Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity
Abstract
:1. Introduction to NK Cell Biology
1.1. NK Cells, MHC Class I and the Missing Self
1.2. NK Cell Immune Crosstalk
2. NK Cells and Cancer Therapy
2.1. Impact of NK Cells on Cancer Patient’s Prognosis
2.2. NK Cells in the Tumor Microenvironment
2.3. Strategies for Restoring NK Cell Anti-Tumor Immunity
2.3.1. Unleashing NK Cell Activity by Antibody-Based Therapeutics
Therapeutic Antibodies Directly Targeting NK Cell Receptors
Bispecific and Trispecific Antibodies: Killer Cell Engagers Targeting NK and Tumor Cell Receptors
Abs Modifying NK Cells’ Activity through an Indirect Effect
2.3.2. Unleashing NK Cell Activity by Cytokine Therapy
Blockage of NKs Immunosuppressive Cytokines
Treatment with Cytokines and Chemokines Inducing NKs Expansion or Recruitment
2.3.3. NK Cell-Based Therapy: NK Adoptive Transfer and CAR-NK
NK Adoptive Transfer Therapy
CAR-NK Cell Therapy
Memory-Like NK Cell Therapy
3. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiessling, R.; Petranyi, G.; Kärre, K.; Jondal, M.; Tracey, D.; Wigzell, H. Killer cells: A functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J. Exp. Med. 1976, 143, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Konjević, G.; Vuletić, A.; Martinović, K.M.; Colović, N.; Čolović, M.; Jurišić, V. Decreased CD161 activating and increased CD158a inhibitory receptor expression on NK cells underlies impaired NK cell cytotoxicity in patients with multiple myeloma. J. Clin. Pathol. 2016, 69, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurisic, V.; Srdic-Rajic, T.; Konjevic, G.; Bogdanovic, G.; Colic, M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J. Membr. Biol. 2011, 239, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; e Sousa, C.R. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [Green Version]
- Badrinath, S.; Dellacherie, M.O.; Li, A.; Zheng, S.; Zhang, X.; Sobral, M.; Pyrdol, J.W.; Smith, K.L.; Lu, Y.; Haag, S. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature 2022, 606, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Sun, K.; Murphy, W.J. Mouse host unlicensed NK cells promote donor allogeneic bone marrow engraftment. Blood J. Am. Soc. Hematol. 2016, 127, 1202–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.; Pierini, A.; Simonetta, F.; Baker, J.; Maas-Bauer, K.; Hirai, T.; Negrin, R.S. Infusion of host-derived unlicensed NK cells improves donor engraftment in non-myeloablative allogeneic hematopoietic cell transplantation. Front. Immunol. 2021, 11, 614250. [Google Scholar] [CrossRef]
- Mancusi, A.; Alvarez, M.; Piccinelli, S.; Velardi, A.; Pierini, A. TNFR2 signaling modulates immunity after allogeneic hematopoietic cell transplantation. Cytokine Growth Factor Rev. 2019, 47, 54–61. [Google Scholar] [CrossRef]
- Tufa, D.M.; Chatterjee, D.; Low, H.Z.; Schmidt, R.E.; Jacobs, R. TNFR2 and IL-12 coactivation enables slanDCs to support NK-cell function via membrane-bound TNF-α. Eur. J. Immunol. 2014, 44, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Theisen, D.J.; Davidson IV, J.T.; Briseño, C.G.; Gargaro, M.; Lauron, E.J.; Wang, Q.; Desai, P.; Durai, V.; Bagadia, P.; Brickner, J.R. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 2018, 362, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.; Vijayan, D.; Putz, E.M.; Aguilera, A.R.; Markey, K.A.; Straube, J.; Kazakoff, S.; Nutt, S.L.; Takeda, K.; Hill, G.R. Interleukin-12 from CD103+ Batf3-dependent dendritic cells required for NK-cell suppression of metastasis. Cancer Immunol. Res. 2017, 5, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melero, I.; Johnston, J.V.; Shufford, W.W.; Mittler, R.S.; Chen, L. NK1. 1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell. Immunol. 1998, 190, 167–172. [Google Scholar] [CrossRef]
- Ochoa, M.C.; Perez-Ruiz, E.; Minute, L.; Oñate, C.; Perez, G.; Rodriguez, I.; Zabaleta, A.; Alignani, D.; Fernandez-Sendin, M.; Lopez, A. Daratumumab in combination with urelumab to potentiate anti-myeloma activity in lymphocyte-deficient mice reconstituted with human NK cells. Oncoimmunology 2019, 8, e1599636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelly, V.S.; Moeini, A.; Roelofsen, L.M.; Bonavita, E.; Bell, C.R.; Hutton, C.; Blanco-Gomez, A.; Banyard, A.; Bromley, C.P.; Flanagan, E. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov. 2021, 11, 2602–2619. [Google Scholar] [CrossRef]
- Tang, Y.-P.; Xie, M.-Z.; Li, K.-Z.; Li, J.-L.; Cai, Z.-M.; Hu, B.-L. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jonge, K.; Ebering, A.; Nassiri, S.; Hajjami, M.-E.; Ouertatani-Sakouhi, H.; Baumgaertner, P.; Speiser, D.E. Circulating CD56bright NK cells inversely correlate with survival of melanoma patients. Sci. Rep. 2019, 9, 4487. [Google Scholar] [CrossRef] [Green Version]
- Rocca, Y.S.; Roberti, M.P.; Juliá, E.P.; Pampena, M.B.; Bruno, L.; Rivero, S.; Huertas, E.; Sanchez Loria, F.; Pairola, A.; Caignard, A. Phenotypic and functional dysregulated blood NK cells in colorectal cancer patients can be activated by cetuximab plus IL-2 or IL-15. Front. Immunol. 2016, 7, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, E.; Godet, Y.; Laheurte, C.; Dosset, M.; Galaine, J.; Beziaud, L.; Loyon, R.; Boullerot, L.; Lauret Marie Joseph, E.; Spehner, L. Circulating NKp46+ Natural Killer cells have a potential regulatory property and predict distinct survival in Non-Small Cell Lung Cancer. Oncoimmunology 2019, 8, e1527498. [Google Scholar] [CrossRef] [PubMed]
- Steppert, C.; Krugmann, J.; Sterlacci, W. Simultaneous endocrine expression and loss of melanoma markers in malignant melanoma metastases, a retrospective analysis. Pathol. Oncol. Res. 2020, 26, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C.; Gravis, G.; Guerin, M.; Granjeaud, S.; Thomassin-Piana, J.; Rocchi, P.; Paciencia-Gros, M.; Poizat, F.; Bentobji, M.; Azario-Cheillan, F. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016, 76, 2153–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.-S.; Zhang, J.-Y.; Teng, Y.-S.; Zhao, Y.-L.; Wang, T.-T.; Mao, F.-Y.; Lv, Y.-P.; Cheng, P.; Li, W.-H.; Chen, N. Tumor-associated monocytes/macrophages impair NK-cell function via TGFβ1 in human gastric cancer. Cancer Immunol. Res. 2017, 5, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delahaye, N.F.; Rusakiewicz, S.; Martins, I.; Ménard, C.; Roux, S.; Lyonnet, L.; Paul, P.; Sarabi, M.; Chaput, N.; Semeraro, M. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat. Med. 2011, 17, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra255. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, M.L.; Idowu, M.O.; Zhao, Y.; Khalak, H.; Payne, K.K.; Wang, X.Y.; Dumur, C.I.; Bedognetti, D.; Tomei, S.; Ascierto, P.A.; et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J. Transl. Med. 2013, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, M.L.; Shrestha, Y.; Zhang, Q.; Wang, J.; Si, H.; Greenlees, L.; Halpin, R.; Achour, I.; Cooper, Z.A.; Raja, R.; et al. Abstract 3245: Durvalumab induces an NK cell response associated with clinical benefit of patients with advanced NSCLC. Cancer Res. 2019, 79, 3245. [Google Scholar] [CrossRef]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- Villegas, F.R.; Coca, S.; Villarrubia, V.G.; Jiménez, R.; Chillón, M.a.J.; Jareño, J.; Zuil, M.; Callol, L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002, 35, 23–28. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z. Natural killer cells in hepatocellular carcinoma: Current status and perspectives for future immunotherapeutic approaches. Front. Med. 2017, 11, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Fauriat, C.; Mallet, F.; Olive, D. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia 2006, 20, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.-M.; Nordström, O.; Johansson, A.; Rydén, L.; Leandersson, K.; Bergenfelz, C. Peripheral Blood Mononuclear Cell Populations Correlate with Outcome in Patients with Metastatic Breast Cancer. Cells 2022, 11, 1639. [Google Scholar] [CrossRef]
