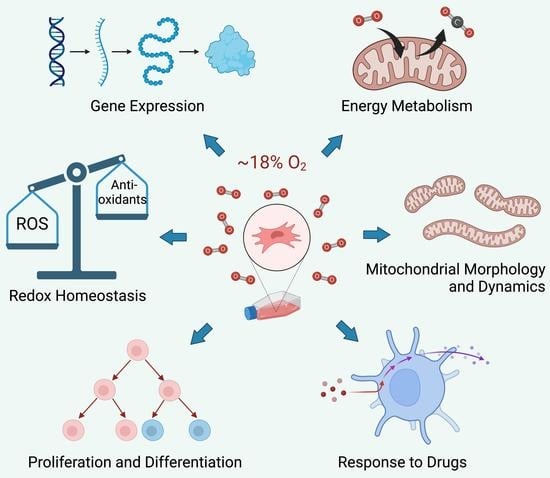
Supraphysiological Oxygen Levels in Mammalian Cell Culture: Current State and Future Perspectives
Abstract
1. Introduction
2. Oxygen and Redox Homeostasis
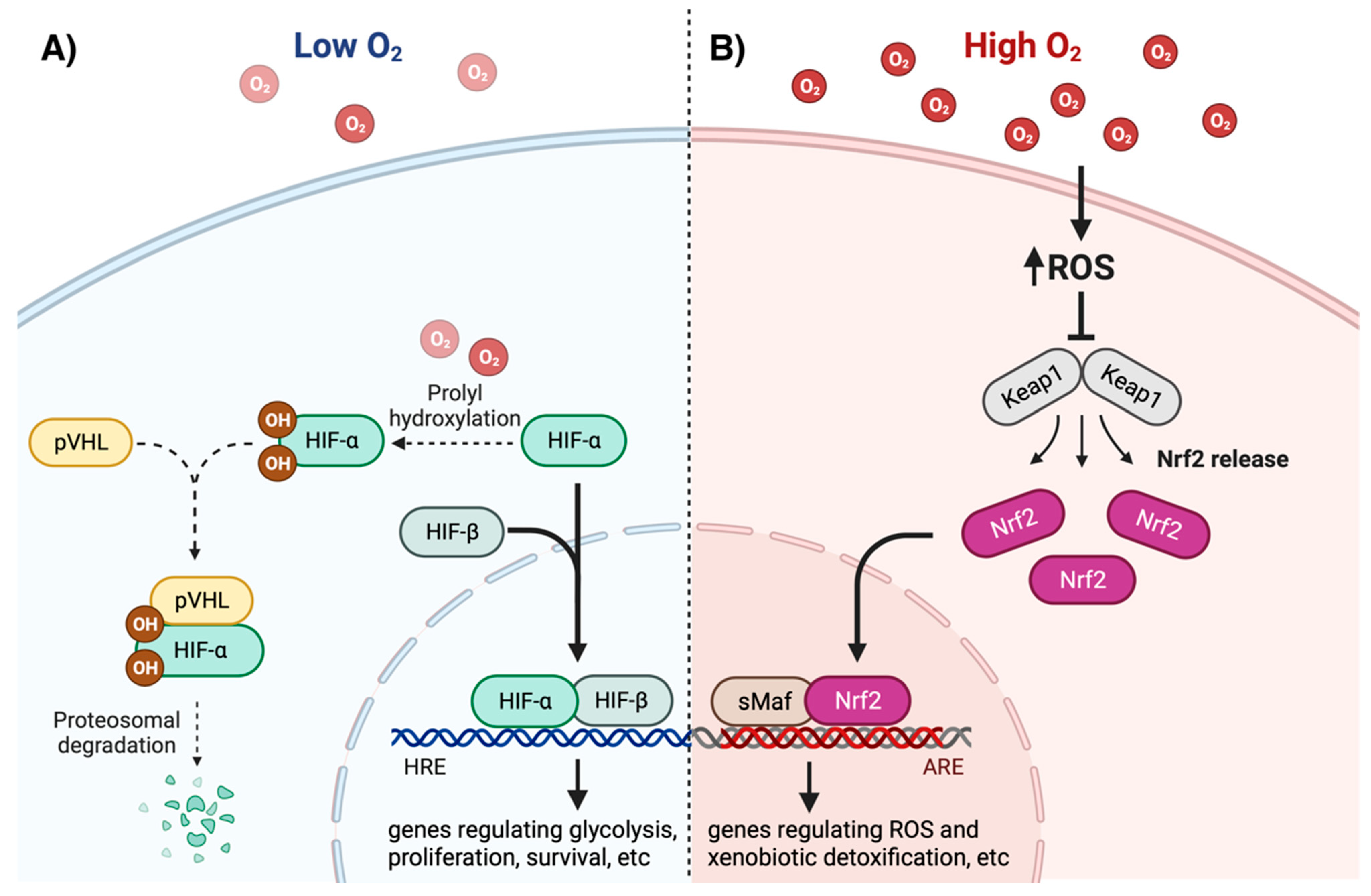
3. Intracellular Oxygen and ROS Sensing
4. Effects of Oxygen and ROS on Gene Expression
5. Oxygen, Proliferation, and Senescence
6. Oxygen and Cell Differentiation
7. Oxygen, Cell Bioenergetics, and Mitochondrial Dynamics
8. Modeling Tissue Physiology and Pathology in Physioxia
9. Cellular Response to Drugs, Hormones, and Toxicants
10. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Moradi, F.; Hu, W.; Regudo, K.L.; Osborne, M.; Pettipas, J.; Atallah, D.S.; Hachem, R.; Ott-Peron, N.; Stuart, J.A. Vertebrate cell culture as an experimental approach – limitations and solutions. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 254, 110570. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxidants Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.L.; Brookes, P.S. Oxygen Sensitivity of Mitochondrial Reactive Oxygen Species Generation Depends on Metabolic Conditions. J. Biol. Chem. 2009, 284, 16236–16245. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Bickerstaff, A.A.; Subramanian, S.V.; Atalay, M.; Bierl, M.; Pendyala, S.; Levy, D.; Sharma, N.; Venojarvi, M.; et al. Oxygen Sensing by Primary Cardiac Fibroblasts. Circ. Res. 2003, 92, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nasto, L.A.; Robinson, A.R.; Ngo, K.; Clauson, C.L.; Dong, Q.; Croix, C.S.; Sowa, G.; Pola, E.; Robbins, P.D.; Kang, J.; et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J. Orthop. Res. 2013, 31, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- El Alami, M.; Viña-Almunia, J.; Gambini, J.; Mas-Bargues, C.; Siow, R.C.; Peñarrocha, M.; Mann, G.E.; Borrás, C.; Viña, J. Activation of p38, p21, and NRF-2 Mediates Decreased Proliferation of Human Dental Pulp Stem Cells Cultured under 21% O2. Stem Cell Rep. 2014, 3, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A Hydrogen Peroxide-Generating Oxygen Sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, L.A.; Selim, S.M.; Fonseca, J.; Messner, H.; McGowan, S.; Stuart, J.A. Hydrogen peroxide production is affected by oxygen levels in mammalian cell culture. Biochem. Biophys. Res. Commun. 2017, 493, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J.; Maddalena, L.A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxidative Med. Cell. Longev. 2018, 2018, 8238459. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Albo, C.; Benguría, A.; Dopazo, A.; López-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; A Enríquez, J.; Bernad, A.; et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2011, 19, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Chrusciel, S.; Fayad-Kobeissi, S.; Dubois-Randé, J.-L.; Azuaje, F.; Boczkowski, J.; Motterlini, R.; Foresti, R. Permanent Culture of Macrophages at Physiological Oxygen Attenuates the Antioxidant and Immunomodulatory Properties of Dimethyl Fumarate. J. Cell. Physiol. 2014, 230, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Aja, S.; Kim, E.-K.; Park, M.J.; Ramamurthy, S.; Jia, J.; Hu, X.; Geng, P.; Ronnett, G.V. Physiological oxygen level is critical for modeling neuronal metabolism in vitro. J. Neurosci. Res. 2011, 90, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Atkuri, K.R.; Herzenberg, L.A.; Niemi, A.-K.; Cowan, T. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc. Natl. Acad. Sci. USA 2007, 104, 4547–4552. