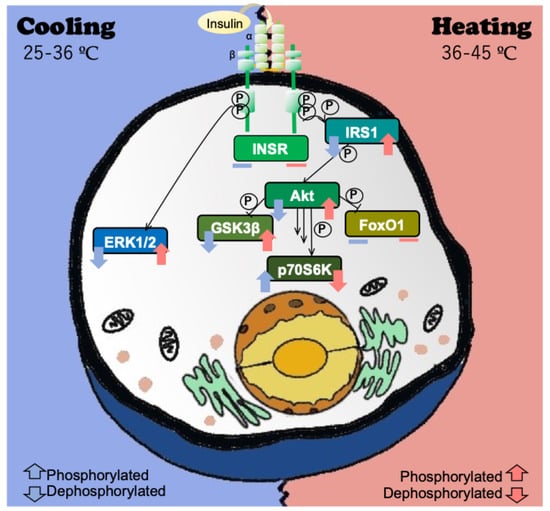
Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.1.1. Chemical Reagents
2.1.2. Antibodies for Western Blot and Immunofluorescence
2.2. Cell Culture
2.3. Western Blotting
2.4. Immunofluorescence
2.5. Statistical Analysis
2.6. Image Processing for Article Figures
3. Results
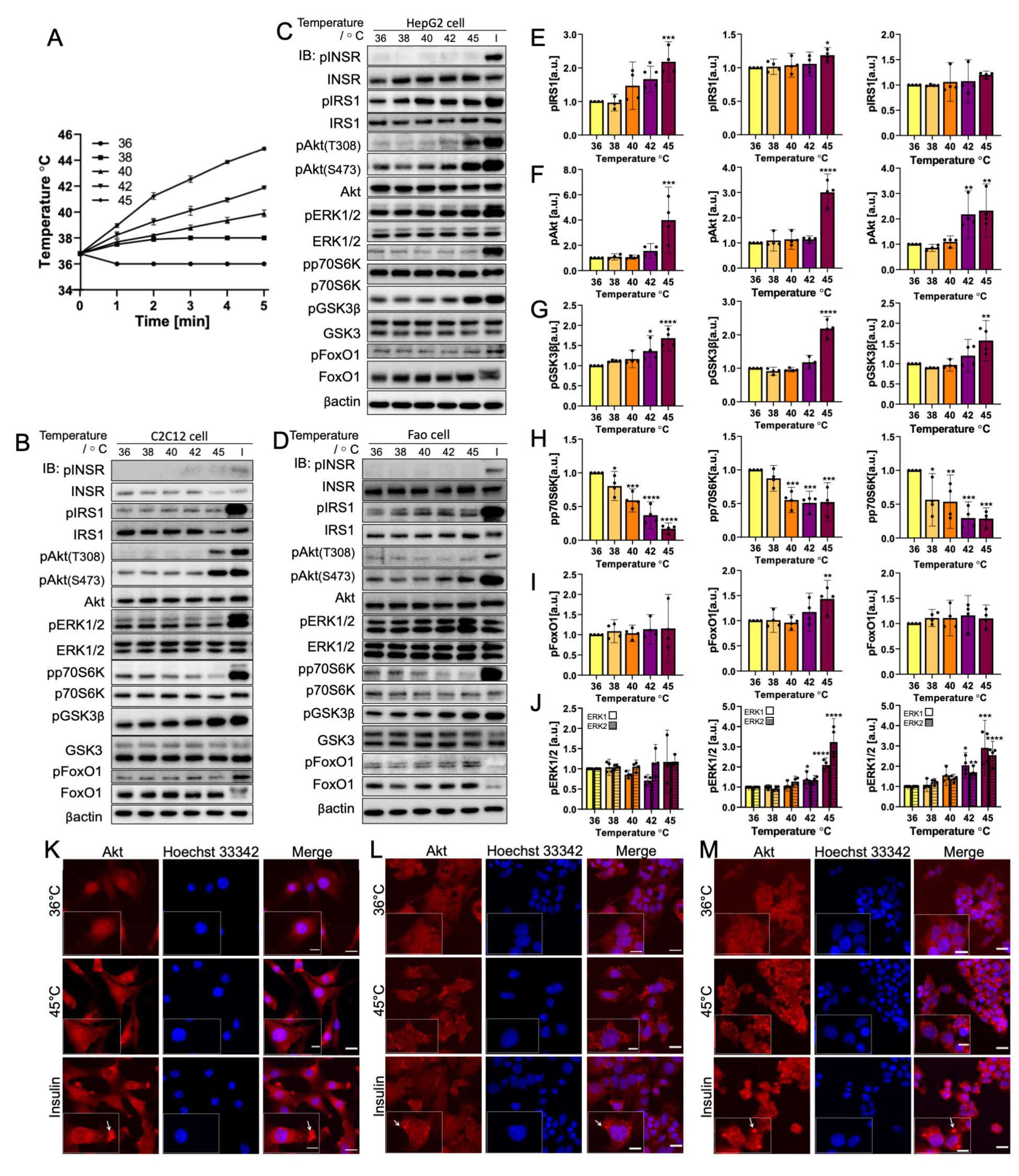
3.1. Rapid Temperature Increase Elevates the Phosphorylation of Insulin Signaling Pathway Biomolecules, Except for p70S6K
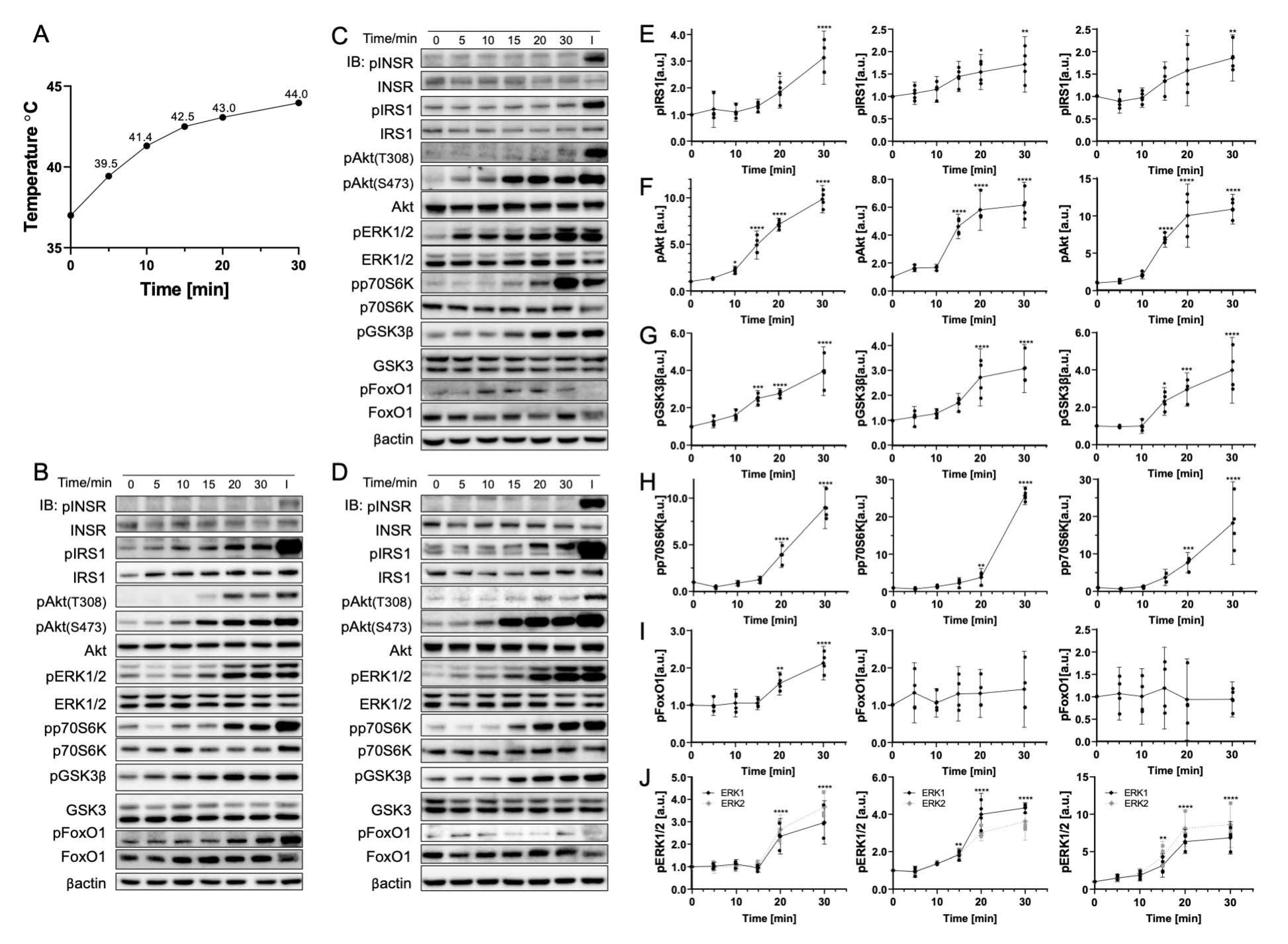
3.2. Gradual Temperature Increase to 45 °C Yielding a Greater Magnitude in the Phosphorylation Increase
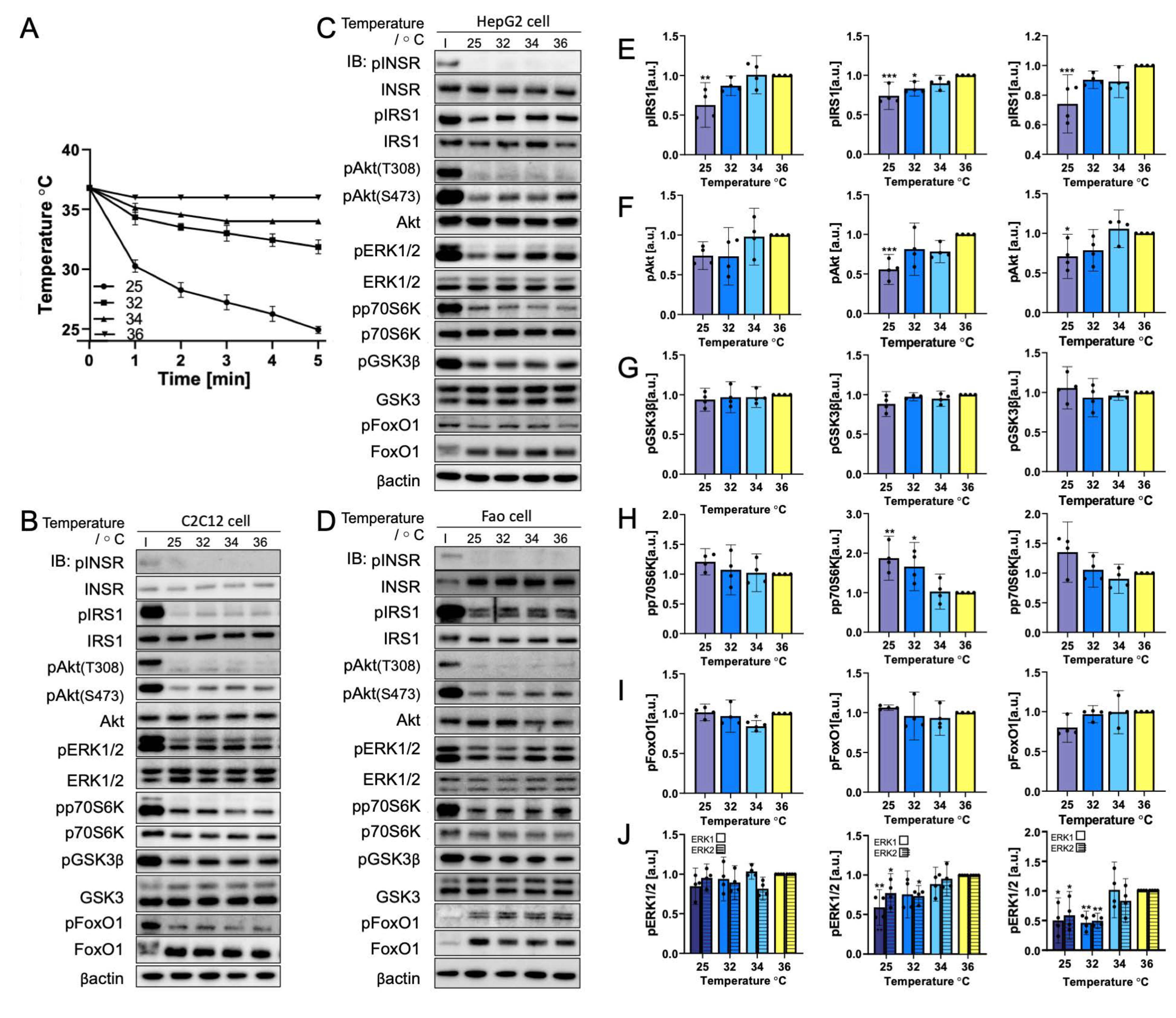
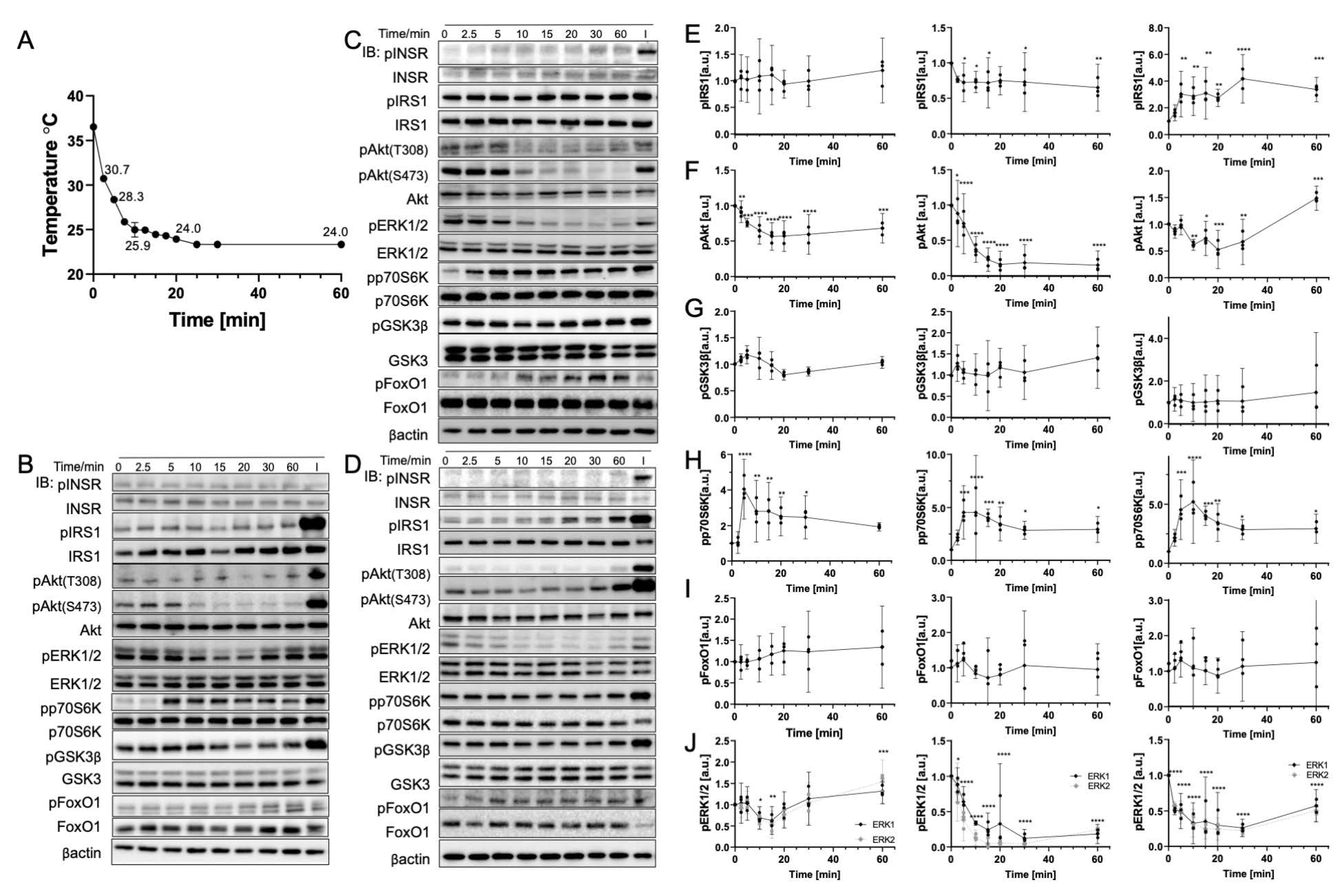
3.3. Rapid Cooling on Cells Causes Minimal Impact on Biomolecule Phosphorylation above 25 °C
3.4. Gradual Cooling to 25 °C Leads to a Continuous Decrease in Phosphorylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Z.; Tang, T.; Wu, T.; Yu, X.; Zhang, Y.; Wang, M.; Zheng, J.; Ying, Y.; Chen, S.; Zhou, J.; et al. Perfecting and Extending the Near-Infrared Imaging Window. Light Sci. Appl. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, e1802394. [Google Scholar] [CrossRef]
- Kaberniuk, A.A.; Baloban, M.; Monakhov, M.V.; Shcherbakova, D.M.; Verkhusha, V.V. Single-Component near-Infrared Optogenetic Systems for Gene Transcription Regulation. Nat. Commun. 2021, 12, 3859. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-Infrared Deep Brain Stimulation via Upconversion Nanoparticle–Mediated Optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernov, K.G.; Redchuk, T.A.; Omelina, E.S.; Verkhusha, V.V. Near-Infrared Fluorescent Proteins, Biosensors, and Optogenetic Tools Engineered from Phytochromes. Chem. Rev. 2017, 117, 6423–6446. [Google Scholar] [CrossRef]