- Pazina, T.; MacFarlane IV, A.W.; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.A.; Robbins, M.D.; Ross, E.A.; Campbell, K.S. Alterations of NK cell phenotype in the disease course of multiple myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef]
- Fang, J.; Li, X.; Ma, D.; Liu, X.; Chen, Y.; Wang, Y.; Lui, V.W.Y.; Xia, J.; Cheng, B.; Wang, Z. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer 2017, 17, 375. [Google Scholar] [CrossRef] [Green Version]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Martinović, K.M.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front. Oncol. 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.-C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood J. Am. Soc. Hematol. 2018, 131, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Dunai, C.; Murphy, W.J. NK cells for PD-1/PD-L1 blockade immunotherapy: Pinning down the NK cell. J. Clin. Investig. 2018, 128, 4251–4253. [Google Scholar] [CrossRef]
- Alvarez, M.; Simonetta, F.; Baker, J.; Morrison, A.R.; Wenokur, A.S.; Pierini, A.; Berraondo, P.; Negrin, R.S. Indirect impact of PD-1/PD-L1 blockade on a murine model of NK cell exhaustion. Front. Immunol. 2020, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Judge, S.J.; Dunai, C.; Aguilar, E.G.; Vick, S.C.; Sturgill, I.R.; Khuat, L.T.; Stoffel, K.M.; Van Dyke, J.; Longo, D.L.; Darrow, M.A. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J. Clin. Investig. 2020, 130, 3051–3068. [Google Scholar] [CrossRef]
- Diniz, M.O.; Schurich, A.; Chinnakannan, S.K.; Duriez, M.; Stegmann, K.A.; Davies, J.; Kucykowicz, S.; Suveizdyte, K.; Amin, O.E.; Alcock, F. NK cells limit therapeutic vaccine–induced CD8+ T cell immunity in a PD-L1–dependent manner. Sci. Transl. Med. 2022, 14, eabi4670. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Facchinetti, F.; Missale, G.; Canetti, D.; Madeddu, D.; Zecca, A.; Veneziani, M.; Gelsomino, F.; Goldoni, M.; Buti, S. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer 2019, 127, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tallerico, R.; Cristiani, C.M.; Staaf, E.; Garofalo, C.; Sottile, R.; Capone, M.; Pico de Coaña, Y.; Madonna, G.; Palella, E.; Wolodarski, M. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology 2017, 6, e1261242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Wu, X.; Ma, S.; Wang, Y.; Nalin, A.P.; Zhu, Z.; Zhang, J.; Benson, D.M.; He, K.; Caligiuri, M.A. The mechanism of Anti–PD-L1 antibody efficacy against PD-L1–Negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019, 9, 1422–1437. [Google Scholar] [CrossRef] [Green Version]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef] [Green Version]
- Catakovic, K.; Gassner, F.J.; Ratswohl, C.; Zaborsky, N.; Rebhandl, S.; Schubert, M.; Steiner, M.; Gutjahr, J.C.; Pleyer, L.; Egle, A. TIGIT expressing CD4+ T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. Oncoimmunology 2018, 7, e1371399. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.; Simonetta, F.; Baker, J.; Pierini, A.; Wenokur, A.S.; Morrison, A.R.; Murphy, W.J.; Negrin, R.S. Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway. JCI Insight 2019, 4, e127729. [Google Scholar] [CrossRef]
- Li, M.; Xia, P.; Du, Y.; Liu, S.; Huang, G.; Chen, J.; Zhang, H.; Hou, N.; Cheng, X.; Zhou, L. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J. Biol. Chem. 2014, 289, 17647–17657. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.-h.T.; Maurer, M.; Korman, A.J. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Inozume, T.; Yaguchi, T.; Furuta, J.; Harada, K.; Kawakami, Y.; Shimada, S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J. Investig. Dermatol. 2016, 136, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriquez-Abreu, D.; Johnson, M.L.; Hussein, M.A.; Cobo, M.; Patel, A.J.; Secen, N.M.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.-H.; et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J. Clin. Oncol. 2020, 38, 9503. [Google Scholar] [CrossRef]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol. Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Waight, J.D.; Chand, D.; Dietrich, S.; Gombos, R.; Horn, T.; Gonzalez, A.M.; Manrique, M.; Swiech, L.; Morin, B.; Brittsan, C. Selective FcγR co-engagement on APCs modulates the activity of therapeutic antibodies targeting T cell antigens. Cancer Cell 2018, 33, 1033–1047.e5. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, J.-M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.-Y.; Ferrone, S. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in MelanomaIL15 and TIGIT Blockade Reverse NK Dysfunction in Melanoma. Clin. Cancer Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.R.; Vick, L.V.; Dunai, C.; Stoffel, K.M. Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas. J. Immunother. Cancer 2020, 8, e001355. [Google Scholar] [CrossRef]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef]
- Alteber, Z.; Kotturi, M.F.; Whelan, S.; Ganguly, S.; Weyl, E.; Pardoll, D.M.; Hunter, J.; Ophir, E. Therapeutic Targeting of Checkpoint Receptors within the DNAM1 AxisTargeting of Checkpoint Receptors within the DNAM-1 Axis. Cancer Discov. 2021, 11, 1040–1051. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; He, F.; Ma, X.; Fan, F.; Meng, M.; Zhuo, Y.; Zhang, L. CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma. Sci. Rep. 2020, 10, 10768. [Google Scholar] [CrossRef] [PubMed]
- Okumura, G.; Iguchi-Manaka, A.; Murata, R.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Tumor-derived soluble CD155 inhibits DNAM-1–mediated antitumor activity of natural killer cells. J. Exp. Med. 2020, 217, e20191290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, R.J.; Hoogstad-van Evert, J.S.; Van der Meer, J.M.; Mekers, V.; Rezaeifard, S.; Korman, A.J.; de Jonge, P.K.; Cany, J.; Woestenenk, R.; Schaap, N.P. TIGIT blockade enhances functionality of peritoneal NK cells with altered expression of DNAM-1/TIGIT/CD96 checkpoint molecules in ovarian cancer. Oncoimmunology 2020, 9, 1843247. [Google Scholar] [CrossRef]
- Lupo, K.B.; Matosevic, S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J. Hematol. Oncol. 2020, 13, 76. [Google Scholar] [CrossRef]
- Wu, B.; Zhong, C.; Lang, Q.; Liang, Z.; Zhang, Y.; Zhao, X.; Yu, Y.; Zhang, H.; Xu, F.; Tian, Y. Poliovirus receptor (PVR)-like protein cosignaling network: New opportunities for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 267. [Google Scholar] [CrossRef]
- Perez-Villar, J.J.; Carretero, M.; Navarro, F.; Melero, I.; Rodríguez, A.; Bottino, C.; Moretta, A.; López-Botet, M. Biochemical and serologic evidence for the existence of functionally distinct forms of the CD94 NK cell receptor. J. Immunol. 1996, 157, 5367–5374. [Google Scholar]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed]
- Mingari, M.C.; Vitale, C.; Cantoni, C.; Bellomo, R.; Ponte, M.; Schiavetti, F.; Bertone, S.; Moretta, A.; Moretta, L. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: Selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur. J. Immunol. 1997, 27, 1374–1380. [Google Scholar] [CrossRef]
- Locatelli, F.; Pende, D.; Falco, M.; Della Chiesa, M.; Moretta, A.; Moretta, L. NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol. 2018, 39, 577–590. [Google Scholar] [CrossRef]
- Mingari, M.C.; Pietra, G.; Moretta, L. Immune checkpoint inhibitors: Anti-NKG2A antibodies on board. Trends Immunol. 2019, 40, 83–85. [Google Scholar] [CrossRef]
