomization
2.3. Demographics, Drinking Profile, and Laboratory Evaluations
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results
3.1. Demographics and Drinking Profile
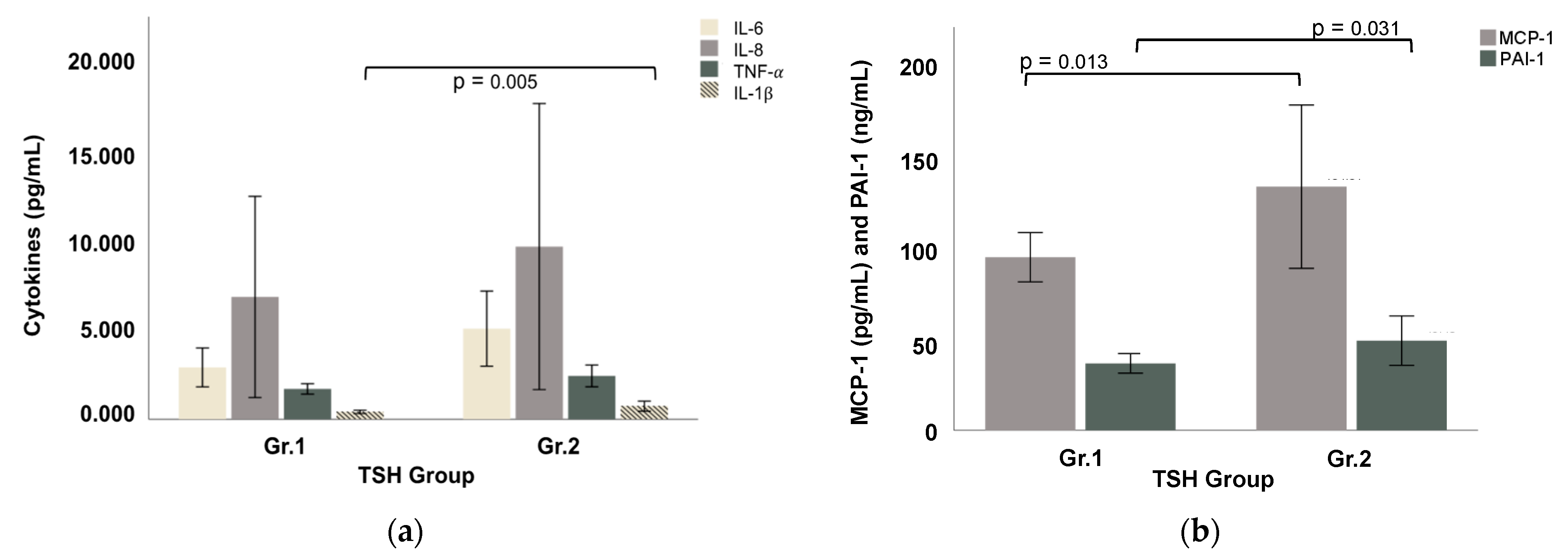
3.2. Gut Dysfunction Markers and Pro-Inflammatory Responses
3.3. Thyroid Function and Non-Specific Inflammatory Markers
3.4. Association of Drinking Markers and Measures of Gut Dysfunction and Pro-Inflammatory Status