- Shcherbakova, D.M.; Baloban, M.; Emelyanov, A.V.; Brenowitz, M.; Guo, P.; Verkhusha, V.V. Bright Monomeric Near-Infrared Fluorescent Proteins as Tags and Biosensors for Multiscale Imaging. Nat. Commun. 2016, 7, 12405. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Baird, M.A.; Allen, J.R.; Howe, E.S.; Klassen, M.P.; Reade, A.; Makhijani, K.; Song, Y.; Liu, S.; Murthy, Z.; et al. A Naturally Monomeric Infrared Fluorescent Protein for Protein Labeling in Vivo. Nat. Methods 2015, 12, 763–765. [Google Scholar] [CrossRef] [Green Version]
- Shcherbakova, D.M.; Cox Cammer, N.; Huisman, T.M.; Verkhusha, V.V.; Hodgson, L. Direct Multiplex Imaging and Optogenetics of Rho GTPases Enabled by Near-Infrared FRET. Nat. Chem. Biol. 2018, 14, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.A.; Tran, G.N.; Gross, L.A.; Crisp, J.L.; Shu, X.; Lin, J.Y.; Tsien, R.Y. A Far-Red Fluorescent Protein Evolved from a Cyanobacterial Phycobiliprotein. Nat. Methods 2016, 13, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Oliinyk, O.S.; Shemetov, A.A.; Pletnev, S.; Shcherbakova, D.M.; Verkhusha, V.V. Smallest Near-Infrared Fluorescent Protein Evolved from Cyanobacteriochrome as Versatile Tag for Spectral Multiplexing. Nat. Commun. 2019, 10, 279. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Oliinyk, O.S.; Verkhusha, V.V. How to Increase Brightness of Near-Infrared Fluorescent Proteins in Mammalian Cells. Cell Chem. Biol. 2017, 24, 758–766.