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.C.; Smerdon, G.R.; Harries, L.W.; Dodd, N.J.; Murphy, M.P.; Curnow, A.; Winyard, P.G. Altered cellular redox homeostasis and redox responses under standard oxygen cell culture conditions versus physioxia. Free Radic. Biol. Med. 2018, 126, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Timpano, S.; Guild, B.D.; Specker, E.J.; Melanson, G.; Medeiros, P.J.; Sproul, S.L.J.; Uniacke, J. Physioxic human cell culture improves viability, metabolism, and mitochondrial morphology while reducing DNA damage. FASEB J. 2019, 33, 5716–5728. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, M.; Pérez, V.; Ríos, C.; Liu, Y.; Lee, S.; Shi, Y.; Van Remmen, H. Effect of oxygen tension on bioenergetics and proteostasis in young and old myoblast precursor cells. Redox Biol. 2013, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via De Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [PubMed]
- Wang, G.L.; Jiang, B.-H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-J.; Wang, L.-Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential Roles of Hypoxia-Inducible Factor 1α (HIF-1α) and HIF-2α in Hypoxic Gene Regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 transcription factors — Similar but not identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The Tumour Suppressor Protein VHL Targets Hypoxia-Inducible Factors for Oxygen-Dependent Pro-teolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Metzen, E.; Ratcliffe, P.J.; Ratcliffe, E.M. HIF hydroxylation and cellular oxygen sensing. Biol. Chem. 2004, 385, 223–230. [Google Scholar] [CrossRef]
- Hirsilä, M.; Koivunen, P.; Günzler, V.; Kivirikko, K.I.; Myllyharju, J. Characterization of the Human Prolyl 4-Hydroxylases That Modify the Hypoxia-inducible Factor. J. Biol. Chem. 2003, 278, 30772–30780. [Google Scholar] [CrossRef]
- Bracken, C.P.; Fedele, A.O.; Linke, S.; Balrak, W.; Lisy, K.; Whitelaw, M.L.; Peet, D.J. Cell-specific Regulation of Hypoxia-inducible Factor (HIF)-1α and HIF-2α Stabilization and Transactivation in a Graded Oxygen Environment. J. Biol. Chem. 2006, 281, 22575–22585. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-M.; Ramachandran, A.; Bajt, M.L.; Lemasters, J.J.; Jaeschke, H. The Oxygen Tension Modulates Acetaminophen-Induced Mitochondrial Oxidant Stress and Cell Injury in Cultured Hepatocytes. Toxicol. Sci. 2010, 117, 515–523. [Google Scholar] [CrossRef]
- Osrodek, M.; Hartman, M.L.; Czyz, M. Physiologically Relevant Oxygen Concentration (6% O2) as an Important Component of the Microenvironment Impacting Melanoma Phenotype and Melanoma Response to Targeted Therapeutics In Vitro. Int. J. Mol. Sci. 2019, 20, 4203. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.A.; Aibueku, O.; Bagshaw, O.; Moradi, F. Hypoxia inducible factors as mediators of reactive oxygen/nitrogen species homeostasis in physiological normoxia. Med Hypotheses 2019, 129, 109249. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.-I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep. 2019, 28, 746–758.e4. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Saito, Y.; Yoshida, Y.; Sekine, A.; Noguchi, N.; Niki, E. 4-Hydroxynonenal Induces Adaptive Response and Enhances PC12 Cell Tolerance Primarily through Induction of Thioredoxin Reductase 1 via Activation of Nrf2. J. Biol. Chem. 2005, 280, 41921–41927. [Google Scholar] [CrossRef]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Alva, R.; Mirza, M.; Baiton, A.; Lazuran, L.; Samokysh, L.; Bobinski, A.; Cowan, C.; Jaimon, A.; Obioru, D.; Al Makhoul, T.; et al. Oxygen toxicity: Cellular mechanisms in normobaric hyperoxia. Cell Biol. Toxicol. 2022, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Adebayo, A.K.; Prasad, M.; Capitano, M.L.; Wang, R.; Bhat-Nakshatri, P.; Anjanappa, M.; Simpson, E.; Chen, D.; Liu, Y.; et al. Tumor collection/processing under physioxia uncovers highly relevant signaling networks and drug sensitivity. Sci. Adv. 2022, 8. [Google Scholar] [CrossRef]