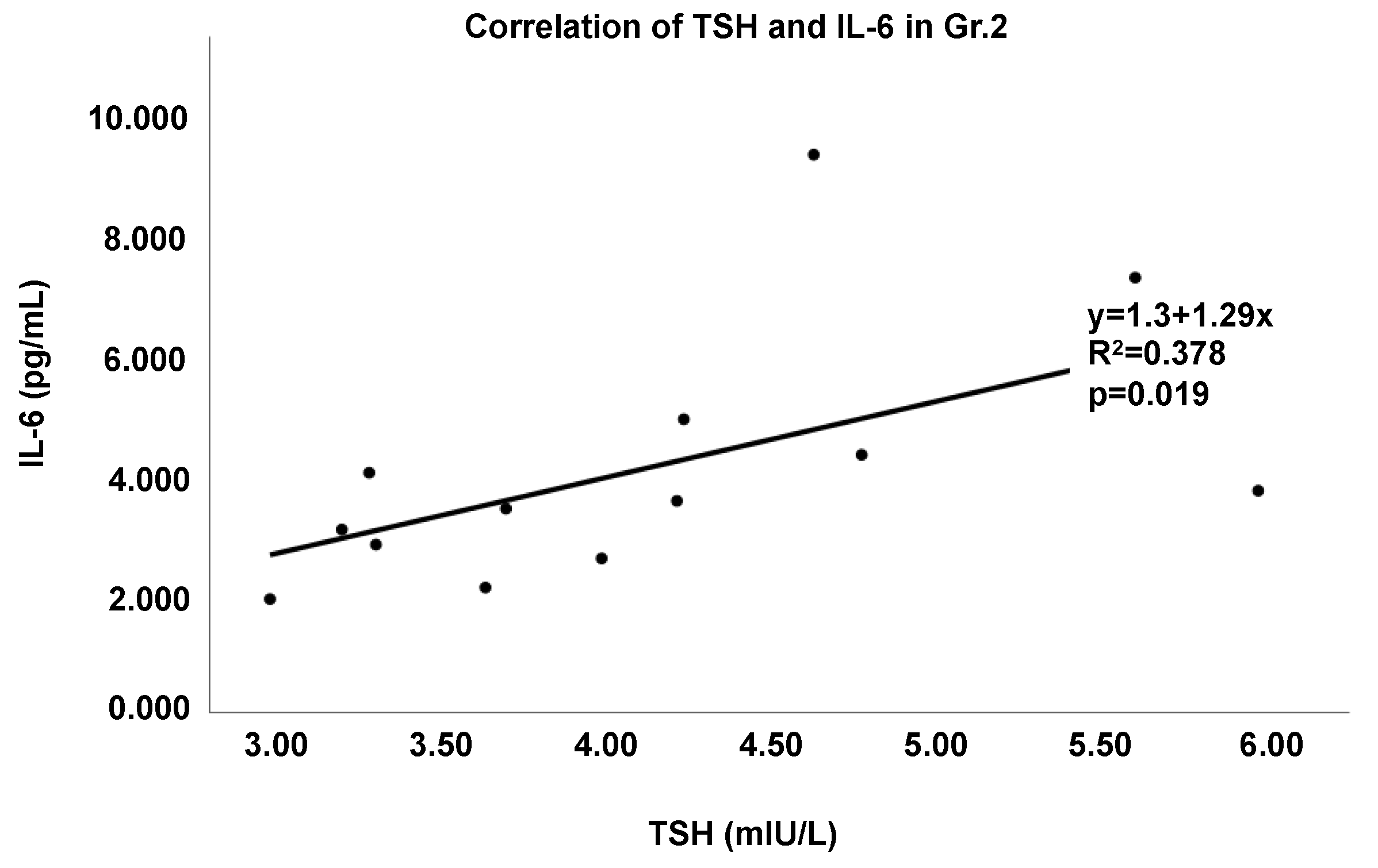
3.5. Characterization of Thyroid Dysregulation by the Gut–Immune–Brain and Gut–Thyroid Axes
3.6. Assessment of Alcohol and Its Effect on the Gut–Brain–Thyroid Axis
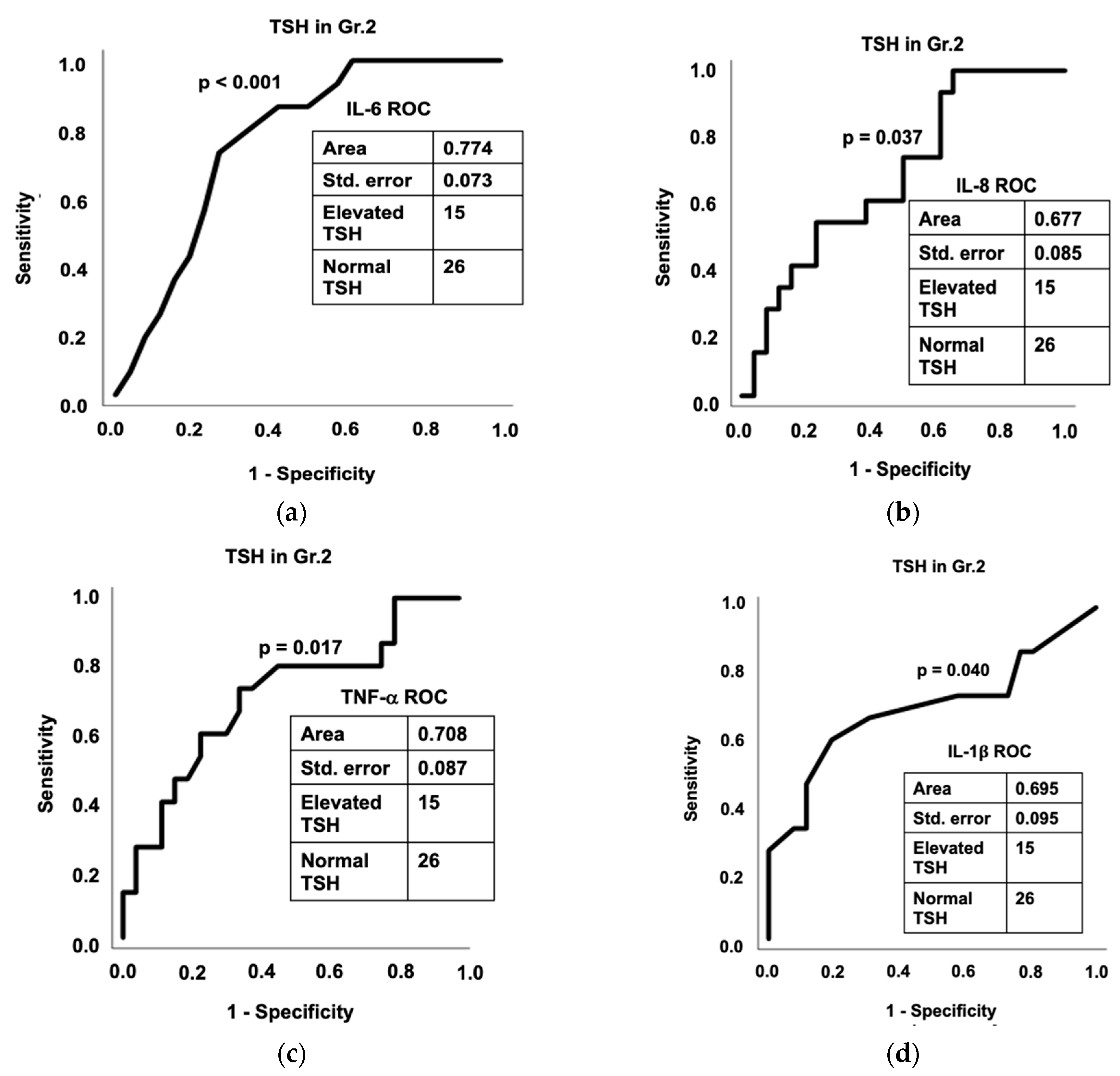
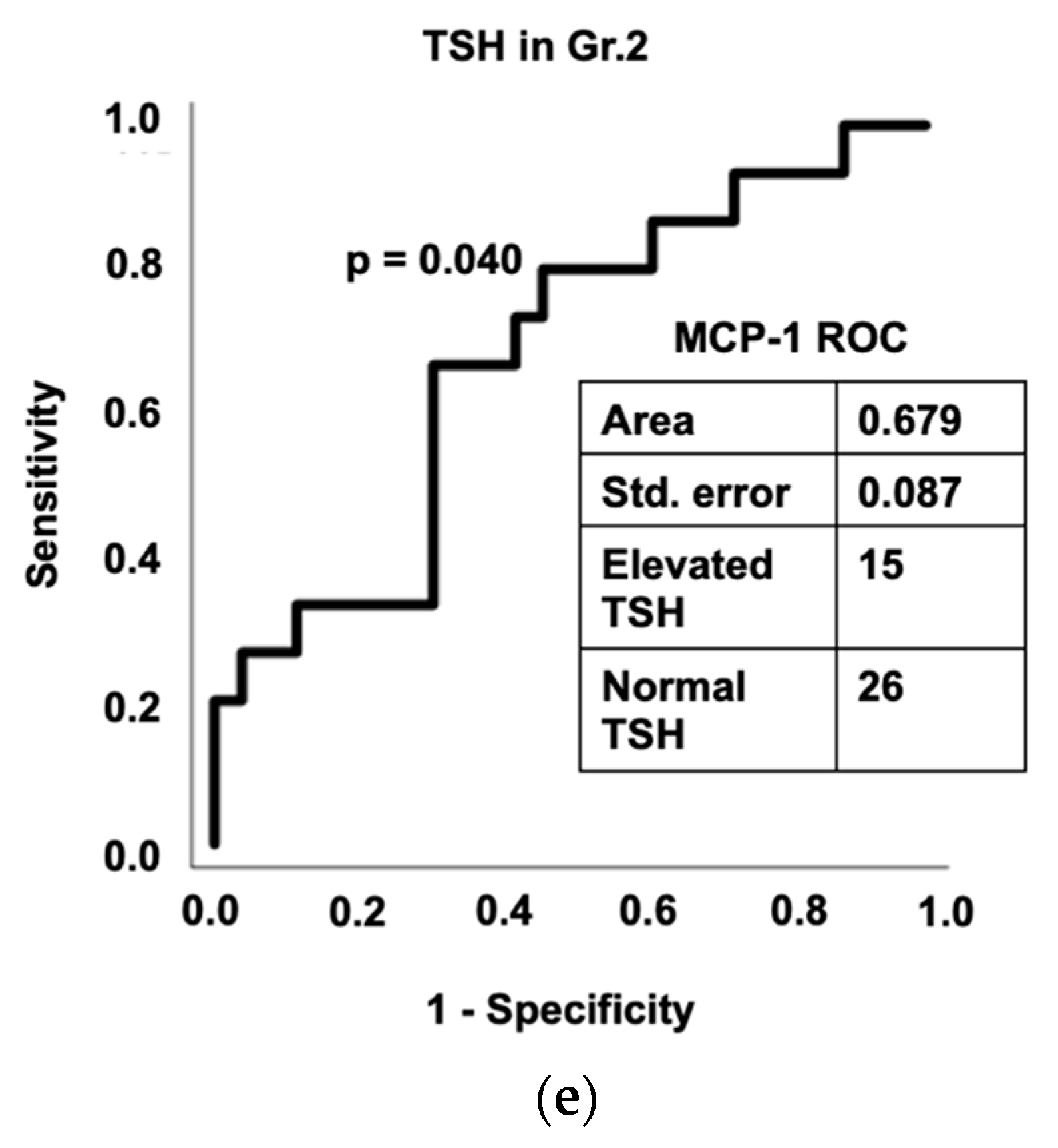
3.7. Diagnostic Assessment of Inflammation and Gut Dysfunction in Thyroid Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tucker, J.A.; Chandler, S.D.; Witkiewitz, K. Epidemiology of recovery from alcohol use disorder. Alcohol Res. Curr. Rev. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Alcohol Use in the United States National Institute on Alcohol Abuse and Alcoholism. 2021. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics#:~:text=Prevalence%20of%20Drinking%3A%20According%20to,in%20this%20age%20group%20and (accessed on 1 July 2022).