- Moretta, A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat. Rev. Immunol. 2002, 2, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Takeda, K.; Kaneda, H.; Matsumoto, H.; Hayakawa, Y.; Raulet, D.H.; Ikarashi, Y.; Kronenberg, M.; Yagita, H.; Kinoshita, K. NKG2A inhibits invariant NKT cell activation in hepatic injury. J. Immunol. 2009, 182, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Mingari, M.C.; Ponte, M.; Bertone, S.; Schiavetti, F.; Vitale, C.; Bellomo, R.; Moretta, A.; Moretta, L. HLA class I-specific inhibitory receptors in human T lymphocytes: Interleukin 15-induced expression of CD94/NKG2A in superantigen-or alloantigen-activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 1998, 95, 1172–1177. [Google Scholar] [CrossRef] [Green Version]
- Cazzetta, V.; Bruni, E.; Terzoli, S.; Carenza, C.; Franzese, S.; Piazza, R.; Marzano, P.; Donadon, M.; Torzilli, G.; Cimino, M. NKG2A expression identifies a subset of human Vδ2 T cells exerting the highest antitumor effector functions. Cell Rep. 2021, 37, 109871. [Google Scholar] [CrossRef] [PubMed]
- Bertone, S.; Schiavetti, F.; Bellomo, R.; Vitale, C.; Ponte, M.; Moretta, L.; Mingari, M.C. Transforming growth factor-β-induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. Eur. J. Immunol. 1999, 29, 23–29. [Google Scholar] [CrossRef]
- Borst, L.; Sluijter, M.; Sturm, G.; Charoentong, P.; Santegoets, S.J.; van Gulijk, M.; van Elsas, M.J.; Groeneveldt, C.; van Montfoort, N.; Finotello, F. NKG2A is a late immune checkpoint on CD8 T cells and marks repeated stimulation and cell division. Int. J. Cancer 2022, 150, 688–704. [Google Scholar] [CrossRef] [PubMed]
- van Montfoort, N.; Borst, L.; Korrer, M.J.; Sluijter, M.; Marijt, K.A.; Santegoets, S.J.; van Ham, V.J.; Ehsan, I.; Charoentong, P.; André, P. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 2018, 175, 1744–1755. [Google Scholar] [CrossRef]
- Cohen, R.; Lefebvre, G.; Posner, M.; Bauman, J.; Salas, S.; Even, C.; Saada-Bouzid, E.; Seiwert, T.; Colevas, D.; Calmels, F. Monalizumab in combination with cetuximab in patients (pts) with recurrent or metastatic (R/M) head and neck cancer (SCCHN) previously treated or not with PD-(L) 1 inhibitors (IO): 1-year survival data. Ann. Oncol. 2019, 30, v460. [Google Scholar] [CrossRef]
- Colevas, D.; Misiukiewicz, K.; Pearson, A.; Fayette, J.; Bauman, J.; Cupissol, D.; Saada-Bouzid, E.; Adkins, D.; Marie, D.; Cornen, S. 123MO Monalizumab, cetuximab and durvalumab in first-line treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): A phase II trial. Ann. Oncol. 2021, 32, S1432. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Diamond, J.R.; Curigliano, G.; Deva, S.; Bendell, J.C.; Han, S.-W.; Kalyan, A.; Naidoo, J.; Kim, R.D.; Patel, S.P. First-line durvalumab + monalizumab, mFOLFOX6, and bevacizumab or cetuximab for metastatic microsatellite-stable colorectal cancer (MSS-CRC). J. Clin. Oncol. 2020, 38, 128. [Google Scholar] [CrossRef]
- Salomé, B.; Sfakianos, J.P.; Ranti, D.; Daza, J.; Bieber, C.; Charap, A.; Hammer, C.; Banchereau, R.; Farkas, A.M.; Ruan, D.F. NKG2A and HLA-E define an alternative immune checkpoint axis in bladder cancer. Cancer Cell 2022, 40, 1027–1043.e1029. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Spira, A.; Campelo, R.G.; Kim, D.-W.; Hamid, O.; Soo-Hoo, Y.; Kumar, R.; Grenga, I.; Forde, P. Abstract CT124: NeoCOAST-2: A randomized, open-label, phase 2 study of neoadjuvant durvalumab plus novel immunotherapies and chemotherapy (CT) followed by adjuvant durvalumab plus novel agents, in patients with resectable non-small-cell lung cancer (NSCLC). Cancer Res. 2022, 82, CT124. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific antibodies: From research to clinical application. Front. Immunol. 2021, 12, 1555. [Google Scholar] [CrossRef]
- Dahlén, E.; Veitonmäki, N.; Norlén, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2018, 6, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.S.; Neri, D. Advances in antibody engineering for rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 197–207. [Google Scholar] [CrossRef]
- Gleason, M.K.; Verneris, M.R.; Todhunter, D.A.; Zhang, B.; McCullar, V.; Zhou, S.X.; Panoskaltsis-Mortari, A.; Weiner, L.M.; Vallera, D.A.; Miller, J.S. Bispecific and Trispecific Killer Cell Engagers Directly Activate Human NK Cells through CD16 Signaling and Induce Cytotoxicity and Cytokine ProductionBiKEs and TriKEs Enhance NK Cell Effector Function. Mol. Cancer Ther. 2012, 11, 2674–2684. [Google Scholar] [CrossRef] [Green Version]
- Vallera, D.A.; Zhang, B.; Gleason, M.K.; Oh, S.; Weiner, L.M.; Kaufman, D.S.; McCullar, V.; Miller, J.S.; Verneris, M.R. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother. Radiopharm. 2013, 28, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B. Targeting Natural Killer Cells to Acute Myeloid Leukemia In Vitro with a CD16 × 33 Bispecific Killer Cell Engager and ADAM17 InhibitionTargeting NK Cells to AML with a BiKE and ADAM17 Inhibitor. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlenzka, J.; Moehler, T.M.; Kipriyanov, S.M.; Kornacker, M.; Benner, A.; Bähre, A.; Stassar, M.J.; Schäfer, H.J.; Little, M.; Goldschmidt, H. Combined effect of recombinant CD19× CD16 diabody and thalidomide in a preclinical model of human B cell lymphoma. Anti-Cancer Drugs 2004, 15, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Glorius, P.; Baerenwaldt, A.; Kellner, C.; Staudinger, M.; Dechant, M.; Stauch, M.; Beurskens, F.; Parren, P.; Van de Winkel, J.; Valerius, T. The novel tribody [(CD20)2xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia 2013, 27, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Bassett, R.; Kerbauy, L.; Basar, R.; Kaplan, M.; Griffin, L.; Esqueda, D.; Ganesh, C. Abstract CT003: Innate cell engager (ICE®) AFM13 combined with preactivated and expanded cord blood (CB)-derived NK cells for patients with refractory/relapsed CD30+ lymphoma. Cancer Res. 2022, 82, CT003. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur. J. Immunol. 2021, 51, 1934–1942. [Google Scholar] [CrossRef]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced FunctionImprovement of Bispecific Antibody by Insertion of IL15. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [Green Version]
- Colomar-Carando, N.; Gauthier, L.; Merli, P.; Loiacono, F.; Canevali, P.; Falco, M.; Galaverna, F.; Rossi, B.; Bosco, F.; Caratini, M. Exploiting Natural Killer cell engagers to control pediatric B-cell precursor acute lymphoblastic leukemia. Cancer Immunol. Res. 2022, 10, 291–302. [Google Scholar] [CrossRef]
- Varchetta, S.; Gibelli, N.; Oliviero, B.; Nardini, E.; Gennari, R.; Gatti, G.; Silva, L.S.; Villani, L.; Tagliabue, E.; Ménard, S. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007, 67, 11991–11999. [Google Scholar] [CrossRef] [Green Version]
- Holgado, E.; Perez-Garcia, J.; Gion, M.; Cortes, J. Is there a role for immunotherapy in HER2-positive breast cancer? NPJ Breast Cancer 2018, 4, 21. [Google Scholar] [CrossRef]
- García-Foncillas, J.; Sunakawa, Y.; Aderka, D.; Wainberg, Z.; Ronga, P.; Witzler, P.; Stintzing, S. Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front. Oncol. 2019, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.M.; Lee, S.C.; Filho, P.A.A.; Lord, C.A.; Jie, H.-B.; Davidson, H.C.; López-Albaitero, A.; Gibson, S.P.; Gooding, W.E.; Ferrone, S. Cetuximab-activated natural killer (NK) and dendritic cells (DC) collaborate to trigger tumor antigen-specific T cell immunity in head and neck cancer patients. Clin. Cancer Res. 2013, 19, 1858–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Ozzo, S.; Tartas, S.; Paintaud, G.; Cartron, G.; Colombat, P.; Bardos, P.; Watier, H.; Thibault, G. Rituximab-dependent cytotoxicity by natural killer cells: Influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004, 64, 4664–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Domaica, C.I. Cytokine regulation of natural killer cell effector functions. Biofactors 2010, 36, 274–288. [Google Scholar] [CrossRef]