- Chapple, S.J.; Keeley, T.P.; Mastronicola, D.; Arno, M.; Vizcay-Barrena, G.; Fleck, R.; Siow, R.C.; Mann, G.E. Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 2015, 92, 152–162. [Google Scholar] [CrossRef]
- Carrera, S.; de Verdier, P.J.; Khan, Z.; Zhao, B.; Mahale, A.; Bowman, K.J.; Zainol, M.; Jones, G.D.D.; Lee, S.W.; Aaronson, S.A.; et al. Protection of Cells in Physiological Oxygen Tensions against DNA Damage-induced Apoptosis. J. Biol. Chem. 2010, 285, 13658–13665. [Google Scholar] [CrossRef]
- Alva, R.; Moradi, F.; Liang, P.; Stuart, J.A. Culture of Cancer Cells at Physiological Oxygen Levels Affects Gene Expression in a Cell-Type Specific Manner. Preprints (Basel) 2022, 2022080497. [Google Scholar] [CrossRef]
- Duś-Szachniewicz, K.; Gdesz-Birula, K.; Zduniak, K.; Wiśniewski, J. Proteomic-Based Analysis of Hypoxia- and Physioxia-Responsive Proteins and Pathways in Diffuse Large B-Cell Lymphoma. Cells 2021, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Chee, N.T.; Lohse, I.; Brothers, S.P. mRNA-to-protein translation in hypoxia. Mol. Cancer 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.G.; Park, C.V.; Kenneth, N.S. Translating the Hypoxic Response—The Role of HIF Protein Translation in the Cellular Response to Low Oxygen. Cells 2019, 8, 114. [Google Scholar] [CrossRef]
- Uniacke, J.; Holterman, C.E.; Lachance, G.; Franovic, A.; Jacob, M.D.; Fabian, M.R.; Payette, J.; Holcik, M.; Pause, A.; Lee, S. An oxygen-regulated switch in the protein synthesis machinery. Nature 2012, 486, 126–129. [Google Scholar] [CrossRef]
- Timpano, S.; Uniacke, J. Human Cells Cultured under Physiological Oxygen Utilize Two Cap-binding Proteins to recruit Distinct mRNAs for Translation. J. Biol. Chem. 2016, 291, 10772–10782. [Google Scholar] [CrossRef]
- Batie, M.; Rocha, S. Gene transcription and chromatin regulation in hypoxia. Biochem. Soc. Trans. 2020, 48, 1121–1128. [Google Scholar] [CrossRef]
- Guillaumet-Adkins, A.; Yañez, Y.; Peris-Diaz, M.D.; Calabria, I.; Palanca-Ballester, C.; Sandoval, J. Epigenetics and Oxidative Stress in Aging. Oxidative Med. Cell. Longev. 2017, 2017, 9175806. [Google Scholar] [CrossRef] [PubMed]
- Lengner, C.; Gimelbrant, A.A.; Erwin, J.; Cheng, A.; Guenther, M.G.; Welstead, G.G.; Alagappan, R.; Frampton, G.M.; Xu, P.; Muffat, J.; et al. Derivation of Pre-X Inactivation Human Embryonic Stem Cells under Physiological Oxygen Concentrations. Cell 2010, 141, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Sun, Y.; Ouyang, Q.; Hu, L.; Tan, Y.; Zhou, X.; Xiong, B.; Zhang, Q.; Yuan, D.; Pan, Y.; et al. Physiological Oxygen Prevents Frequent Silencing of the DLK1-DIO3 Cluster during Human Embryonic Stem Cells Culture. Stem Cells 2013, 32, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.G.; Cliff, T.S.; Gammilonghi, A.; Ryall, J.G.; Dalton, S.; Gardner, D.K.; Harvey, A.J. Oxygen Regulates Human Pluripotent Stem Cell Metabolic Flux. Stem Cells Int. 2019, 2019, 8195614. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, B.; Steinbacher, J.; Zhao, H.; D’Anna, F.; Kuchnio, A.; Ploumakis, A.; Hermans, E. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016, 537, 63–68. [Google Scholar] [CrossRef]
- Lin, G.; Sun, W.; Yang, Z.; Guo, J.; Liu, H.; Liang, J. Hypoxia induces the expression of TET enzymes in HepG2 cells. Oncol. Lett. 2017, 14, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Dogan, F.; Aljumaily, R.; Kitchen, M.; Forsyth, N. DNMT3B Is an Oxygen-Sensitive De Novo Methylase in Human Mesenchymal Stem Cells. Cells 2021, 10, 1032. [Google Scholar] [CrossRef]
- Dogan, F.; Aljumaily, R.M.K.; Kitchen, M.; Forsyth, N.R. Physoxia Influences Global and Gene-Specific Methylation in Pluripotent Stem Cells. Int. J. Mol. Sci. 2022, 23, 5854. [Google Scholar] [CrossRef]