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Piatkevich, K.D.; Mc Larney, B.; Abdelfattah, A.S.; Mehta, S.; Murdock, M.H.; Gottschalk, S.; Molina, R.S.; Zhang, W.; Chen, Y.; et al. A Genetically Encoded Near-Infrared Fluorescent Calcium Ion Indicator. Nat. Methods 2019, 16, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Xin, H.; Wan, T.; Ping, Y. Near-Infrared Optogenetic Engineering of Photothermal NanoCRISPR for Programmable Genome Editing. Proc. Natl. Acad. Sci. USA 2020, 117, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Weihs, F.; Dacres, H. Red-shifted bioluminescence Resonance Energy Transfer: Improved tools and materials for analytical in vivo approaches—ScienceDirect. TrAC Trends Anal. Chem. 2019, 116, 61–73. [Google Scholar] [CrossRef]
- Sun, J.; Tian, M.; Lin, W. A Two-Photon Excited Red-Emissive Probe for Imaging Mitochondria with High Fidelity and Its Application in Monitoring Mitochondrial Depolarization via FRET. Analyst 2019, 144, 2387–2392. [Google Scholar] [CrossRef]
- Rumfeldt, J.A.; Takala, H.; Liukkonen, A.; Ihalainen, J.A. UV-Vis Spectroscopy Reveals a Correlation Between Y263 and BV Protonation States in Bacteriophytochromes. Photochem. Photobiol. 2019, 95, 969–979. [Google Scholar] [CrossRef] [Green Version]
- Subach, O.M.; Barykina, N.V.; Anokhin, K.V.; Piatkevich, K.D.; Subach, F.V. Near-Infrared Genetically Encoded Positive Calcium Indicator Based on GAF-FP Bacterial Phytochrome. Int. J. Mol. Sci. 2019, 20, 3488. [Google Scholar] [CrossRef] [Green Version]
- Hososhima, S.; Yuasa, H.; Ishizuka, T.; Hoque, M.R.; Yamashita, T.; Yamanaka, A.; Sugano, E.; Tomita, H.; Yawo, H. Near-Infrared (NIR) up-Conversion Optogenetics. Sci. Rep. 2015, 5, 16533. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, H.; Wang, Y.; Si, Y.; Zhang, H.; Li, X.; Zhang, Z.; Yan, B.; Jiang, S.; Wang, F.; et al. Near-Infrared Manipulation of Multiple Neuronal Populations via Trichromatic Upconversion. Nat. Commun. 2021, 12, 5662. [Google Scholar] [CrossRef]