- Keyes, K.M. Age, period, and cohort effects in alcohol use in the United States in the 20th and 21st centuries: Implications for the coming decades. Alcohol Res. Curr. Rev. 2022, 42, 2. [Google Scholar] [CrossRef]
- Goldberg, M. Thyroid function in chronic alcoholism. Lancet 1962, 2, 746–749. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Fletcher, D.L.; Simonyl, A.; Rudeen, P.K. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcohol. Clin. Exp. Res. 1996, 20, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Loosen, P.T.; Wilson, I.C.; Dew, B.W.; Tipermas, A. Thyrotropin-releasing hormone (TRH) in abstinent alcoholic men. Am. J. Psychiatry 1983, 140, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Casacchia, M.; Rossi, A.; Stratta, P. Thyrotropin-releasing hormone test in recently abstinent alcoholics. Psychiatry Res. 1985, 16, 249–251. [Google Scholar] [CrossRef]
- Ozsoy, S.; Esel, E.; Izgi, H.B.; Sofuoglu, S. Thyroid function in early and late alcohol withdrawal: Relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcohol. 2006, 41, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Hermann, D.; Heinz, A.; Mann, K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction 2002, 97, 1369–1381. [Google Scholar] [CrossRef]
- Canesso, M.C.C.; Lacerda, N.L.; Ferreira, C.M.; Goncalves, J.L.; Almeida, D.; Gamba, C.; Cassali, G.; Pedroso, S.H.; Moreira, C.; Martins, F.S.; et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: The role of the gut microbiota. BMC Microbiol. 2014, 14, 240. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, S.; De Saeger, C.; Delzenne, N.; de Timary, P.; Starkel, P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 2014, 76, 725–733. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef] [PubMed]
- Pijls, K.E.; Jonkers, D.M.; Elamin, E.E.; Masclee, A.A.; Koek, G.H. Intestinal epithelial barrier function in liver cirrhosis: An extensive review of the literature. Liver Int. 2013, 33, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Giordano, M.; Nunnari, G.; Bertino, G.; Malaguarnera, M. Gut microbiota in alcoholic liver disease: Pathogenetic role and therapeutic perspectives. World J. Gastroenterol. 2014, 20, 16639–16648. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Voigt, R.M.; Keshavarzian, A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014, 3, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, S.; Toratani, S.; Shea-Donohue, T.; Kashiwabara, Y.; Vogel, S.N.; Metcalf, E.S. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol. Clin. Exp. Res. 2001, 25, 579–589. [Google Scholar] [CrossRef]
- Uesugi, T.; Froh, M.; Arteel, G.E.; Bradford, B.U.; Thurman, R.G. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 2001, 34, 101–108. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.J.; Yang, Y.; Xiao, Z.; Wan, J.B. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef]
- Vatsalya, V.; Song, M.; Schwandt, M.L.; Cave, M.C.; Barve, S.S.; George, D.T.; Ramchandani, V.A.; McClain, C.J. Effects of sex, drinking history, and omega-3 and omega-6 fatty acids dysregulation on the onset of liver injury in very heavy drinking alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2016, 40, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Wartofsky, L.; Dickey, R.A. The evidence for a narrower thyrotropin reference range is compelling. J. Clin. Endocrinol. Metab. 2005, 90, 5483–5488. [Google Scholar] [CrossRef]
