Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions
Abstract
1. Introduction
2. Methods
2.1. Animals and Surgical Procedures
2.2. Intermittent Binge EtOH Exposure
2.3. Immunohistochemistry (IHC)
2.3.1. Slice Preparation
2.3.2. PSD-95 and GFAP
2.3.3. Neuroligin 1, 3, and Neurexin
2.4. Data Acquisition and Processing
2.4.1. Astrocyte-Synaptic Co-Localization
2.4.2. Neuroligin 1, 3, and Neurexin
2.4.3. Golgi-Cox Staining
2.4.4. Dendritic Spine Analysis
2.4.5. Blood EtOH Concentrations (BECs)
2.5. Statistical Analysis
3. Results
3.1. Blood EtOH Concentrations (BECs)
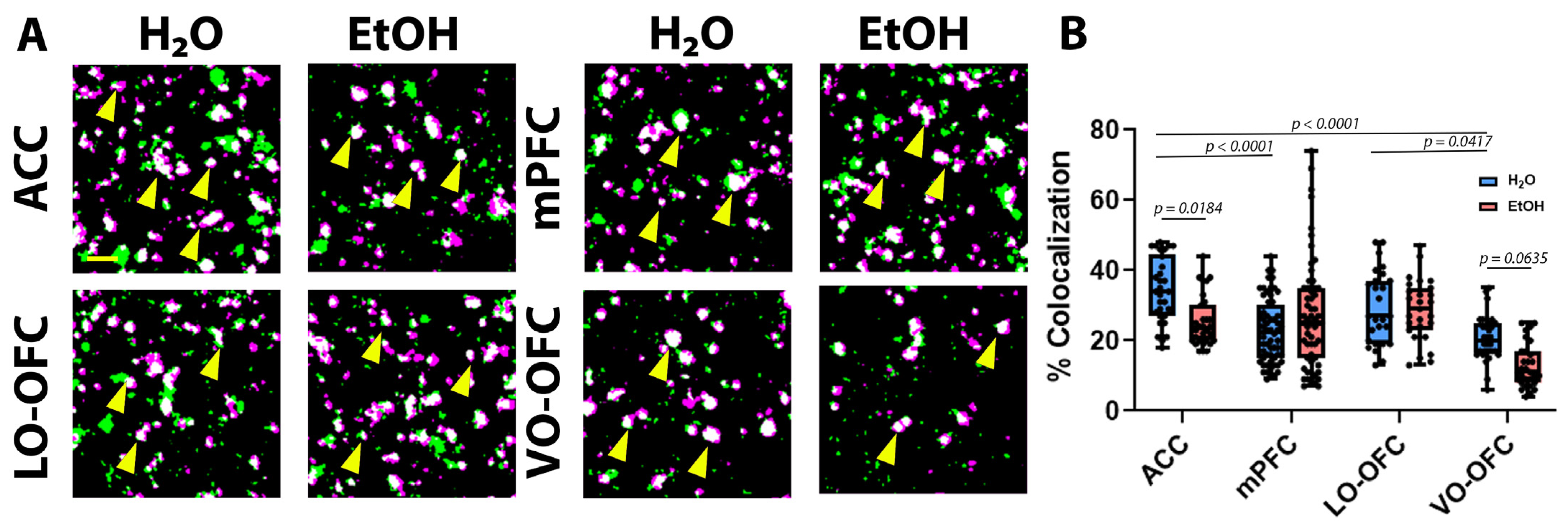
3.2. AIE Induced Changes in PFC Astrocyte Morphology and PAP-Synaptic Proximity in a Subregion-Dependent Manner, but Only after a Period of Forced Abstinence
3.3. AIE Results in Cortical Subregion Dependent Shifts in Dendritic Spine Maturation after Forced Abstinence
3.4. AIE-Induced loss of PAP-Synaptic Co-localization Is Not Driven by Changes in Expression of Synaptic Stabilization Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrick, M.E.; Schulenberg, J.E. Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res. 2013, 35, 193–200. [Google Scholar] [PubMed]
- White, A.M.; Castle, I.P.; Powell, P.A.; Hingson, R.W.; Koob, G.F. Alcohol-Related Deaths During the COVID-19 Pandemic. JAMA 2022, 327, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018; p. 450. [Google Scholar]
- White, A.M.; Castle, I.P.; Hingson, R.W.; Powell, P.A. Using Death Certificates to Explore Changes in Alcohol-Related Mortality in the United States, 1999 to 2017. Alcohol. Clin. Exp. Res. 2020, 44, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Creswell, K.G.; Bachrach, R.; Clark, D.B.; Martin, C.S. Adolescent Binge Drinking: Developmental context and opportunities for prevention. Alcohol Res. Curr. Rev. 2018, 39, 5–15. [Google Scholar]
- Hingson, R.; White, A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J. Stud. Alcohol Drugs 2014, 75, 158–169. [Google Scholar] [CrossRef]
- Grifasi, I.R.; McIntosh, S.E.; Thomas, R.D.; Lysle, D.T.; Thiele, T.E.; Marshall, S.A. Characterization of the Hippocampal Neuroimmune Response to Binge-Like Ethanol Consumption in the Drinking in the Dark Model. Neuroimmunomodulation 2019, 26, 19–32. [Google Scholar] [CrossRef]
- Kandel, D.B.; Johnson, J.G.; Bird, H.R.; Canino, G.; Goodman, S.H.; Lahey, B.B.; Regier, D.A.; Schwab-Stone, M. Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J. Abnorm. Child Psychol. 1997, 25, 121–132. [Google Scholar] [CrossRef]
- Kuntsche, E.; Kuntsche, S.; Thrul, J.; Gmel, G. Binge drinking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 976–1017. [Google Scholar] [CrossRef]
- Miller, J.W.; Naimi, T.S.; Brewer, R.D.; Jones, S.E. Binge drinking and associated health risk behaviors among high school students. Pediatrics 2007, 119, 76–85. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Wood, J.N.; Grafman, J. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 2003, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.L.; Mendoza, M.P.; Cruz, F.C.; Leao, R.M.; Caprioli, D.; Rubio, F.J.; Whitaker, L.R.; McPherson, K.B.; Bossert, J.M.; Shaham, Y.; et al. Distinct Fos-Expressing Neuronal Ensembles in the Ventromedial Prefrontal Cortex Mediate Food Reward and Extinction Memories. J. Neurosci. 2016, 36, 6691–6703. [Google Scholar] [CrossRef]