- Johnston, H.; Dickinson, P.; Ivens, A.; Buck, A.H.; Levine, R.D.; Remacle, F.; Campbell, C.J. Intracellular redox potential is correlated with miRNA expression in MCF7 cells under hypoxic conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 19753–19759. [Google Scholar] [CrossRef]
- Shi, R.; Yang, H.; Lin, X.; Cao, Y.; Zhang, C.; Fan, Z.; Hou, B. Analysis of the characteristics and expression profiles of coding and noncoding RNAs of human dental pulp stem cells in hypoxic conditions. Stem Cell Res. Ther. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Burt, A.M.; Wilson, J.N. Critical Effect of Oxygen Tension on Rate of Growth of Animal Cells in Continuous Suspended Culture. Nature 1958, 182, 1508–1509. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 1977, 267, 423–425. [Google Scholar] [CrossRef]
- Balin, A.K.; Fisher, A.J.; Carter, D.M. Oxygen modulates growth of human cells at physiologic partial pressures. J. Exp. Med. 1984, 160, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Kirsner, T.S. Low Oxygen Stimulates Proliferation of Fibroblasts Seeded as Single Cells. J. Cell Physiol. 1993, 154, 506510. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2017, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.A.; Rubio, M.; Dollé, M.E.T.; Campisi, J.; Vijg, J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell 2003, 2, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Samper, E.; Krtolica, A.; Goldstein, J.; Melov, S.; Campisi, J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Lees, S.J.; Childs, T.E.; Booth, F.W. P21 Cip1 Expression Is Increased in Ambient Oxygen, Compared to Estimated Physi-ological (5%) Levels in Rat Muscle Precursor Cell Culture. Cell Prolif. 2008, 41, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Bon-Mathier, A.-C.; Rignault-Clerc, S.; Bielmann, C.; Rosenblatt-Velin, N. Oxygen as a key regulator of cardiomyocyte proliferation: New results about cell culture conditions! Biochim. Biophys. Acta 2019, 1867, 118460. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, L.; Meyer, A.K.; Wagenführ, L.; Storch, A. Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol. Cell. Neurosci. 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Covello, K.L.; Kehler, J.; Yu, H.; Gordan, J.D.; Arsham, A.M.; Hu, C.-J.; Labosky, P.A.; Simon, M.C.; Keith, B. HIF-2α regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006, 20, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.D.; Sachdev, S.; Das, P.; Hearne, L.B.; Hannink, M.; Roberts, R.M.; Ezashi, T. Identification of Oxygen-Sensitive Transcriptional Programs in Human Embryonic Stem Cells. Stem Cells Dev. 2008, 17, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Simon, M.C. Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Cooper, S.; Gabigbad, T. The Effects of Oxidizing Species Derived from Molecular Oxygen on the Pro-liferation In Vitro of Human Granulocyte-Macrophage Progenitor Cells. Ann. N. Y. Acad. Sci. 1989, 554, 177–184. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Cooper, S.; Lu, L.; Miller, M.E.; Langefeld, C.D.; Ralph, P. Enhanced Stimulation of Human Bone Marrow Macrophage Colony Formation In Vitro by Recombinant Human Macrophage Colony-Stimulating Factor in Agarose Medium and at Low Oxygen Tension. Blood 1990, 76, 323–329. [Google Scholar] [CrossRef]
- Fehrer, C.; Brunauer, R.; Laschober, G.; Unterluggauer, H.; Reitinger, S.; Kloss, F.; Gülly, C.; Gaßner, R.; Lepperdinger, G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 2007, 6, 745–757. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Glowacki, J. Low Oxygen Tension Enhances Chondroinduction by Demineralized Bone Matrix in Human Dermal Fibroblasts in vitro. Cells Tissues Organs 2005, 180, 151–158. [Google Scholar] [CrossRef]