- Spencer, C.A.; Hollowell, J.G.; Kazarosyan, M.; Braverman, L.E. National health and nutrition examination survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J. Clin. Endocrinol. Metab. 2007, 92, 4236–4240. [Google Scholar] [CrossRef] [Green Version]
- Sobell, L.C.; Sobell, M.B. Timeline follow-back. In Measuring Alcohol Consumption; Springer: Berlin, Germany, 1992; pp. 41–72. [Google Scholar]
- Skinner, H.A.; Sheu, W.-J. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J. Stud. Alcohol 1982, 43, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Barve, S.; Deaciuc, I.; Kugelmas, M.; Hill, D. Cytokines in alcoholic liver disease. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1999; Volume 19, pp. 205–219. [Google Scholar] [CrossRef]
- Means, T.K.; Lien, E.; Yoshimura, A.; Wang, S.; Golenbock, D.T.; Fenton, M.J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 1999, 163, 6748–6755. [Google Scholar] [PubMed]
- Lien, E.; Sellati, T.J.; Yoshimura, A.; Flo, T.H.; Rawadi, G.; Finberg, R.W.; Carroll, J.D.; Espevik, T.; Ingalls, R.R.; Radolf, J.D.; et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 1999, 274, 33419–33425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.K.; Seth, A.; Sheth, P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G881–G884. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, Z.; Ozdemirci, S.; Erden, B.F.; Dundar, V. The effect of long-term ethanol feeding on Brucella melitensis infection of rats. Alcohol Alcohol. 2001, 36, 314–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanaugh, M.J.; Clark, C.; Goto, M.; Kovacs, E.J.; Gamelli, R.L.; Sayeed, M.M.; Choudhry, M.A. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns 2005, 31, 290–296. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Szabo, G.; Lippai, D. Converging actions of alcohol on liver and brain immune signaling. Int. Rev. Neurobiol. 2014, 118, 359–380. [Google Scholar] [CrossRef]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 77–84. [Google Scholar] [CrossRef] [Green Version]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, K.; Hara, H. The inflammasome and its regulation. Crit. Rev. Immunol. 2014, 34, 41–80. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Brogioni, S.; Grasso, L.; Martino, E. Increased serum interleukin-6 concentration in patients with subacute thyroiditis: Relationship with concomitant changes in serum T4-binding globulin concentration. J. Endocrinol. Investig. 1993, 16, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Monson, J.P.; Wood, D.F.; Besser, G.M.; Burrin, J.M. Serum cytokines in thyrotoxicosis. J. Clin. Endocrinol. Metab. 1999, 84, 435–439. [Google Scholar] [CrossRef]
- Kobawala, T.P.; Patel, G.H.; Gajjar, D.R.; Patel, K.N.; Thakor, P.B.; Parekh, U.B.; Patel, K.M.; Shukla, S.N.; Shah, P.M. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J. Thyroid. Res. 2011, 2011, 270149. [Google Scholar] [CrossRef] [Green Version]
- Fabris, N.; Pierpaoli, W.; Sorkin, E. Hormones and the immunological capacity. IV. Restorative effects of developmental hormones or of lymphocytes on the immunodeficiency syndrome of the dwarf mouse. Clin. Exp. Immunol. 1971, 9, 227–240. [Google Scholar] [PubMed]
- Comsa, J.; Leonhardt, H.; Ozminski, K. Hormonal influences on the secretion of the thymus. Thymus 1979, 1, 81–93. [Google Scholar]
- King, D.B.; Scanes, C.G. Effect of mammalian growth hormone and prolactin on the growth of hypophysectomized chickens. Proc. Soc. Exp. Biol. Med. 1986, 182, 201–207. [Google Scholar] [CrossRef]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef]