- Müller, L.; Aigner, P.; Stoiber, D. Type I interferons and natural killer cell regulation in cancer. Front. Immunol. 2017, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Becknell, B.; Caligiuri, M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005, 86, 209–239. [Google Scholar]
- Mirjačić Martinović, K.; Babović, N.; Džodić, R.; Jurišić, V.; Matković, S.; Konjević, G. Favorable in vitro effects of combined IL-12 and IL-18 treatment on NK cell cytotoxicity and CD25 receptor expression in metastatic melanoma patients. J. Transl. Med. 2015, 13, 120. [Google Scholar] [CrossRef] [Green Version]
- Jost, S.; Quillay, H.; Reardon, J.; Peterson, E.; Simmons, R.P.; Parry, B.A.; Bryant, N.N.; Binder, W.D.; Altfeld, M. Changes in cytokine levels and NK cell activation associated with influenza. PLoS ONE 2011, 6, e25060. [Google Scholar] [CrossRef] [Green Version]
- Viel, S.; Marçais, A.; Guimaraes, F.S.-F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuletić, A.; Jovanić, I.; Jurišić, V.; Milovanović, Z.; Nikolić, S.; Spurnić, I.; Konjević, G. Decreased interferon γ production in CD3+ and CD3−CD56+ lymphocyte subsets in metastatic regional lymph nodes of melanoma patients. Pathol. Oncol. Res. 2015, 21, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Laouar, Y.; Sutterwala, F.S.; Gorelik, L.; Flavell, R.A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 2005, 6, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-S.; Terabe, M.; Mamura, M.; Kang, M.-J.; Chae, H.; Stuelten, C.; Kohn, E.; Tang, B.; Sabzevari, H.; Anver, M.R. An anti–transforming growth factor β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008, 68, 3835–3843. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.; Bouchlaka, M.N.; Sckisel, G.D.; Sungur, C.M.; Chen, M.; Murphy, W.J. Increased antitumor effects using IL-2 with anti–TGF-β reveals competition between mouse NK and CD8 T cells. J. Immunol. 2014, 193, 1709–1716. [Google Scholar] [CrossRef] [Green Version]
- Marcoe, J.P.; Lim, J.R.; Schaubert, K.L.; Fodil-Cornu, N.; Matka, M.; McCubbrey, A.L.; Farr, A.R.; Vidal, S.M.; Laouar, Y. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat. Immunol. 2012, 13, 843–850. [Google Scholar] [CrossRef]
- Alvarez, M.; Dunai, C.; Khuat, L.T.; Aguilar, E.G.; Barao, I.; Murphy, W.J. IL-2 and Anti-TGF-β Promote NK Cell Reconstitution and Anti-tumor Effects after Syngeneic Hematopoietic Stem Cell Transplantation. Cancers 2020, 12, 3189. [Google Scholar] [CrossRef]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I study of GC1008 (fresolimumab): A human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 2014, 9, e90353. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Qu, H.; Qu, H.; Theilhaber, J.; Shapiro, G.; Gregory, R.; Winter, C.; Malkova, N.; Sun, F.; Jaworski, J. Pan-TGFβ inhibition by SAR439459 relieves immunosuppression and improves antitumor efficacy of PD-1 blockade. OncoImmunology 2020, 9, 1811605. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J.; Datta, A.; Littlefield, C.; Kalra, A.; Chapron, C.; Wawersik, S.; Dagbay, K.B.; Brueckner, C.T.; Nikiforov, A.; Danehy, F.T., Jr. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 2020, 12, eaay8456. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Daste, A.; Ravaud, A.; Salas, S.; Isambert, N.; McClay, E.; Awada, A.; Borel, C.; Ojalvo, L.S.; Helwig, C. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: Results from a phase I cohort. J. Immunother. Cancer 2020, 8, e000664. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Bang, Y.-J.; Kondo, S.; Chung, H.C.; Muro, K.; Dussault, I.; Helwig, C.; Osada, M. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin. Cancer Res. 2020, 26, 3202–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Ares, L.; Kim, T.M.; Vicente, D.; Felip, E.; Lee, D.H.; Lee, K.H.; Lin, C.-C.; Flor, M.J.; Di Nicola, M.; Alvarez, R.M. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: Results from an expansion cohort of a phase 1 trial. J. Thorac. Oncol. 2020, 15, 1210–1222. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Kong, L.; Wang, M.; Spanoudis, C.; Chaturvedi, P.; George, V.; Jiao, J.-A.; You, L.; Egan, J.O. Bifunctional TGF-β trap/IL-15 protein complex elicits potent NK cell and CD8+ T Cell immunity against solid tumors. Mol. Ther. 2021, 29, 2949–2962. [Google Scholar] [CrossRef]
- Ni, G.; Zhang, L.; Yang, X.; Li, H.; Ma, B.; Walton, S.; Wu, X.; Yuan, J.; Wang, T.; Liu, X. Targeting interleukin-10 signalling for cancer immunotherapy, a promising and complicated task. Hum. Vaccines Immunother. 2020, 16, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.S.; Corrigan, A.E.; Dadah, H.; George, A.M.; Keshwara, S.M.; Sideris, M.; Szabados, B. Cytokine-based cancer immunotherapy: Challenges and opportunities for IL-10. Anticancer. Res. 2021, 41, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, M.; Cui, B.; Lu, C.; Liu, X.; Zhang, P.; Liu, B.; Ma, C.; Shen, Y.; Lu, Z. CD4+CD25+Foxp3+ regulatory T cells suppress NKG2D-mediated NK cell cytotoxicity in peripheral blood. Medicine 2019, 98, e15722. [Google Scholar] [CrossRef] [PubMed]
- Littwitz-Salomon, E.; Malyshkina, A.; Schimmer, S.; Dittmer, U. The cytotoxic activity of natural killer cells is suppressed by IL-10+ regulatory T cells during acute retroviral infection. Front. Immunol. 2018, 9, 1947. [Google Scholar] [CrossRef]
- Spallanzani, R.G.; Torres, N.I.; Avila, D.E.; Ziblat, A.; Iraolagoitia, X.L.R.; Rossi, L.E.; Domaica, C.I.; Fuertes, M.B.; Rabinovich, G.A.; Zwirner, N.W. Regulatory dendritic cells restrain NK cell IFN-γ production through mechanisms involving NKp46, IL-10, and MHC class I–specific inhibitory receptors. J. Immunol. 2015, 195, 2141–2148. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Fu, K.; Yang, L.; Wu, S.; Cen, Z.; Meng, X.; Huang, Q.; Xie, Z. Hypoxia-inducible gene 2 promotes the immune escape of hepatocellular carcinoma from nature killer cells through the interleukin-10-STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 229. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, J.; Liu, Q.; Song, W.; Zhang, X.; Tiruthani, K.; Hu, H.; Das, M.; Goodwin, T.J.; Liu, R. Local blockade of interleukin 10 and CXC motif chemokine ligand 12 with nano-delivery promotes antitumor response in murine cancers. Acs Nano 2018, 12, 9830–9841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.-P. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 Signaling. Front. Immunol. 2021, 12, 619195. [Google Scholar] [CrossRef]
- Dunai, C.; Ames, E.; Ochoa, M.C.; Fernandez-Sendin, M.; Melero, I.; Simonetta, F.; Baker, J.; Alvarez, M. Killers on the loose: Immunotherapeutic strategies to improve NK cell-based therapy for cancer treatment. Int. Rev. Cell Mol. Biol. 2022, 370, 65–122. [Google Scholar] [PubMed]
- Hecht, J.R.; Lonardi, S.; Bendell, J.; Sim, H.-W.; Macarulla, T.; Lopez, C.D.; Van Cutsem, E.; Martin, A.J.M.; Park, J.O.; Greil, R. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J. Clin. Oncol. 2021, 39, 1108. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): A multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef]
- Tannir, N.M.; Papadopoulos, K.P.; Wong, D.J.; Aljumaily, R.; Hung, A.; Afable, M.; Kim, J.S.; Ferry, D.; Drakaki, A.; Bendell, J. Pegilodecakin as monotherapy or in combination with anti-PD-1 or tyrosine kinase inhibitor in heavily pretreated patients with advanced renal cell carcinoma: Final results of cohorts A, G, H and I of IVY Phase I study. Int. J. Cancer 2021, 149, 403–408. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Tessmer-Tuck, J.; Pierson, B.A.; Weisdorf, D.; McGlave, P.; Blazar, B.R.; Katsanis, E.; Verfaillie, C.; Lebkowski, J.; Radford, J., Jr. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 1997, 3, 34–44. [Google Scholar]