- Ao, Y.; Zeng, K.; Yu, B.; Miao, Y.; Hung, W.; Yu, Z.; Xue, Y.; Tan, T.T.Y.; Xu, T.; Zhen, M.; et al. An Upconversion Nanoparticle Enables Near Infrared-Optogenetic Manipulation of the Caenorhabditis elegans Motor Circuit. ACS Nano 2019, 13, 3373–3386. [Google Scholar] [CrossRef]
- Nourhashemi, M.; Mahmoudzadeh, M.; Wallois, F. Thermal Impact of Near-Infrared Laser in Advanced Noninvasive Optical Brain Imaging. Neurophotonics 2016, 3, 015001. [Google Scholar] [CrossRef] [PubMed]
- Ritossa, F. A New Puffing Pattern Induced by Temperature Shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Creagh, E.M.; Sheehan, D.; Cotter, T.G. Heat Shock Proteins—Modulators of Apoptosis in Tumour Cells. Leukemia 2000, 14, 1161–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.J.; Fort, P.E. Heat Shock Proteins Regulatory Role in Neurodevelopment. Front. Neurosci. 2018, 12, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obayashi, T.; Funasaka, K.; Ohno, E.; Miyahara, R.; Hirooka, Y.; Hamaguchi, M.; Goto, H.; Senga, T. Treatment with Near-Infrared Radiation Promotes Apoptosis in Pancreatic Cancer Cells. Oncol. Lett. 2015, 10, 1836–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer: A New Approach That Kills Cancer Cells and Enhances Anti-Cancer Host Immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef]
- Ali, A.; Bharadwaj, S.; O’Carroll, R.; Ovsenek, N. HSP90 Interacts with and Regulates the Activity of Heat Shock Factor 1 in Xenopus Oocytes. Mol. Cell. Biol. 1998, 18, 4949–4960. [Google Scholar] [CrossRef] [Green Version]