- Betre, H.; Ong, S.R.; Guilak, F.; Chilkoti, A.; Fermor, B.; Setton, L.A. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 2006, 27, 91–99. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; E Hardingham, T. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef]
- Dennis, J.E.; Whitney, G.A.; Rai, J.; Fernandes, R.J.; Kean, T.J. Physioxia Stimulates Extracellular Matrix Deposition and Increases Mechanical Properties of Human Chondrocyte-Derived Tissue-Engineered Cartilage. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Studer, L.; Csete, M.; Lee, S.-H.; Kabbani, N.; Walikonis, J.; Wold, B.; McKay, R. Enhanced Proliferation, Survival, and Dopaminergic Differentiation of CNS Precursors in Lowered Oxygen. J. Neurosci. 2000, 20, 7377–7383. [Google Scholar] [CrossRef]
- Antebi, B.; Ii, L.A.R.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, Q.; Zhang, Y.; Yu, M.; Jing, W.; Tian, W. Physioxia: A more effective approach for culturing human adipose-derived stem cells for cell transplantation. Stem Cell Res. Ther. 2018, 9, 148. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef]
- Grodzki, A.C.G.; Giulivi, C.; Lein, P.J. Oxygen Tension Modulates Differentiation and Primary Macrophage Functions in the Human Monocytic THP-1 Cell Line. PLoS ONE 2013, 8, e54926. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Gonzalez, B.C.; Paone, N.; Mueller, C.; Floss, J.C.; Sousa, M.E.; Shi, M.Y. Effect of Physiological Oxygen on Primary Human Corneal Endothelial Cell Cultures. Transl. Vis. Sci. Technol. 2022, 11, 33. [Google Scholar] [CrossRef]
- Alva, R.; Abbas, M.; Bagshaw, O.R.; Moffatt, C.; Gardner, G.; Stuart, J.A. Mitochondrial Oxygen Toxicity. In Mitochondrial Intoxication; de Oliveira, M.R., Ed.; Academic Press: Amsterdam, The Netherlands, in press; ISBN 9780323884624.
- Jaber, S.M.; Bordt, E.A.; Bhatt, N.M.; Lewis, D.M.; Gerecht, S.; Fiskum, G.; Polster, B.M. Sex differences in the mitochondrial bioenergetics of astrocytes but not microglia at a physiologically relevant brain oxygen tension. Neurochem. Int. 2018, 117, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Moffatt, C.; Stuart, J. The Effect of Oxygen and Micronutrient Composition of Cell Growth Media on Cancer Cell Bioenergetics and Mitochondrial Networks. Biomolecules 2021, 11, 1177. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Tiede, L.M.; A Cook, E.; Morsey, B.; Fox, H.S. Oxygen matters: Tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis. 2011, 2, e246. [Google Scholar] [CrossRef]
- Zhou, L.; Dosanjh, A.; Chen, H.; Karasek, M. Divergent Effects of Extracellular Oxygen on the Growth, Morphology, and Function of Human Skin Microvascular Endothelial Cells. J. Cell Physiol. 2000, 182, 134–140. [Google Scholar] [CrossRef]
- Piossek, F.; Beneke, S.; Schlichenmaier, N.; Mucic, G.; Drewitz, S.; Dietrich, D.R. Physiological oxygen and co-culture with human fibroblasts facilitate in vivo-like properties in human renal proximal tubular epithelial cells. Chem. Interactions 2022, 361. [Google Scholar] [CrossRef]
- Guo, R.; Xu, X.; Lu, Y.; Xie, X. Physiological oxygen tension reduces hepatocyte dedifferentiation in in vitro culture. Sci. Rep. 2017, 7, 5923. [Google Scholar] [CrossRef]
- Keeley, T.P.; Siow, R.C.M.; Jacob, R.; Mann, G.E. A PP2A-mediated feedback mechanism controls Ca 2+ -dependent NO synthesis under physiological oxygen. FASEB J. 2017, 31, 5172–5183. [Google Scholar] [CrossRef] [PubMed]
- Keeley, T.P.; Siow, R.C.M.; Jacob, R.; Mann, G.E. Reduced SERCA activity underlies dysregulation of Ca2+ homeostasis under atmospheric O2 levels. FASEB J. 2017, 32, 2531–2538. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.; Fraser, P.A.; Mann, G.E. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox Biol. 2020, 37, 101708. [Google Scholar] [CrossRef] [PubMed]