- Nguyen, L.T.; Saibil, S.D.; Sotov, V.; Le, M.X.; Khoja, L.; Ghazarian, D.; Bonilla, L.; Majeed, H.; Hogg, D.; Joshua, A.M. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol. Immunother. 2019, 68, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Glauser, F.L.; DeBlois, G.; Bechard, D.; Fowler, A.A.; Merchant, R.; Fairman, R.P. Cardiopulmonary toxicity of adoptive immunotherapy. Am. J. Med. Sci. 1988, 296, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.; Glenn, L.; Hoen, H.; Richards, B.; Smith, J.W.; Lufkin, R.; Crocenzi, T.S.; Urba, W.J.; Curti, B.D. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J. Immunother. Cancer 2014, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Bendickova, K.; Fric, J. Roles of IL-2 in bridging adaptive and innate immunity, and as a tool for cellular immunotherapy. J. Leukoc. Biol. 2020, 108, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Hallett, W.H.; Ames, E.; Álvarez, M.; Barao, I.; Taylor, P.A.; Blazar, B.R.; Murphy, W.J. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell–mediated antitumor responses. Biol. Blood Marrow Transplant. 2008, 14, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Licona Limon, P. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Kovar, M.; Rubinstein, M.P.; Surh, C.D.; Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006, 311, 1924–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, D.; Arenas-Ramirez, N.; Rath, M.; Karakus, U.; Hümbelin, M.; van Gogh, M.; Borsig, L.; Boyman, O. An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer. Nat. Commun. 2020, 11, 6440. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Gonzalez-Nicolini, V.; Freimoser-Grundschober, A.; Nayak, T.K.; Fahrni, L.; Hosse, R.J.; Gerrits, D.; Geven, E.J.; Sam, J.; Lang, S. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. mAbs 2021, 13, 1913791. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Alvarez, E.C.; Mau-Sorensen, M.; Lassen, U.; Lolkema, M.; Robbrecht, D.; Gomez-Roca, C.; Martin-Liberal, J.; Tabernero, J.; Ros, W. Clinical activity, safety, and PK/PD from a phase I study of RO6874281, a fibroblast activation protein (FAP) targeted interleukin-2 variant (IL-2v). Ann. Oncol. 2018, 29, viii134–viii135. [Google Scholar] [CrossRef]
- Cooper, M.A.; Bush, J.E.; Fehniger, T.A.; VanDeusen, J.B.; Waite, R.E.; Liu, Y.; Aguila, H.L.; Caligiuri, M.A. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood J. Am. Soc. Hematol. 2002, 100, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Mazzolini, G.; Hervas-Stubbs, S.; de Sanmamed, M.F.; Berraondo, P.; Melero, I. Interleukin-15 in gene therapy of cancer. Curr. Gene Ther. 2013, 13, 15–30. [Google Scholar] [CrossRef]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid TumorsPhase I Trial of SC rhIL15 in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Bessard, A.; Solé, V.; Bouchaud, G.; Quéméner, A.; Jacques, Y. High antitumor activity of RLI, an interleukin-15 (IL-15)–IL-15 receptor α fusion protein, in metastatic melanoma and colorectal cancerRLI, an IL-15–IL-15Rα Fusion Protein, in Tumor Therapy. Mol. Cancer Ther. 2009, 8, 2736–2745. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Martínez, A.; Fernández, L.; Valentín, J.; Martínez-Romera, I.; Corral, M.D.; Ramírez, M.; Abad, L.; Santamaría, S.; González-Vicent, M.; Sirvent, S. A phase I/II trial of interleukin-15–stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 2015, 17, 1594–1603. [Google Scholar] [CrossRef]
- Fujii, R.; Jochems, C.; Tritsch, S.R.; Wong, H.C.; Schlom, J.; Hodge, J.W. An IL-15 superagonist/IL-15Rα fusion complex protects and rescues NK cell-cytotoxic function from TGF-β1-mediated immunosuppression. Cancer Immunol. Immunother. 2018, 67, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid TumorsPhase I Trial of ALT-803 in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Fu, S.; Zhao, Q. 2022 update on the scientific premise and clinical trials for IL-15 agonists as cancer immunotherapy. J. Leukoc. Biol. 2022, 112, 823–834. [Google Scholar] [CrossRef]
- Cooley, S.; Parham, P.; Miller, J.S. Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood J. Am. Soc. Hematol. 2018, 131, 1053–1062. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Desai, S.; Pence, P.; Cooley, S. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood J. Am. Soc. Hematol. 2022, 139, 1177–1183. [Google Scholar] [CrossRef]
- Miyazaki, T.; Maiti, M.; Hennessy, M.; Chang, T.; Kuo, P.; Addepalli, M.; Obalapur, P.; Sheibani, S.; Wilczek, J.; Pena, R. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer 2021, 9, e002024. [Google Scholar] [CrossRef]
- Conlon, K.; Watson, D.C.; Waldmann, T.A.; Valentin, A.; Bergamaschi, C.; Felber, B.K.; Peer, C.J.; Figg, W.D.; Potter, E.L.; Roederer, M. Phase I study of single agent NIZ985, a recombinant heterodimeric IL-15 agonist, in adult patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2021, 9, e003388. [Google Scholar] [CrossRef]
- Barao, I.; Alvarez, M.; Redelman, D.; Weiss, J.M.; Ortaldo, J.R.; Wiltrout, R.H.; Murphy, W.J. Hydrodynamic delivery of human IL-15 cDNA increases murine natural killer cell recovery after syngeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2011, 17, 1754–1764. [Google Scholar] [CrossRef] [Green Version]
- Marrero, B.; Shirley, S.; Heller, R. Delivery of interleukin-15 to B16 melanoma by electroporation leads to tumor regression and long-term survival. Technol. Cancer Res. Treat. 2014, 13, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, C.; Kulkarni, V.; Rosati, M.; Alicea, C.; Jalah, R.; Chen, S.; Bear, J.; Sardesai, N.Y.; Valentin, A.; Felber, B.K. Intramuscular delivery of heterodimeric IL-15 DNA in macaques produces systemic levels of bioactive cytokine inducing proliferation of NK and T cells. Gene Ther. 2015, 22, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, M.C.; Melero, I.; Berraondo, P. High-density lipoproteins delivering interleukin-15. Oncoimmunology 2013, 2, e23410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, M.C.; Fioravanti, J.; Rodriguez, I.; Hervas-Stubbs, S.; Azpilikueta, A.; Mazzolini, G.; Gúrpide, A.; Prieto, J.; Pardo, J.; Berraondo, P. Antitumor Immunotherapeutic and Toxic Properties of an HDL-Conjugated Chimeric IL-15 Fusion ProteinHDL Carrying an IL-15 Fusion Protein. Cancer Res. 2013, 73, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Fioravanti, J.; Duitman, E.H.; Medina-Echeverz, J.; Palazon, A.; Arina, A.; Dubrot, J.; Alfaro, C.; Morales-Kastresana, A.; Murillo, O. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS ONE 2012, 7, e52370. [Google Scholar] [CrossRef]
- Alvarez, M.; Ochoa, M.C.; Minute, L.; Melero, I.; Berraondo, P. Rapid isolation and enrichment of mouse NK cells for experimental purposes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 631, pp. 257–275. [Google Scholar]
- Ochoa, M.C.; Minute, L.; López, A.; Pérez-Ruiz, E.; Gomar, C.; Vasquez, M.; Inoges, S.; Etxeberria, I.; Rodriguez, I.; Garasa, S. Enhancement of antibody-dependent cellular cytotoxicity of cetuximab by a chimeric protein encompassing interleukin-15. Oncoimmunology 2018, 7, e1393597. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.; Barra, N.; Davies, E.; Ashkar, A.; Lichty, B. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012, 19, 238–246. [Google Scholar] [CrossRef]
- Niu, Z.; Bai, F.; Sun, T.; Tian, H.; Yu, D.; Yin, J.; Li, S.; Li, T.; Cao, H.; Yu, Q. Recombinant Newcastle disease virus expressing IL15 demonstrates promising antitumor efficiency in melanoma model. Technol. Cancer Res. Treat. 2015, 14, 607–615. [Google Scholar] [CrossRef]
- Tosic, V.; Thomas, D.L.; Kranz, D.M.; Liu, J.; McFadden, G.; Shisler, J.L.; MacNeill, A.L.; Roy, E.J. Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PLoS ONE 2014, 9, e109801. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.-f.; Qi, W.-x.; Liu, M.-y.; Li, Y. The combination of NK and CD8+ T cells with CCL20/IL15-armed oncolytic adenoviruses enhances the growth suppression of TERT-positive tumor cells. Cell. Immunol. 2017, 318, 35–41. [Google Scholar] [CrossRef]