- Blalock, J.E.; Johnson, H.M.; Smith, E.M.; Torres, B.A. Enhancement of the in vitro antibody response by thyrotropin. Biochem. Biophys. Res. Commun. 1984, 125, 30–34. [Google Scholar] [CrossRef]
- Pienaar, W.P.; Roberts, M.C.; Emsley, R.A.; Aalbers, C.; Taljaard, F.J. The thyrotropin releasing hormone stimulation test in alcoholism. Alcohol Alcohol. 1995, 30, 661–667. [Google Scholar]
- Sellman, J.D.; Joyce, P.R. The clinical significance of the thyrotropin-releasing hormone test in alcoholic men. Aust. N. Z. J. Psychiatry 1992, 26, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Valimaki, M.; Pelkonen, R.; Harkonen, M.; Ylikahri, R. Hormonal changes in noncirrhotic male alcoholics during ethanol withdrawal. Alcohol Alcohol. 1984, 19, 235–242. [Google Scholar] [PubMed]
- Winokur, A.; Caroff, S.N.; Amsterdam, J.D.; Maislin, G. Administration of thyrotropin-releasing hormone at weekly intervals results in a diminished thyrotropin response. Biol. Psychiatry 1984, 19, 695–702. [Google Scholar] [PubMed]
- Sheppard, M.C.; Shennan, K.I. Desensitization of rat anterior pituitary gland to thyrotrophin releasing hormone. J. Endocrinol. 1984, 101, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, L. Decreased thyroid gland volume in alcoholic cirrhosis of the liver. J. Clin. Endocrinol. Metab. 1984, 58, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, L.; Rasmussen, N.; Ravn, V.; Kastrup, J.; Krogsgaard, K.; Aldershvile, J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: The possibility of a toxic effect of alcohol on the thyroid gland. Metabolism 1988, 37, 229–233. [Google Scholar] [CrossRef]
- Knudsen, N.; Bulow, I.; Laurberg, P.; Perrild, H.; Ovesen, L.; Jorgensen, T. Alcohol consumption is associated with reduced prevalence of goitre and solitary thyroid nodules. Clin. Endocrinol. 2001, 55, 41–46. [Google Scholar] [CrossRef]
- Livraghi, T.; Paracchi, A.; Ferrari, C.; Reschini, E.; Macchi, R.M.; Bonifacino, A. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: 4-year experience. Radiology 1994, 190, 529–533. [Google Scholar] [CrossRef]
- Rao, N.L.; Shetty, S.; Upadhyaya, K.; R.M, P.; Lobo, E.C.; Kedilaya, H.P.; Prasad, G. Salivary C-reactive protein in Hashimoto’s thyroiditis and subacute thyroiditis. Int. J. Inflam. 2010, 2010, 514659. [Google Scholar] [CrossRef] [Green Version]
- Baruah, M.P.; Bhattacharya, B. Significant role of serum CRP in differentiating inflammatory from non-inflammatory causes of thyrotoxicosis. Indian J. Endocrinol. Metab. 2012, 16, 976–981. [Google Scholar] [CrossRef]
- Pearce, E.N.; Bogazzi, F.; Martino, E.; Brogioni, S.; Pardini, E.; Pellegrini, G.; Parkes, A.B.; Lazarus, J.H.; Pinchera, A.; Braverman, L.E. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid 2003, 13, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Maas, M.A.; Lowik, C.W.; Platvoet, M.C.; Wiersinga, W.M. Induced illness in interleukin-6 (IL-6) knock-out mice: A causal role of IL-6 in the development of the low 3,5,3′-triiodothyronine syndrome. Endocrinology 1996, 137, 5250–5254. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.H.; Black, E.G.; Sheppard, M.C.; Franklyn, J.A. Relation between serum interleukin-6 and thyroid hormone concentrations in 270 hospital in-patients with non-thyroidal illness. Clin. Endocrinol. 1996, 44, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Moura Neto, A.; Zantut-Wittmann, D.E. Abnormalities of thyroid hormone metabolism during systemic illness: The low T3 syndrome in different clinical settings. Int. J. Endocrinol. 2016, 2016, 2157583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-pituitary-thyroid axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef]