- Danilov, C.A.; Fiskum, G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia 2008, 56, 801–808. [Google Scholar] [CrossRef]
- Fonseca, J.; Moradi, F.; Valente, A.J.F.; Stuart, J.A. Oxygen and Glucose Levels in Cell Culture Media Determine Resveratrol’s Effects on Growth, Hydrogen Peroxide Production, and Mitochondrial Dynamics. Antioxidants 2018, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dailey, L.A.; Swedrowska, M.; Siow, R.; Mann, G.E.; Vizcay-Barrena, G.; Arno, M.; Mudway, I.S.; Forbes, B. Quantifying the magnitude of the oxygen artefact inherent in culturing airway cells under atmospheric oxygen versus physiological levels. FEBS Lett. 2015, 590, 258–269. [Google Scholar] [CrossRef]
- Otto-Ślusarczyk, D.; Graboń, W.; Mielczarek-Puta, M.; Chrzanowska, A. Teriflunomide – The common drug with underestimated oxygen - Dependent anticancer potential. Biochem. Biophys. Rep. 2021, 28, 101141. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Muir-Howie, H.; Hu, H.-L.; Evans, A.; Green, M.R.; Alaluf, S.; Muir-Howie, H.; Hu, H.-H.; Evans, A.; Green, M.R. Atmospheric Oxygen Accelerates the Induction of a Post-Mitotic Phenotype in Human Dermal Fibroblasts: The Key Pro-tective Role of Glutathione. Differentiation 2000, 66, 147–155. [Google Scholar] [CrossRef]
- DiProspero, T.J.; Dalrymple, E.; Lockett, M.R. Physiologically relevant oxygen tensions differentially regulate hepatotoxic responses in HepG2 cells. Toxicol. Vitr. 2021, 74, 105156. [Google Scholar] [CrossRef]
- Albert, I.; Hefti, M.; Luginbuehl, V. Physiological oxygen concentration alters glioma cell malignancy and responsiveness to photodynamic therapy in vitro. Neurol. Res. 2014, 36, 1001–1010. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, D.-Y.; Kim, W. Cultivation of human skin cells under physiological oxygen concentration modulates expression of skin significant genes and response to hydroxy acids. Biochem. Biophys. Res. Commun. 2021, 551, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Fiocchetti, M.; Marino, M.; Moffatt, C.; Stuart, J.A. Media composition and O2 levels determine effects of 17β-Estradiol on mitochondrial bioenergetics and cellular reactive oxygen species. Am. J. Physiol. Cell Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, L.; Tiede, L.M.; Morsey, B.; Fox, H.S. Quantitative Proteomics Reveals Oxygen-Dependent Changes in Neuronal Mitochondria Affecting Function and Sensitivity to Rotenone. J. Proteome Res. 2013, 12, 4599–4606. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.P.; Rinaldi, R.J.; Johnson, G.T.; Bourgeois, M.M.; McCluskey, J.D.; Harbison, R.D. Reduced oxygen tension culturing conditionally alters toxicogenic response of differentiated H9c2 cardiomyoblasts to acrolein. Toxicol. Mech. Methods 2018, 28, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Trepiana, J.; Meijide, S.; Navarro, R.; Hernández, M.L.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B. Influence of oxygen partial pressure on the characteristics of human hepatocarcinoma cells. Redox Biol. 2017, 12, 103–113. [Google Scholar] [CrossRef]
- Spyrou, J.; Gardner, D.K.; Harvey, A.J. Metabolomic and Transcriptional Analyses Reveal Atmospheric Oxygen During Human Induced Pluripotent Stem Cell Generation Impairs Metabolic Reprogramming. Stem Cells 2019, 37, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y.; Ungrin, M. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS ONE 2018, 13, e0204269. [Google Scholar] [CrossRef]
- Samokhin, P.; Gardner, G.L.; Moffatt, C.; Stuart, J.A. An Inexpensive Incubator for Mammalian Cell Culture Capable of Regulating O2, CO2, and Temperature. Oxygen 2022, 2, 22–30. [Google Scholar] [CrossRef]