- Laurent, V.; Westbrook, R.F. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn. Mem. 2009, 16, 520–529. [Google Scholar] [CrossRef]
- Sotres-Bayon, F.; Quirk, G.J. Prefrontal control of fear: More than just extinction. Curr. Opin. Neurobiol. 2010, 20, 231–235. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- Lei, H.; Lai, J.; Sun, X.; Xu, Q.; Feng, G. Lateral orbitofrontal dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. J. Psychiatry Neurosci. 2019, 44, 120–131. [Google Scholar] [CrossRef]
- Nogueira, R.; Abolafia, J.M.; Drugowitsch, J.; Balaguer-Ballester, E.; Sanchez-Vives, M.V.; Moreno-Bote, R. Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat. Commun. 2017, 8, 14823. [Google Scholar] [CrossRef]
- Wood, R.L.; Worthington, A. Neurobehavioral Abnormalities Associated with Executive Dysfunction after Traumatic Brain Injury. Front. Behav. Neurosci. 2017, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; Stenger, A.; Fein, G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb. Cortex 2013, 23, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Vollstadt-Klein, S.; Hermann, D.; Rabinstein, J.; Wichert, S.; Klein, O.; Ende, G.; Mann, K. Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol. Clin. Exp. Res. 2010, 34, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, L.; Balleine, B.W.; Corbit, L.H.; Killcross, S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 2013, 1282, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Van den Oever, M.C.; Spijker, S.; Smit, A.B.; De Vries, T.J. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci. Biobehav. Rev. 2010, 35, 276–284. [Google Scholar] [CrossRef]
- Boettiger, C.A.; Mitchell, J.M.; Tavares, V.C.; Robertson, M.; Joslyn, G.; D’Esposito, M.; Fields, H.L. Immediate reward bias in humans: Fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J. Neurosci. 2007, 27, 14383–14391. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Overholser, J.C.; Meltzer, H.Y.; Stockmeier, C.A.; Rajkowska, G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol. Clin. Exp. Res. 2006, 30, 1845–1855. [Google Scholar] [CrossRef]
- Crews, F.T.; Braun, C.J.; Hoplight, B.; Switzer, R.C., 3rd; Knapp, D.J. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol. Clin. Exp. Res. 2000, 24, 1712–1723. [Google Scholar] [CrossRef]
- DeWit, D.J.; Adlaf, E.M.; Offord, D.R.; Ogborne, A.C. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am. J. Psychiatry 2000, 157, 745–750. [Google Scholar] [CrossRef]
- Lyon, K.A.; Allen, N.J. From Synapses to Circuits, Astrocytes Regulate Behavior. Front. Neural Circuits 2021, 15, 786293. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ozawa, K.; Yahagi, K.; Mishima, T.; Akther, S.; Vo, C.T.; Lee, A.B.; Tanaka, M.; Itohara, S.; Hirase, H. Transient Astrocytic Gq Signaling Underlies Remote Memory Enhancement. Front. Neural Circuits 2021, 15, 658343. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.K.; DaCosta, A.J.; Mason, S.C.; Blednov, Y.A.; Mayfield, R.D.; Harris, R.A. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology 2021, 46, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.D.; Risher, W.C.; Risher, M.L. Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse. Cells 2020, 9, 297. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Kim, S.K.; Nabekura, J.; Koizumi, S. Astrocyte-mediated synapse remodeling in the pathological brain. Glia 2017, 65, 1719–1727. [Google Scholar] [CrossRef]