- Mazalouskas, M.D.; Godoy-Ruiz, R.; Weber, D.J.; Zimmer, D.B.; Honkanen, R.E.; Wadzinski, B.E. Small G Proteins Rac1 and Ras Regulate Serine/Threonine Protein Phosphatase 5 (PP5)·Extracellular Signal-Regulated Kinase (ERK) Complexes Involved in the Feedback Regulation of Raf1. J. Biol. Chem. 2014, 289, 4219–4232. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt Kinase Activity by Binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.; Chang, X.; Ma, D. Hsp90 Maintains the Stability and Function of the Tau Phosphorylating Kinase GSK3β. Int. J. Mol. Sci. 2007, 8, 51–60. [Google Scholar] [CrossRef]
- Banz, V.M.; Medová, M.; Keogh, A.; Furer, C.; Zimmer, Y.; Candinas, D.; Stroka, D. Hsp90 Transcriptionally and Post-Translationally Regulates the Expression of NDRG1 and Maintains the Stability of Its Modifying Kinase GSK3β. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2009, 1793, 1597–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fittipaldi, S.; Dimauro, I.; Mercatelli, N.; Caporossi, D. Role of Exercise-Induced Reactive Oxygen Species in the Modulation of Heat Shock Protein Response. Free Radic. Res. 2014, 48, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive Oxygen Species, Heat Stress and Oxidative-Induced Mitochondrial Damage. A Review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Wade, S.A.; Brown, W.G.; Stoddart, P.R. Modeling of Light Absorption in Tissue during Infrared Neural Stimulation. J. Biomed. Opt. 2012, 17, 075002. [Google Scholar] [CrossRef]
- Oehler-Jänne, C.; von Bueren, A.O.; Vuong, V.; Hollenstein, A.; Grotzer, M.A.; Pruschy, M. Temperature Sensitivity of Phospho-Ser473-PKB/AKT. Biochem. Biophys. Res. Commun. 2008, 375, 399–404. [Google Scholar] [CrossRef]