- Kowalsky, S.J.; Liu, Z.; Feist, M.; Berkey, S.E.; Ma, C.; Ravindranathan, R.; Dai, E.; Roy, E.J.; Guo, Z.S.; Bartlett, D.L. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 2018, 26, 2476–2486. [Google Scholar] [CrossRef] [Green Version]
- Backhaus, P.S.; Veinalde, R.; Hartmann, L.; Dunder, J.E.; Jeworowski, L.M.; Albert, J.; Hoyler, B.; Poth, T.; Jäger, D.; Ungerechts, G. Immunological effects and viral gene expression determine the efficacy of oncolytic measles vaccines encoding IL-12 or IL-15 agonists. Viruses 2019, 11, 914. [Google Scholar] [CrossRef] [Green Version]
- McArdel, S.L.; Dugast, A.-S.; Hoover, M.E.; Bollampalli, A.; Hong, E.; Castano, Z.; Leonard, S.C.; Pawar, S.; Mellen, J.; Muriuki, K. Anti-tumor effects of RTX-240: An engineered red blood cell expressing 4-1BB ligand and interleukin-15. Cancer Immunol. Immunother. 2021, 70, 2701–2719. [Google Scholar] [CrossRef]
- Cohen, J. IL-12 deaths: Explanation and a puzzle. Science 1995, 270, 908. [Google Scholar] [CrossRef]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood J. Am. Soc. Hematol. 1997, 90, 2541–2548. [Google Scholar]
- Lenzi, R.; Edwards, R.; June, C.; Seiden, M.V.; Garcia, M.E.; Rosenblum, M.; Freedman, R.S. Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease < 1 cm) associated with ovarian cancer or primary peritoneal carcinoma. J. Transl. Med. 2007, 5, 66. [Google Scholar]
- Cirella, A.; Luri-Rey, C.; Di Trani, C.A.; Teijeira, A.; Olivera, I.; Bolaños, E.; Castañón, E.; Palencia, B.; Brocco, D.; Fernández-Sendin, M. Novel strategies exploiting interleukin-12 in cancer immunotherapy. Pharmacol. Ther. 2022, 239, 108189. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef]
- Shi, F.; Rakhmilevich, A.L.; Heise, C.P.; Oshikawa, K.; Sondel, P.M.; Yang, N.-S.; Mahvi, D.M. Intratumoral injection of interleukin-12 plasmid DNA, either naked or in complex with cationic lipid, results in similar tumor regression in a murine model. Mol. Cancer Ther. 2002, 1, 949–957. [Google Scholar]
- Heinzerling, L.; Burg, G.; Dummer, R.; Maier, T.; Oberholzer, P.A.; Schultz, J.; Elzaouk, L.; Pavlovic, J.; Moelling, K. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: Clinical efficacy. Hum. Gene Ther. 2005, 16, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Anwer, K.; Barnes, M.; Fewell, J.; Lewis, D.; Alvarez, R. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010, 17, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Thaker, P.H.; Brady, W.E.; Lankes, H.A.; Odunsi, K.; Bradley, W.H.; Moore, K.N.; Muller, C.Y.; Anwer, K.; Schilder, R.J.; Alvarez, R.D. A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 147, 283–290. [Google Scholar]
- Di Trani, C.A.; Fernandez-Sendin, M.; Cirella, A.; Segués, A.; Olivera, I.; Bolaños, E.; Melero, I.; Berraondo, P. Advances in mRNA-based drug discovery in cancer immunotherapy. Expert Opin. Drug Discov. 2022, 17, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.L.; Bailey, D.; Zielinski, J.; Apte, A.; Musenge, F.; Karp, R.; Burke, S.; Garcon, F.; Mishra, A.; Gurumurthy, S. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 2020, 26, 6284–6298. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Hellman, M.; Carneiro, B.; Marron, T.; Subbiah, V.; Mehmi, I.; Eyles, J.; Dubois, V.; Ridgway, B.; Hernandez, A.G. 19O Preliminary safety, antitumor activity and pharmacodynamics results of HIT-IT MEDI1191 (mRNA IL-12) in patients with advanced solid tumours and superficial lesions. Ann. Oncol. 2021, 32, S9. [Google Scholar] [CrossRef]
- Alkayyal, A.A.; Tai, L.-H.; Kennedy, M.A.; de Souza, C.T.; Zhang, J.; Lefebvre, C.; Sahi, S.; Ananth, A.A.; Mahmoud, A.B.; Makrigiannis, A.P. NK-Cell Recruitment Is Necessary for Eradication of Peritoneal Carcinomatosis with an IL12-Expressing Maraba Virus Cellular VaccineIL12-Expressing Oncolytic Virus as Tumor Cell Vaccine. Cancer Immunol. Res. 2017, 5, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-J.; Zhang, S.-N.; Choi, I.-K.; Kim, J.-S.; Yun, C.-O. Strengthening of antitumor immune memory and prevention of thymic atrophy mediated by adenovirus expressing IL-12 and GM-CSF. Gene Ther. 2012, 19, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quetglas, J.I.; Labiano, S.; Aznar, M.Á.; Bolaños, E.; Azpilikueta, A.; Rodriguez, I.; Casales, E.; Sánchez-Paulete, A.R.; Segura, V.; Smerdou, C. Virotherapy with a Semliki Forest Virus–Based Vector Encoding IL12 Synergizes with PD-1/PD-L1 BlockadeVirotherapy+ PD-1 Blockade. Cancer Immunol. Res. 2015, 3, 449–454. [Google Scholar] [CrossRef]
- Marotel, M.; Hasim, M.; Hagerman, A.; Ardolino, M. The two-faces of NK cells in oncolytic virotherapy. Cytokine Growth Factor Rev. 2020, 56, 59–68. [Google Scholar] [CrossRef]
- Paoloni, M.; Mazcko, C.; Selting, K.; Lana, S.; Barber, L.; Phillips, J.; Skorupski, K.; Vail, D.; Wilson, H.; Biller, B. Defining the pharmacodynamic profile and therapeutic index of NHS-IL12 immunocytokine in dogs with malignant melanoma. PLoS ONE 2015, 10, e0129954. [Google Scholar] [CrossRef]
- Schilbach, K.; Alkhaled, M.; Welker, C.; Eckert, F.; Blank, G.; Ziegler, H.; Sterk, M.; Müller, F.; Sonntag, K.; Wieder, T. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology 2015, 4, e1014760. [Google Scholar] [CrossRef]
- Eckert, F.; Schmitt, J.; Zips, D.; Krueger, M.A.; Pichler, B.J.; Gillies, S.D.; Strittmatter, W.; Handgretinger, R.; Schilbach, K. Enhanced binding of necrosis-targeting immunocytokine NHS-IL12 after local tumour irradiation in murine xenograft models. Cancer Immunol. Immunother. 2016, 65, 1003–1013. [Google Scholar] [CrossRef]
- Eckert, F.; Jelas, I.; Oehme, M.; Huber, S.M.; Sonntag, K.; Welker, C.; Gillies, S.D.; Strittmatter, W.; Zips, D.; Handgretinger, R. Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology 2017, 6, e1323161. [Google Scholar] [CrossRef] [Green Version]
- Strauss, J.; Heery, C.R.; Kim, J.W.; Jochems, C.; Donahue, R.N.; Montgomery, A.S.; McMahon, S.; Lamping, E.; Marté, J.L.; Madan, R.A. First-in-Human Phase I Trial of a Tumor-Targeted Cytokine (NHS-IL12) in Subjects with Metastatic Solid TumorsPhase I NHS-IL12. Clin. Cancer Res. 2019, 25, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Marelli, B.; Qi, J.; Qin, G.; Yu, H.; Wang, H.; Jenkins, M.H.; Lo, K.-M.; Lan, Y. NHS-IL12 and bintrafusp alfa combination therapy enhances antitumor activity in preclinical cancer models. Transl. Oncol. 2022, 16, 101322. [Google Scholar] [CrossRef]
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Cancer Res. 2008, 68, 8437–8445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2021, 29, 10–21. [Google Scholar] [CrossRef]
- Su, L.; Hu, Z.; Yang, Y.G. Role of CXCR4 in the progression and therapy of acute leukaemia. Cell Prolif. 2021, 54, e13076. [Google Scholar] [CrossRef]
- Mehrpouri, M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, K. MSX-122: Is an effective small molecule CXCR4 antagonist in cancer therapy? Int. Immunopharmacol. 2022, 108, 108863. [Google Scholar] [CrossRef] [PubMed]
- Floranović, M.P.; Veličković, L.J. Effect of CXCL12 and its receptors on unpredictable renal cell carcinoma. Clin. Genitourin. Cancer 2020, 18, e337–e342. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Beider, K.; Nagler, A.; Wald, O.; Franitza, S.; Dagan-Berger, M.; Wald, H.; Giladi, H.; Brocke, S.; Hanna, J.; Mandelboim, O. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood 2003, 102, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced bone marrow homing of natural killer cells following mRNA transfection with gain-of-function variant CXCR4R334X. Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, Y.Y.; Du, Z.; Zhang, X.; Chng, W.J.; Wang, S. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther. 2022, 29, 475–483. [Google Scholar] [CrossRef]