- Nishida, H.; Okabe, S. Direct astrocytic contacts regulate local maturation of dendritic spines. J. Neurosci. 2007, 27, 331–340. [Google Scholar] [CrossRef]
- Risher, W.C.; Patel, S.; Kim, I.H.; Uezu, A.; Bhagat, S.; Wilton, D.K.; Pilaz, L.J.; Singh Alvarado, J.; Calhan, O.Y.; Silver, D.L.; et al. Astrocytes refine cortical connectivity at dendritic spines. Elife 2014, 3, e04047. [Google Scholar] [CrossRef]
- Witcher, M.R.; Kirov, S.A.; Harris, K.M. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 2007, 55, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Zhang, C.; Arac, D.; Boucard, A.A.; Brunger, A.T.; Sudhof, T.C. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J. 2009, 28, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Testen, A.; Ali, M.; Sexton, H.G.; Hodges, S.; Dubester, K.; Reissner, K.J.; Swartzwelder, H.S.; Risher, M.L. Region-Specific Differences in Morphometric Features and Synaptic Colocalization of Astrocytes During Development. Neuroscience 2019, 400, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Carty, N.; Nash, K.R.; Brownlow, M.; Cruite, D.; Wilcock, D.; Selenica, M.L.; Lee, D.C.; Gordon, M.N.; Morgan, D. Intracranial injection of AAV expressing NEP but not IDE reduces amyloid pathology in APP+PS1 transgenic mice. PLoS ONE 2013, 8, e59626. [Google Scholar] [CrossRef]
- Benediktsson, A.M.; Schachtele, S.J.; Green, S.H.; Dailey, M.E. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J. Neurosci. Methods 2005, 141, 41–53. [Google Scholar] [CrossRef]
- Shigetomi, E.; Bushong, E.A.; Haustein, M.D.; Tong, X.; Jackson-Weaver, O.; Kracun, S.; Xu, J.; Sofroniew, M.V.; Ellisman, M.H.; Khakh, B.S. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 2013, 141, 633–647. [Google Scholar] [CrossRef]
- Zlatkine, P.; Mehul, B.; Magee, A.I. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J. Cell Sci. 1997, 110 Pt 5, 673–679. [Google Scholar] [CrossRef]
- Scofield, M.D.; Li, H.; Siemsen, B.M.; Healey, K.L.; Tran, P.K.; Woronoff, N.; Boger, H.A.; Kalivas, P.W.; Reissner, K.J. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol. Psychiatry 2016, 80, 207–215. [Google Scholar] [CrossRef]
- Testen, A.; Sepulveda-Orengo, M.T.; Gaines, C.H.; Reissner, K.J. Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front. Cell. Neurosci. 2018, 12, 246. [Google Scholar] [CrossRef]
- Risher, M.L.; Sexton, H.G.; Risher, W.C.; Wilson, W.A.; Fleming, R.L.; Madison, R.D.; Moore, S.D.; Eroglu, C.; Swartzwelder, H.S. Adolescent Intermittent Alcohol Exposure: Dysregulation of Thrombospondins and Synapse Formation are Associated with Decreased Neuronal Density in the Adult Hippocampus. Alcohol. Clin. Exp. Res. 2015, 39, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.E. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics 2009, 123, e975–e981. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Sun, Z.; Lee, T.; Fusco, F.R.; Kimble, T.D.; Meade, C.A.; Cuthbertson, S.; Reiner, A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J. Neurosci. Methods 1999, 93, 149–162. [Google Scholar] [CrossRef]