- Marchus, C.R.N.; Knudson, J.A.; Morrison, A.E.; Strawn, I.K.; Hartman, A.J.; Shrestha, D.; Pancheri, N.M.; Glasgow, I.; Schiele, N.R. Low-Cost, Open-Source Cell Culture Chamber for Regulating Physiologic Oxygen Levels Specifications Table. HardwareX 2022, 11, e00253. [Google Scholar] [CrossRef]

| Cell Types | Experimental Conditions | Methods | Outcomes | Reference |
|---|---|---|---|---|
| Metabolic Effects | ||||
| Stem cells | hMSCs/18% or 3% O2 | Seahorse XF analysis, microarray, RT-PCR | ↑ OCR/ECAR ratio at 18% O2 ↑ expression HIF targets involved in glucose metabolism at 3% O2 | [19] |
| hPSCs/18% or 5% O2 | Seahorse XF analysis, NMR spectroscopy, RNA-seq, RT-PCR | ↑ OCR/ECAR ratio at 18% O2 ↑ glycolytic intermediates at 3% O2 ↑ expression HIF targets involved in glucose metabolism at 3% O2 | [60] | |
| MPCs from old mice/18% or 3% O2 | Seahorse XF analysis | ↓ OCR at 18% O2 ↑ ECAR at 18% O2 | [25] | |
| Primary differentiated cells | hCEnCs/18% or 2.5% O2 | Seahorse XF analysis | ↑ ECAR at 2.5% O2 | [94] |
| Rat primary cortical neurons/18% or 5% O2 | ATP bioluminescent assay, LSC, lactate assay | ↑ glucose uptake at 5% O2 ↓ glucose oxidation at 5% O2 ↑ lactate levels at 5% O2 | [21] | |
| HRPTEC/18–3% O2 | Resazurin assay | ↑ metabolic activity at 18% O2, compared to 15% and 12% O2 | [24] | |
| Cancer/immortalized cells | U87MG/18–3% O2 | Resazurin assay | ↓ metabolic activity at 18% O2, compared to 8–3% O2 | |
| MCF-7/18–3% O2 | Resazurin assay | ↓ metabolic activity at 18% O2, compared to 8% O2 | ||
| MCF-7/18% or 5% O2 | Seahorse XF analysis | ↓ basal and maximal OCR at 18% O2 | [97] | |
| LNCaP/18% or 5% O2 | Seahorse XF analysis | ↓ basal OCR at 18% O2 ↑ maximal OCR at 18% O2 | ||
| Huh-7/18% or 5% O2 | Seahorse XF analysis | ↓ basal and maximal OCR at 18% O2 | ||
| SaOS2/18% or 5% O2 | Seahorse XF analysis | ↓ maximal OCR at 18% O2 | ||
| Effects on mitochondrial morphology, abundance, and dynamics | ||||
| Primary differentiated cells | rat primary neurons/18%, 5%, or 2% O2 | TEM and confocal microscopy; Image J | Globular-shaped mitochondria at 18% O2 (versus elongated at 2% and 5% O2) ↓ mitochondrial network size, mitochondrial fraction, and mitochondrial perimeter at 18% O2 | [99] |
| Cancer/immortalized cells | U87MG/18–3% O2 | Confocal microscopy; Volocity | Rounder mitochondria at 18% O2 | [24] |
| HEK293/18–3% O2 | Confocal microscopy; Volocity | Rounder mitochondria at 18% O2 | ||
| MCF-7/18–3% O2 | Confocal microscopy; Volocity | Elongated mitochondria at 18% O2 | ||
| LNCaP/18% or 5% O2 | Confocal microscopy; MiNA | ↓ mitochondrial footprint at 18% O2 ↓ mean network size 18% O2 | [97] | |
| Huh-7/18% or 5% O2 | Confocal microscopy; MiNA | ↑ mitochondrial footprint at 18% O2 | ||
| SaOS2/18% or 5% O2 | Confocal microscopy; MiNA | ↑ mitochondrial footprint at 18% O2 ↓ mean network size 18% O2 | ||
| Molecule | Mechanism | Conditions | Outcomes | Reference |
|---|---|---|---|---|
| Drugs | ||||
| resveratrol | ROS scavenger, multiple targets | PC-3 and C2C12 cells 18% or 5% O2 | Differential H2O2 production, proliferation, and mitochondrial network dynamics | [107] |
| sulforaphane | ROS scavenger, multiple targets | bEnd.3 cells 18% or 5% O2 H/R | Attenuated reoxygenation-induced ROS production at 18% O2 but not at 5% O2 | [105] |
| quercetin | ROS scavenger, multiple targets | human neonatal foreskin fibroblasts 18% or 4% O2 | GSH depletion and loss of type I cells at 18% O2 but not at 4% O2 | [110] |
| doxorubicin | DNA intercalating agent | HCT116, IMR90, U2OS, and MCF-7 cells 18% O2 or 5% O2 | ↑ apoptosis at 18% O2 | [49] |
| acetaminophen | COX inhibitor | mouse hepatocytes 18%, 10%, or 5% O2 | ↑ hepatotoxicity at 18% O2 ↑ mROS and RNS production at 18% O2 | [36] |
| HepG2 cells 18%, 8%, or 3% O2 | ↓ hepatotoxicity at 18% O2 differential regulation of phase I and II enzymes | [111] | ||