| Group 1 (Normal TSH, Gr. 1) | Group 2 (Elevated TSH, Gr. 2) | Between Group p–Value | |||||
|---|---|---|---|---|---|---|---|
| Males (n = 21; 75%) | Females (n = 7; 25%) | Total (n = 28; 63.6%) | Males (n = 10; 62.5%) | Females (n = 6; 37.5%) | Total (n = 16; 36.4%) | ||
| Demographics | |||||||
| Age c (years) | 41.6 ± 8.5 | 41.3 ± 13.8 | 41.5 ± 9.8 | 47.3 ± 12.1 | 44.7 ± 11.6 | 46.3 ± 11.6 | ns |
| BMI bc (kg/m2) | 26.3 ± 4.4 | 29.5 ± 10.5 | 27.1 ± 6.5 | 27.8 ± 4.6 | 26.3 ± 2.7 | 27.3 ± 4.0 | ns |
| Drinking History | |||||||
| TD90 | 1091 ± 450 | 915 ± 664 | 1052 ± 496 | 1159 ± 686 | 1084 ± 604 | 1131 ± 637 | ns |
| HDD90 | 71.43 ± 22.02 | 61.33 ± 21.26 | 69.19 ± 21.87 | 73.60 ± 21.58 | 75.83 ± 18.44 | 74.44 ± 19.85 | ns |
| AvgDPD90 ac | 14.32 ± 3.89 | 14.91 ± 9.26 | 14.45 ± 5.31 | 15.68 ± 7.65 | 13.57 ± 6.10 | 14.89 ± 6.97 | ns |
| NDD90 | 75.14 ± 18.26 | 62.50 ± 20.93 | 72.33 ± 19.22 | 75.50 ± 22.48 | 77.67 ± 16.07 | 76.31 ± 19.76 | ns |
| LTDH | 15.80 ± 9.60 | 9.86 ± 5.21 | 14.26 ± 8.98 | 21.90 ± 9.69 | 11.83 ± 9.00 | 18.13 ± 10.42 | ns |
| Liver Panel | |||||||
| ALT (IU/L) | 81.86 ± 40.93 | 58.57 ± 77.88 | 76.04 ± 51.90 | 80.00 ± 41.00 | 94.50 ± 108.70 | 85.44 ± 70.71 | ns |
| AST (IU/L) | 92.57 ± 72.12 | 88.14 ± 134.12 | 91.46 ± 88.62 | 114.40 ± 103.13 | 158.33 ± 122.50 | 130.88 ± 108.93 | ns |
| AST:ALT | 1.091 ± 0.553 | 1.408 ± 0.459 | 1.170 ± 0.541 | 1.300 ± 0.626 | 1.970 ± 0.937 | 1.551 ± 0.800 | ns |
| TBili (mg/dL) | 0.786 ± 0.869 | 0.743 ± 0.365 | 0.775 ± 0.767 | 0.690 ± 0.247 | 0.733 ± 0.398 | 0.706 ± 0.300 | ns |
| Albumin (g/dL) | 4.181 ± 0.423 | 4.029 ± 0.206 | 4.143 ± 0.382 | 4.080 ± 0.358 | 4.217 ± 0.458 | 4.131 ± 0.389 | ns |
| Thyroid Function | |||||||
| TSH (mIU/L) | 1.781 ± 0.609 | 1.793 ± 0.711 | 1.784 ± 0.622 | 3.931 ± 0.951 | 4.656 ± 1.001 | 4.173 ± 0.996 | 0.046 |
| T3 (ng/dL) | 106.45 ± 19.22 | 109.33 ± 30.12 | 107.47 ± 22.72 | 131.88 ± 20.32 | 146.25 ± 17.75 | 136.67 ± 19.97 | ns |
| fT4 (ng/dL) | 5.782 ± 1.154 | 5.700 ± 1.101 | 5.753 ± 1.101 | 5.575 ± 1.456 | 7.950 ± 0.507 | 6.367 ± 1.669 | 0.037 |
| Nutritional Status | |||||||
| Zinc (ug/dL) | 79.53 ± 12.19 | 64.43 ± 12.30 | 75.46 ± 13.78 | 71.90 ± 13.37 | 92.67 ± 46.26 | 79.69 ± 30.47 | ns |
| Blood Cell Measures | |||||||
| AMC c (K/uL) | 0.492 ± 0.233 | 0.489 ± 0.126 | 0.491 ± 0.209 | 0.535 ± 0.268 | 0.518 ± 0.076 | 0.528 ± 0.212 | ns |
| ANC (K/uL) | 3.684 ± 2.063 | 5.216 ± 1.872 | 4.067 ± 2.095 | 3.266 ± 1.799 | 3.370 ± 1.280 | 3.305 ± 1.578 | ns |
| Inflammatory markers | |||||||
| CRP c (mg/L) | 2.07 ± 1.61 | 0.57 ± 0.04 | 1.42 ± 1.39 | 4.47 ± 7.16 | 0.88 ** | 3.75 ± 6.40 | 0.039 |
| IgA (pg/mL) | 295.9 ± 113.7 | 268.3 ± 128.4 | 289.0 ± 115.7 | 281.1 ± 173.7 | 249.7 ± 86.0 | 269.3 ± 144.3 | ns |
| IgG (pg/mL) | 1102 ± 439 | 1152 ± 213 | 1114 ± 391 | 1169 ± 314 | 1010 ± 254 | 1109 ± 295 | ns |
| IgM d (pg/mL) | 123.4 ± 63.5 | 106.9 ± 48.7 | 119.3 ± 59.7 | 113.8 ± 41.6 | 156.8 ± 90.2 | 129.9 ± 64.9 | ns |
| Candidate Cytokine Response | |||||||
| IL-6 (pg/mL) | 2.588 ± 2.537 | 3.771 ± 3.101 | 2.861 ± 2.659 | 4.268 ± 2.250 | 6.102 ± 5.357 | 5.002 ± 3.743 | ns |