- Risher, W.C.; Ustunkaya, T.; Singh Alvarado, J.; Eroglu, C. Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS ONE 2014, 9, e107591. [Google Scholar] [CrossRef]
- Bourne, J.N.; Harris, K.M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008, 31, 47–67. [Google Scholar] [CrossRef]
- Matsuzaki, M. Factors critical for the plasticity of dendritic spines and memory storage. Neurosci Res. 2007, 57, 1–9. [Google Scholar] [CrossRef]
- Asato, M.R.; Terwilliger, R.; Woo, J.; Luna, B. White Matter Development in Adolescence: A DTI Study. Cereb. Cortex 2010, 20, 2122–2131. [Google Scholar] [CrossRef]
- Cressman, V.L.; Balaban, J.; Steinfeld, S.; Shemyakin, A.; Graham, P.; Parisot, N.; Moore, H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol. 2010, 518, 2693–2709. [Google Scholar] [CrossRef]
- Cunningham, M.G.; Bhattacharyya, S.; Benes, F.M. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002, 453, 116–130. [Google Scholar] [CrossRef]
- Liston, C.; Watts, R.; Tottenham, N.; Davidson, M.C.; Niogi, S.; Ulug, A.M.; Casey, B.J. Frontostriatal Microstructure Modulates Efficient Recruitment of Cognitive Control. Cereb. Cortex 2005, 16, 553–560. [Google Scholar] [CrossRef]
- Markham, J.A.; Morris, J.R.; Juraska, J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 2007, 144, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry 2013, 22, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Uddin, L.Q.; Prater, K.; Amin, H.; Greicius, M.D.; Menon, V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage 2010, 52, 290–301. [Google Scholar] [CrossRef]
- Broadwater, M.A.; Lee, S.H.; Yu, Y.; Zhu, H.; Crews, F.T.; Robinson, D.L.; Shih, Y.I. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict. Biol. 2018, 23, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; He, J.; Lee, J.; Styner, M.; Crews, F.T. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Liu, W.; Oguz, I.; Styner, M.; Crews, F.T. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav. 2014, 116, 142–151. [Google Scholar] [CrossRef]
- Gass, J.T.; Glen, W.B., Jr.; McGonigal, J.T.; Trantham-Davidson, H.; Lopez, M.F.; Randall, P.K.; Yaxley, R.; Floresco, S.B.; Chandler, L.J. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 2014, 39, 2570–2583. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience 2012, 226, 475–488. [Google Scholar] [CrossRef]
- Hwang, S.N.; Lee, J.S.; Seo, K.; Lee, H. Astrocytic Regulation of Neural Circuits Underlying Behaviors. Cells 2021, 10, 296. [Google Scholar] [CrossRef]
- Allen, N.J. Astrocyte regulation of synaptic behavior. Annu. Rev. Cell Dev. Biol. 2014, 30, 439–463. [Google Scholar] [CrossRef]
- Blanco-Suarez, E.; Caldwell, A.L.; Allen, N.J. Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 2017, 595, 1903–1916. [Google Scholar] [CrossRef]