| cyclophosphamide | DNA cross-linking agent | HepG2 cells 18%, 8%, or 3% O2 | ↓ hepatotoxicity at 18% O2 | [111] |
| teriflunomide | pyrimidine synthesis inhibitor | SW480 and SW620 cells 18% or 10% O2 | ↓ proapoptotic effect at 18% O2 ↓ antiproliferative effect at 18% O2 | [109] |
| oxaliplatin | DNA synthesis inhibitor | SW480 and SW620 cells 18% or 10% O2 | ↓ antiproliferative effect at 18% O2 | [109] |
| paclitaxel | microtubule stabilizer | mouse mammary tumors 18% or 3–5% O2 | ↑ cytotoxicity at 18% O2 | [47] |
| alpelisib | PI3K inhibitor | mouse mammary tumors 18% or 3–5% O2 | ↑ cytotoxicity at 18% O2 | [47] |
| erlotinib | EGFR inhibitor | mouse mammary tumors 18% or 3–5% O2 | ↑ cytotoxicity at 18% O2 | [47] |
| vemurafenib | BRAFV600 inhibitor | patient-derived melanoma cells 18% or 6% O2 | ↓ Ki-67-positive cells at 18% O2 ↓ reduction of VEGF, PCG-1α, and SLC7A11 levels at 18% O2 | [37] |
| trametinib | MEK1/2 inhibitor | patient-derived melanoma cells 18% or 6% O2 | ↓ Ki-67-positive cells at 18% O2 ↓ reduction of VEGF, PCG-1α, and SLC7A11 levels at 18% O2 | [37] |
| camptothecin | topoisomerase inhibitor | U87MG cells 18% O2 or 9% O2 | ↑ cytotoxicity at 18% O2 | [112] |
| dimethyl fumarate | Nrf2 inducer | RAW 264.7 cells 18% O2 or 5% O2 | ↑ expression of Nrf2 targets and antioxidant response | [20] |
| glycolic acid | keratolytic, antioxidant | Hs68 and HaCaT cells 18% or 2% O2 | Differential regulation of skin barrier and dermal network-related genes | [113] |
| gluconolactone | keratolytic, antioxidant | Hs68 and HaCaT cells 18% or 2% O2 | Differential regulation of skin barrier and dermal network-related genes | [113] |
| salicylic acid | keratolytic, AMPKactivator | Hs68 and HaCaT cells 18% or 2% O2 | Differential regulation of skin barrier and dermal network-related genes | [113] |
| Hormones | ||||
| 17β-estradiol | ER antagonist | C2C12 cells 18% O2 or 5% O2 | Differential H2O2 production, metabolism, and mitochondrial network dynamics | [114] |
| Toxicants | ||||
| LPS | TLR4 agonist | RAW 264.7 cells 18% O2 or 5% O2 | ↑ production of inflammatory mediators | [20] |
| rotenone | complex I inhibitor | SH-SY5Y cells 18% O2 or 5% O2 | ↓ cytotoxicity at 18% O2 No inhibition of ATP synthesis with 0.2 µM rotenone at 18% O2 (with effects observed at 5%) | [115] |
| acrolein | DNA and protein adduct inducer | differentiated H9c2 cells 18% O2 and 5% O2 | ↑ cytotoxicity at 18% O2 | [116] |
| aflatoxin B | DNA adduct inducer | HepG2 cells 18%, 8%, or 3% O2 | ↓ hepatotoxicity at 18% O2 | [111] |
| Other | ||||
| V. baccifera leaf extract | Prooxidant, cytotoxic | HepG2 cells 18% O2 or 8% O2 | ↑ cytotoxicity at 18% O2 | [117] |
| CuO NPs | Prooxidant, genotoxic, cytotoxic | A549 cells 18% O2 or 13% O2 | ↓ NP-induced oxidative stress at 18% O2 ↓ cytotoxicity at 18% O2 | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alva, R.; Gardner, G.L.; Liang, P.; Stuart, J.A. Supraphysiological Oxygen Levels in Mammalian Cell Culture: Current State and Future Perspectives. Cells 2022, 11, 3123. https://doi.org/10.3390/cells11193123
Alva R, Gardner GL, Liang P, Stuart JA. Supraphysiological Oxygen Levels in Mammalian Cell Culture: Current State and Future Perspectives. Cells. 2022; 11(19):3123. https://doi.org/10.3390/cells11193123
Chicago/Turabian StyleAlva, Ricardo, Georgina L. Gardner, Ping Liang, and Jeffrey A. Stuart. 2022. "Supraphysiological Oxygen Levels in Mammalian Cell Culture: Current State and Future Perspectives" Cells 11, no. 19: 3123. https://doi.org/10.3390/cells11193123
APA StyleAlva, R., Gardner, G. L., Liang, P., & Stuart, J. A. (2022). Supraphysiological Oxygen Levels in Mammalian Cell Culture: Current State and Future Perspectives. Cells, 11(19), 3123. https://doi.org/10.3390/cells11193123