| IL-8 cd (pg/mL) | 4.013 ± 3.247 | 15.886 ± 27.849 | 6.753 ± 13.753 | 5.581 ± 2.410 | 15.463 + 22.137 | 9.534 ± 14.263 | ns |
| TNF-α d (pg/mL) | 1.743 ± 0.693 | 1.458 ± 0.779 | 1.677 ± 0.708 | 2.215 ± 0.562 | 2.659 ± 1.624 | 2.393 ± 1.083 | ns |
| IL-1β b (pg/mL) | 0.451 ± 0.227 | 0.268 ± 0.196 | 0.409 ± 0.230 | 0.688 ± 0.378 | 0.772 ± 0.689 | 0.722 ± 0.503 | 0.005 |
| MCP-1 a (pg/mL) | 96.62 ± 32.57 | 91.47 ± 39.87 | 95.43 ± 33.60 | 123.92 ± 63.64 | 149.90 ± 107.31 | 134.31 ± 81.24 | 0.013 |
| PAI-1 a (ng/mL) | 35.91 ± 13.80 | 40.36 ± 11.98 | 36.94 ± 13.31 | 56.86 ± 24.65 | 38.34 ± 21.69 | 49.46 ± 24.56 | 0.031 |
| Candidate Gut Dysfunction Markers | |||||||
| LPS (EU/mL) | 0.106 ± 0.060 | 0.091 ± 0.058 | 0.102 ± 0.059 | 0.097 ± 0.064 | 0.109 ± 0.069 | 0.102 ± 0.064 | ns |
| LBP abcd (ng/mL) | 1119 ± 1430 | 3989 ± 4989 | 1781 ± 2838 | 2822 ± 3480 | 751 ± 624 | 2132 ± 2986 | ns |
| sCD14 d (ng/mL) | 9063 ± 1970 | 9375 ± 1323 | 9141 ± 1812 | 8554 ± 1840 | 11321 ± 733 | 9592 ± 2031 | ns |
| LBP + sCD14 | LPS + LBP + sCD14 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-8 | IL-1β | MCP-1 | PAI-1 | IL-1β | ||||||||
| TD90 | ns | ns | ns | ns | ns | ns | 0.827 | p = 0.007 | ns | ns | ns | ns | 0.827 | p = 0.023 |
| NDD90 | ns | ns | 0.736 | p = 0.043 | ns | ns | 0.741 | p = 0.040 | ns | ns | 0.786 | p = 0.018 | ns | ns |
| HDD | 0.759 | p = 0.030 | 0.788 | p = 0.018 | 0.769 | p = 0.025 | 0.774 | p = 0.023 | 0.757 | p = 0.031 | 0.798 | p = 0.014 | ns | ns |
| LPS + LBP | LBP + sCD14 | LPS + LBP + sCD14 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | PAI-1 | IL-6 | PAI-1 | IL-6 | PAI-1 | |||||||
| TSH | 0.756 | p = 0.046 | 0.800 | p = 0.022 | 0.816 | p = 0.016 | 0.896 | p = 0.002 | 0.819 | p = 0.043 | 0.905 | p = 0.005 |
| T3 | ns | ns | 0.878 | p = 0.012 | ns | ns | 0.914 | p = 0.004 | ns | ns | 0.919 | p = 0.013 |
| fT4 | ns | ns | 0.854 | p = 0.021 | ns | ns | 0.903 | p = 0.006 | ns | ns | 0.906 | p = 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagaram, M.; Royer, A.J.; Hu, H.; Rajhans, A.; Parthasarathy, R.; Krishnasamy, S.S.; Mokshagundam, S.P.; Kong, M.; Schwandt, M.L.; Parajuli, D.; et al. Illustration of Gut–Thyroid Axis in Alcohol Use Disorder: Interplay of Gut Dysfunction, Pro-Inflammatory Responses, and Thyroid Function. Cells 2022, 11, 3100. https://doi.org/10.3390/cells11193100
Sagaram M, Royer AJ, Hu H, Rajhans A, Parthasarathy R, Krishnasamy SS, Mokshagundam SP, Kong M, Schwandt ML, Parajuli D, et al. Illustration of Gut–Thyroid Axis in Alcohol Use Disorder: Interplay of Gut Dysfunction, Pro-Inflammatory Responses, and Thyroid Function. Cells. 2022; 11(19):3100. https://doi.org/10.3390/cells11193100
Chicago/Turabian StyleSagaram, Manasa, Amor J. Royer, Huirong Hu, Abhas Rajhans, Ranganathan Parthasarathy, Sathya Sridevi Krishnasamy, Sri Prakash Mokshagundam, Maiying Kong, Melanie L. Schwandt, Dipendra Parajuli, and et al. 2022. "Illustration of Gut–Thyroid Axis in Alcohol Use Disorder: Interplay of Gut Dysfunction, Pro-Inflammatory Responses, and Thyroid Function" Cells 11, no. 19: 3100. https://doi.org/10.3390/cells11193100
APA StyleSagaram, M., Royer, A. J., Hu, H., Rajhans, A., Parthasarathy, R., Krishnasamy, S. S., Mokshagundam, S. P., Kong, M., Schwandt, M. L., Parajuli, D., Cave, M. C., & Vatsalya, V. (2022). Illustration of Gut–Thyroid Axis in Alcohol Use Disorder: Interplay of Gut Dysfunction, Pro-Inflammatory Responses, and Thyroid Function. Cells, 11(19), 3100. https://doi.org/10.3390/cells11193100