- McGuier, N.S.; Padula, A.E.; Lopez, M.F.; Woodward, J.J.; Mulholland, P.J. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol 2015, 49, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.J.; Pancaroglu, R.; Kang, Y.; Rooyakkers, A.; Craig, A.M. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J. Neurosci. 2010, 30, 7495–7506. [Google Scholar] [CrossRef] [PubMed]
- Dagar, S.; Gottmann, K. Differential Properties of the Synaptogenic Activities of the Neurexin Ligands Neuroligin1 and LRRTM2. Front. Mol. Neurosci. 2019, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.; Sylwestrak, E.; O’Sullivan, M.L.; Otto, S.; Tiglio, K.; Savas, J.N.; Yates, J.R., 3rd; Comoletti, D.; Taylor, P.; Ghosh, A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 2009, 64, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. GFAP versus S100B in serum after traumatic brain injury: Relationship to brain damage and outcome. J. Neurotrauma 2004, 21, 1553–1561. [Google Scholar] [CrossRef]
- Reilly, J.F.; Maher, P.A.; Kumari, V.G. Regulation of astrocyte GFAP expression by TGF-beta1 and FGF-2. Glia 1998, 22, 202–210. [Google Scholar] [CrossRef]
- Nwachukwu, K.N.; Evans, W.A.; Sides, T.R.; Trevisani, C.P.; Davis, A.; Marshall, S.A. Chemogenetic manipulation of astrocytic signaling in the basolateral amygdala reduces binge-like alcohol consumption in male mice. J. Neurosci. Res. 2021, 99, 1957–1972. [Google Scholar] [CrossRef]
- Nwachukwu, K.N.; King, D.M.; Healey, K.L.; Swartzwelder, H.S.; Marshall, S.A. Sex-specific effects of adolescent intermittent ethanol exposure-induced dysregulation of hippocampal glial cells in adulthood. Alcohol 2022, 100, 31–39. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Sheng, J.G.; Griffin, W.S. Glial cytokines in Alzheimer’s disease: Review and pathogenic implications. Hum. Pathol. 1995, 26, 816–823. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Wang, W.; Eberhardt, A.; Vinitsky, H.S.; Reeves, B.C.; Peng, S.; Lou, N.; Hussain, R.; Nedergaard, M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci. Rep. 2018, 8, 2246. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol. Med. 2020, 26, 285–295. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Yin, Q.; Zhu, W.; Zhang, R.; Fan, X. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin. Neurosci. 2009, 63, 43–49. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, C.D.; Sexton, H.G.; Hyde, J.; Greene, B.; Risher, M.-L. Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions. Cells 2022, 11, 3111. https://doi.org/10.3390/cells11193111
Walker CD, Sexton HG, Hyde J, Greene B, Risher M-L. Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions. Cells. 2022; 11(19):3111. https://doi.org/10.3390/cells11193111
Chicago/Turabian StyleWalker, Christopher Douglas, Hannah Gray Sexton, Jentre Hyde, Brittani Greene, and Mary-Louise Risher. 2022. "Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions" Cells 11, no. 19: 3111. https://doi.org/10.3390/cells11193111
APA StyleWalker, C. D., Sexton, H. G., Hyde, J., Greene, B., & Risher, M.-L. (2022). Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions. Cells, 11(19), 3111. https://doi.org/10.3390/cells11193111