- Meinhardt, K.; Kroeger, I.; Bauer, R.; Ganss, F.; Ovsiy, I.; Rothamer, J.; Büttner, M.; Atreya, I.; Waldner, M.; Bittrich, M. Identification and characterization of the specific murine NK cell subset supporting graft-versus-leukemia-and reducing graft-versus-host-effects. Oncoimmunology 2015, 4, e981483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2022, 1–16. [Google Scholar] [CrossRef]
- Ames, E.; Murphy, W.J. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol. Immunother. 2014, 63, 21–28. [Google Scholar] [CrossRef]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Lupo, K.B.; Matosevic, S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef] [Green Version]
- Stringaris, K.; Sekine, T.; Khoder, A.; Alsuliman, A.; Razzaghi, B.; Sargeant, R.; Pavlu, J.; Brisley, G.; de Lavallade, H.; Sarvaria, A. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014, 99, 836. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015, 13, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor RegressionNK Adoptive Transfer in Cancer Patients. Clin. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Ko, M.-W.; Chen, F.; Jewett, A. Defective NK cell expansion, cytotoxicity, and lack of ability to differentiate tumors from a pancreatic cancer patient in a long term follow-up: Implication in the progression of cancer. Cancer Immunol. Immunother. 2022, 71, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the dysfunctional NK cell: Assessing the clinical relevance of exhaustion, anergy, and senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.M., Jr.; Bakan, C.E.; Zhang, S.; Collins, S.M.; Liang, J.; Srivastava, S.; Hofmeister, C.C.; Efebera, Y.; Andre, P.; Romagne, F. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood J. Am. Soc. Hematol. 2011, 118, 6387–6391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romagné, F.; André, P.; Spee, P.; Zahn, S.; Anfossi, N.; Gauthier, L.; Capanni, M.; Ruggeri, L.; Benson, D.M., Jr.; Blaser, B.W. Preclinical characterization of 1-7F9, a novel human anti–KIR receptor therapeutic antibody that augments natural killer–mediated killing of tumor cells. Blood J. Am. Soc. Hematol. 2009, 114, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood J. Am. Soc. Hematol. 2014, 123, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; André, P.; Paturel, C. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675. [Google Scholar] [CrossRef] [Green Version]
- Korde, N.; Carlsten, M.; Lee, M.-J.; Minter, A.; Tan, E.; Kwok, M.; Manasanch, E.; Bhutani, M.; Tageja, N.; Roschewski, M. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica 2014, 99, e81. [Google Scholar] [CrossRef] [Green Version]
- Sönmez, C.; Wölfer, J.; Holling, M.; Brokinkel, B.; Stummer, W.; Wiendl, H.; Thomas, C.; Schulte-Mecklenbeck, A.; Grauer, O.M. Blockade of inhibitory killer cell immunoglobulin-like receptors and IL-2 triggering reverses the functional hypoactivity of tumor-derived NK-cells in glioblastomas. Sci. Rep. 2022, 12, 6769. [Google Scholar] [CrossRef]
- Burns, L.; Weisdorf, D.; DeFor, T.; Vesole, D.; Repka, T.; Blazar, B.; Burger, S.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.; Zhang, M. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: A phase I/II trial. Bone Marrow Transplant. 2003, 32, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, L.; Santos, S.; Vesga, M.; Anguita, J.; Martin-Ruiz, I.; Carrascosa, T.; Juan, M.; Eguizabal, C. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci. Rep. 2019, 9, 18729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural killer cells for immunotherapy–advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD 19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Aversa, F.; Terenzi, A.; Tabilio, A.; Falzetti, F.; Carotti, A.; Ballanti, S.; Felicini, R.; Falcinelli, F.; Velardi, A.; Ruggeri, L. Full haplotype-mismatched hematopoietic stem-cell transplantation: A phase II study in patients with acute leukemia at high risk of relapse. J. Clin. Oncol. 2005, 23, 3447–3454. [Google Scholar] [CrossRef]
- Moretta, A.; Locatelli, F.; Moretta, L. Human NK cells: From HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol. Rev. 2008, 224, 58–69. [Google Scholar] [CrossRef]
- Aversa, F.; Tabilio, A.; Terenzi, A.; Velardi, A.; Falzetti, F.; Giannoni, C.; Zei, T.; Martelli, M.P.; Gambelunghe, C.; Rossetti, M. Successful engraftment of T-cell–depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 1994, 84, 3948–3955. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, L.; Vago, L.; Eikema, D.-J.; de Wreede, L.C.; Ciceri, F.; Diaz, M.A.; Locatelli, F.; Jindra, P.; Milone, G.; Diez-Martin, J.L. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: A study on behalf of the CTIWP of the EBMT. Bone Marrow Transplant. 2021, 56, 1900–1907. [Google Scholar] [CrossRef]
- Pende, D.; Marcenaro, S.; Falco, M.; Martini, S.; Bernardo, M.E.; Montagna, D.; Romeo, E.; Cognet, C.; Martinetti, M.; Maccario, R. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood J. Am. Soc. Hematol. 2009, 113, 3119–3129. [Google Scholar] [CrossRef] [Green Version]
- Falco, M.; Romeo, E.; Marcenaro, S.; Martini, S.; Vitale, M.; Bottino, C.; Mingari, M.C.; Moretta, L.; Moretta, A.; Pende, D. Combined genotypic and phenotypic killer cell Ig-like receptor analyses reveal KIR2DL3 alleles displaying unexpected monoclonal antibody reactivity: Identification of the amino acid residues critical for staining. J. Immunol. 2010, 185, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood J. Am. Soc. Hematol. 2010, 115, 4293–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazar, B.R.; MacDonald, K.P.; Hill, G.R. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood J. Am. Soc. Hematol. 2018, 131, 2651–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluckman, E. Cord blood transplantation. Biol. Blood Marrow Transplant. 2006, 12, 808–812. [Google Scholar] [CrossRef] [Green Version]
- Biederstädt, A.; Rezvani, K. Engineering the next generation of CAR-NK immunotherapies. Int. J. Hematol. 2021, 114, 554–571. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Cadavid, J.V.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z. Expansion, persistence, and efficacy of donor memory-like NK cells infused for post-transplant relapse. J. Clin. Investig. 2022, 132, e154334. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Firor, A.E.; Pinz, K.G.; Jares, A.; Liu, H.; Salman, H.; Golightly, M.; Lan, F.; Jiang, X. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016, 7, 56219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, C.; Mihara, K.; Andreansky, M.; Nicholson, I.; Pui, C.; Geiger, T.; Campana, D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004, 18, 676–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Klein Wolterink, R.G.; Wang, J.; Bos, G.M.; Germeraad, W.T. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar]
- Xu, Y.; Liu, Q.; Zhong, M.; Wang, Z.; Chen, Z.; Zhang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 2019, 12, 49. [Google Scholar] [CrossRef]
- Chen, K.; Wada, M.; Pinz, K.; Liu, H.; Lin, K.; Jares, A.; Firor, A.; Shuai, X.; Salman, H.; Golightly, M. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017, 31, 2151–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, F.; Wang, Y.; Jiang, L.; Zhu, X.; Chen, D.; Yuan, L.; An, G.; Meng, H.; Yang, L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 2019, 9, 64. [Google Scholar] [PubMed]
- Oelsner, S.; Friede, M.E.; Zhang, C.; Wagner, J.; Badura, S.; Bader, P.; Ullrich, E.; Ottmann, O.G.; Klingemann, H.; Tonn, T. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy 2017, 19, 235–249. [Google Scholar] [CrossRef]