- Ananthanarayanan, B.; Ni, Q.; Zhang, J. Signal propagation from membrane messengers to nuclear effectors revealed by reporters of phosphoinositide dynamics and Akt activity. Proc. Natl. Acad. Sci. USA 2005, 102, 15081–15086. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.; McGraw, T.E. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 7004–7009. [Google Scholar] [CrossRef] [Green Version]
- Bang, O.-S.; Ha, B.-G.; Park, E.K.; Kang, S.-S. Activation of Akt Is Induced by Heat Shock and Involved in Suppression of Heat-Shock-Induced Apoptosis of NIH3T3 Cells. Biochem. Biophys. Res. Commun. 2000, 278, 306–311. [Google Scholar] [CrossRef]
- Zhou, J.; Schmid, T.; Frank, R.; Brüne, B. PI3K/Akt Is Required for Heat Shock Proteins to Protect Hypoxia-Inducible Factor 1α from PVHL-Independent Degradation. J. Biol. Chem. 2004, 279, 13506–13513. [Google Scholar] [CrossRef] [Green Version]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt Forms an Intracellular Complex with Heat Shock Protein 90 (Hsp90) and Cdc37 and Is Destabilized by Inhibitors of Hsp90 Function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef]
- Chen, M.; Choi, S.; Wen, T.; Chen, C.; Thapa, N.; Lee, J.H.; Cryns, V.L.; Anderson, R.A. A P53–Phosphoinositide Signalosome Regulates Nuclear AKT Activation. Nat. Cell Biol. 2022, 24, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhang, W.; Zheng, G.; Meng, S.; Che, H.; Ke, T.; Yang, J.; Chen, J.; Luo, W. Akt Activation Protects Liver Cells from Apoptosis in Rats during Acute Cold Exposure. Int. J. Biol. Sci. 2013, 9, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurivich, D.A.; Chung, J.; Blenis, J. Heat Shock Induces Two Distinct S6 Protein Kinase Activities in Quiescent Mammalian Fibroblasts. J. Cell. Physiol. 1991, 148, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.; Dennis, P.B.; Andjelkovic, M.; Dufner, A.; Kozma, S.C.; Hemmings, B.A.; Thomas, G. Phosphorylation and Activation of P70s6k by PDK1. Science 1998, 279, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Yoshihara, T.; Kakigi, R.; Ichinoseki-Sekine, N.; Tsuzuki, T. Heat Stress-Induced Changes in Skeletal Muscle: Heat Shock Proteins and Cell Signaling Transduction. J. Phys. Fit. Sports Med. 2012, 1, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Zhang, Y.; Gu, L.; Cui, J.; Duan, B.; Wang, Y.; Du, J. Heat Shock Protein 27 Downstream of P38-PI3K/Akt Signaling Antagonizes Melatonin-Induced Apoptosis of SGC-7901 Gastric Cancer Cells. Cancer Cell Int. 2016, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Zhang, X.; Dai, X.; Han, S.; Wu, X.; Wang, L.; Wei, W.; Zhang, N.; Xie, W.; Guo, J. S6K1-Mediated Phosphorylation of PDK1 Impairs AKT Kinase Activity and Oncogenic Functions. Nat. Commun. 2022, 13, 1548. [Google Scholar] [CrossRef]
- Parrott, L.A.; Templeton, D.J. Osmotic Stress Inhibits p70/85 S6 Kinase through Activation of a Protein Phosphatase. J. Biol. Chem. 1999, 274, 24731–24736. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Endo, M.; Kawamura, G.; Ozawa, T. Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation. Cells 2022, 11, 3136. https://doi.org/10.3390/cells11193136
Dong Q, Endo M, Kawamura G, Ozawa T. Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation. Cells. 2022; 11(19):3136. https://doi.org/10.3390/cells11193136
Chicago/Turabian StyleDong, Qi, Mizuki Endo, Genki Kawamura, and Takeaki Ozawa. 2022. "Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation" Cells 11, no. 19: 3136. https://doi.org/10.3390/cells11193136
APA StyleDong, Q., Endo, M., Kawamura, G., & Ozawa, T. (2022). Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation. Cells, 11(19), 3136. https://doi.org/10.3390/cells11193136