- Boissel, L.; Betancur, M.; Lu, W.; Krause, D.; Van Etten, R.; Wels, W.; Klingemann, H. Retargeting NK-92 cells by means of CD19-and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2013, 2, e26527. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Uherek, C.; Maki, G.; Chow, K.U.; Schimpf, A.; Klingemann, H.-G.; Tonn, T.; Wels, W.S. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol. Immunother. 2008, 57, 411–423. [Google Scholar] [CrossRef]
- Hambach, J.; Riecken, K.; Cichutek, S.; Schütze, K.; Albrecht, B.; Petry, K.; Röckendorf, J.L.; Baum, N.; Kröger, N.; Hansen, T. Targeting CD38-expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody-based chimeric antigen receptors (Nb-CARs). Cells 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luanpitpong, S.; Poohadsuan, J.; Klaihmon, P.; Issaragrisil, S. Selective cytotoxicity of single and dual anti-CD19 and anti-CD138 chimeric antigen receptor-natural killer cells against hematologic malignancies. J. Immunol. Res. 2021, 2021, 5562630. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Deng, Y.; Benson, D.M.; He, S.; Hughes, T.; Zhang, J.; Peng, Y.; Mao, H.; Yi, L.; Ghoshal, K. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014, 28, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Chu, J.; Keung Chan, W.; Zhang, J.; Wang, Y.; Cohen, J.B.; Victor, A.; Meisen, W.H.; Kim, S.-h.; Grandi, P. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015, 5, 11483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Han, J.; Chu, J.; Zhang, L.; Zhang, J.; Chen, C.; Chen, L.; Wang, Y.; Wang, H.; Yi, L. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 2016, 7, 27764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhang, H.; Ding, J.; Liu, H.; Li, H.; Li, H.; Lu, M.; Miao, Y.; Li, L.; Zheng, J. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res. 2018, 2018, 4263520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083. [Google Scholar] [PubMed]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Li, H.-K.; Hsiao, C.-W.; Yang, S.-H.; Yang, H.-P.; Wu, T.-S.; Lee, C.-Y.; Lin, Y.-L.; Pan, J.; Cheng, Z.-F.; Lai, Y.-D. A novel off-the-shelf trastuzumab-armed NK cell therapy (ACE1702) using antibody-cell-conjugation technology. Cancers 2021, 13, 2724. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood J. Am. Soc. Hematol. 2013, 121, 3599–3608. [Google Scholar] [CrossRef] [Green Version]
- Mishra, H.K.; Dixon, K.J.; Pore, N.; Felices, M.; Miller, J.S.; Walcheck, B. Activation of ADAM17 by IL-15 Limits Human NK Cell Proliferation. Front. Immunol. 2021, 12, 711621. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kumagai, A.; Iriguchi, S.; Yasui, Y.; Miyasaka, T.; Nakagoshi, K.; Nakane, K.; Saito, K.; Takahashi, M.; Sasaki, A. Non–clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti–glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020, 111, 1478–1490. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti–PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Perales, M.-A.; Abramson, J.S. Modern management of relapsed and refractory aggressive B-cell lymphoma: A perspective on the current treatment landscape and patient selection for CAR T-cell therapy. Blood Rev. 2020, 40, 100640. [Google Scholar] [CrossRef] [PubMed]
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef]
- Ma, R.; Lu, T.; Li, Z.; Teng, K.-Y.; Mansour, A.G.; Yu, M.; Tian, L.; Xu, B.; Ma, S.; Zhang, J. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 2021, 81, 3635–3648. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, e142116. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Boieri, M.; Ulvmoen, A.; Sudworth, A.; Lendrem, C.; Collin, M.; Dickinson, A.M.; Kveberg, L.; Inngjerdingen, M. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology 2017, 6, e1274478. [Google Scholar] [CrossRef] [Green Version]
- Uppendahl, L.D.; Felices, M.; Bendzick, L.; Ryan, C.; Kodal, B.; Hinderlie, P.; Boylan, K.L.M.; Skubitz, A.P.N.; Miller, J.S.; Geller, M.A. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol. Oncol. 2019, 153, 149–157. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.C.; Beilke, J.N.; Bezman, N.A.; Lanier, L.L. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 2011, 208, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Muntasell, A.; Vilches, C.; Angulo, A.; López-Botet, M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: A different perspective of the host-pathogen interaction. Eur. J. Immunol. 2013, 43, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Llano, M.; Lee, N.; Navarro, F.; García, P.; Albar, J.P.; Geraghty, D.E.; López-Botet, M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: Preferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 1998, 28, 2854–2863. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Ciurea, S.; Schafer, J.; Bassett, R. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2018, 132, 2782. [Google Scholar] [CrossRef] [Green Version]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.; Foley, B.; Mattsson, K. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Della Chiesa, M.; Falco, M.; Podesta, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood J. Am. Soc. Hematol. 2012, 119, 399–410. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Vergès, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood J. Am. Soc. Hematol. 2012, 119, 2665–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood J. Am. Soc. Hematol. 2018, 131, 247–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Ham, J.D.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
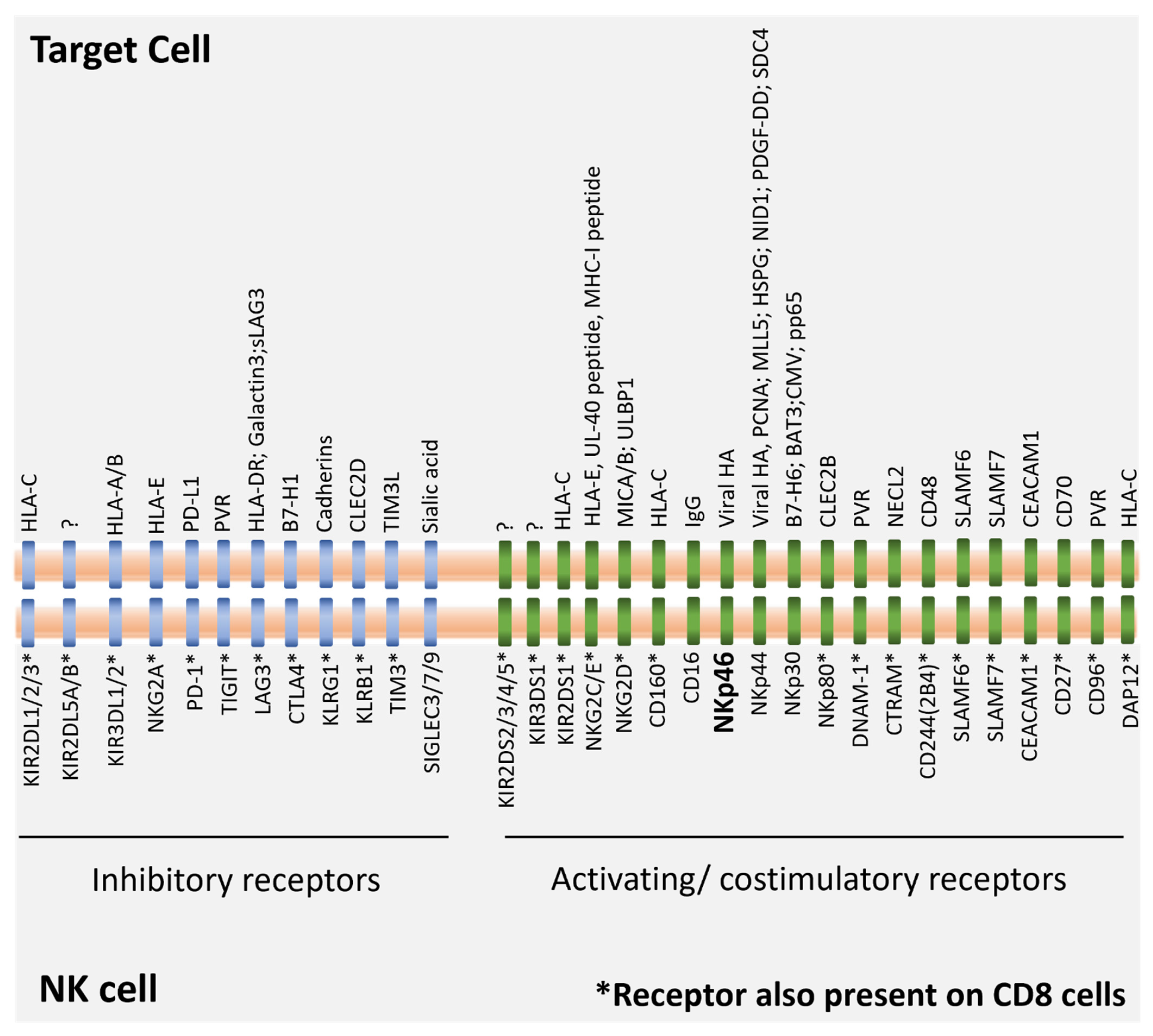
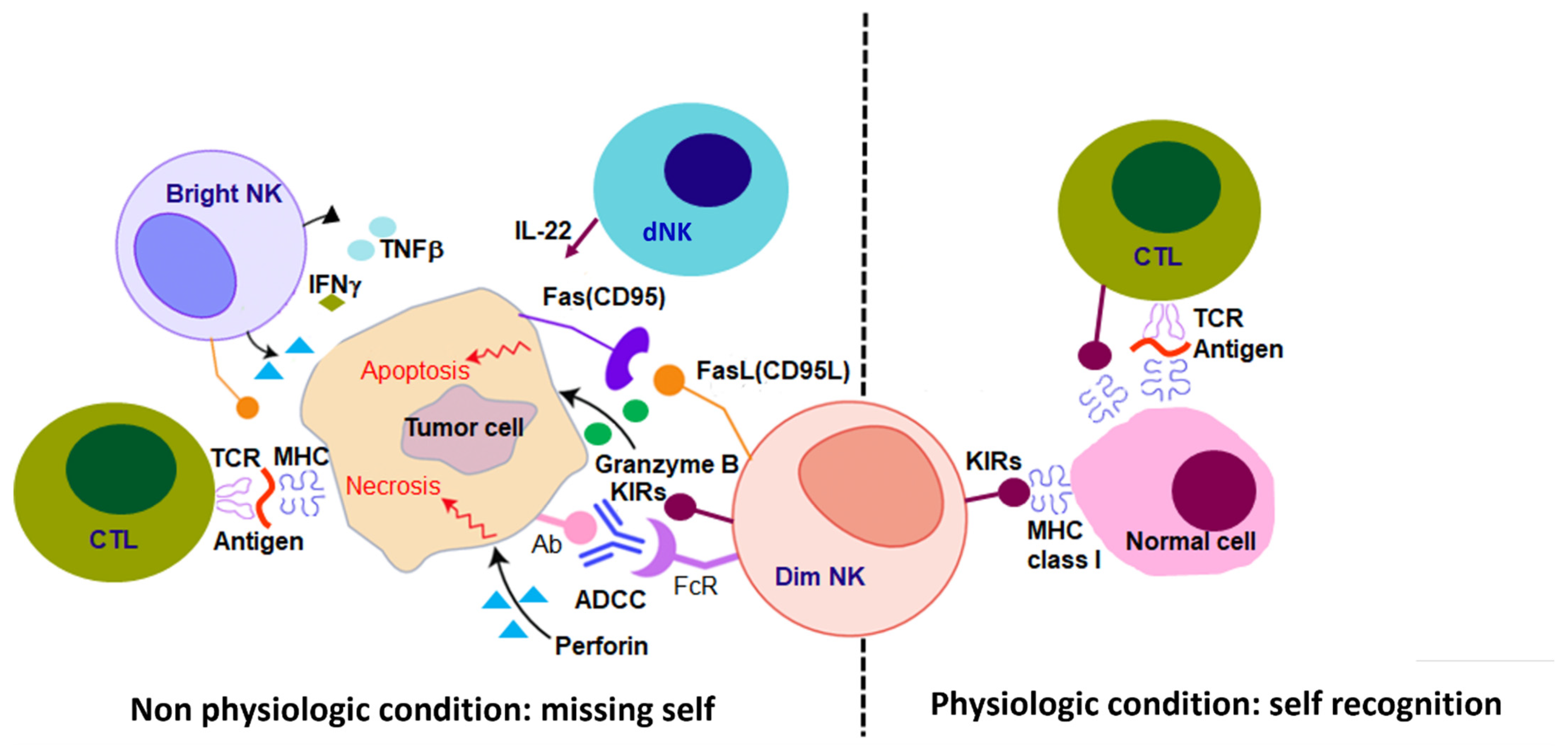
| NK Localization | Cancer Type | Technical Information | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM | NSCLC | SKCM | HNSC | Breast | CRC | BR | Sample Type | NK Cell Markers | Factors Associated with Better Prognosis | Detection Method | References | |
| Periphery | ++ | NA | NA | NA | NA | NA | NA | PBMC | CD56, NKp30, NKp44, NKp46, NKG2D, CD16, 2B4/CD244 | ↑ CD16 NK cells ↑ 2B4/CD244 NK cells | Flow cytometry | Fauriat et al., 2006 [34] |
| NA | ++ | NA | NA | NA | NA | NA | PBMC | CD56, CD16, NKp30, NKp44, NKp46, NKG2D | ↓ NKp46+ CD56dim CD16+ NK cells | Flow cytometry | Picard et al., 2019 [20] | |
| NA | NA | ++ | NA | NA | NA | NA | PBMC | CD56, CD16, NKp46, NKG2D, NKG2A, CD95, CD11a, CD38, PD-1, CD158b, KLRG1 | ↓ CD56bright NK cells | Flow cytometry | De Jonde et al., 2019 [18] | |
| NA | NA | NA | NA | ++ | NA | NA | PBMC | CD56 | ↓ total NK cells | Flow cytometry | Larsson et al., 2022 [35] | |
| NA | NA | NA | NA | NA | ++ | NA | PB | CD56, CD16 | ↑ % NK cells | Flow cytometry | Tang et al., 2020 [17] | |
| NA | NA | NA | NA | NA | NA | ++ | PBMC | CD56, CD16, NKp30, NKp44, NKp46, NKp80, NKG2D, DNAM-1 | ↓ NKp30 i3 expression | Flow cytometry RT-PCR | Semerano M et al., 2015 [27] | |
| TME | ++ | NA | NA | NA | NA | NA | NA | BM | CD56, CD57, KIR2DL1/S1, CD69, CD16, DNAM-1, NKG2D, SLAMF7, CD11a, NKp30, NKp46, NKp44 | ↓ SLAMF7 NK cells | Flow cytometry | Pazina T et al., 2021 [36] |
| NA | ++ | NA | NA | NA | NA | NA | FFPE tissues | CD57 | ↑ NK cell infiltration | IHC | Villegas et al., 2002 [32] | |
| NA | NA | ++ | NA | ++ | NA | NA | In silico analysis (TCGA) | NCR1, KLRF1, KLRD1, PRF1, FCGR3A, CCL4, CCL3, CD247 | ↑ expression NK cell specific signature | scRNAseq RNAseq Microarray | Ascierto et al., 2019 [30] | |
| NA | NA | NA | ++ | NA | NA | NA | FFPE tissues | CD57 | ↑ CD57+ NK cell infiltration | IHC | Fang et al., 2017 [37] | |
| NA | NA | NA | NA | ++ | NA | NA | Frozen tissues, FFPE tissues | CRTAM, CD96, CD1d, LFA-1, CD56, CD16, NKG2D, DNAM1, NKp30, NKp44, NKp46 | ↑ NKp30, NKp46, NKG2D, CRTAM DNAM1, CD96 expression | Microarray analysis RT-PCR | Ascierto et al., 2013 [29] | |
| NA | NA | NA | NA | NA | ++ | NA | FFPE tissues | CD57 | ↑ NK cell infiltration | IHC | Coca et al., 1997 [31] | |
| NA | NA | NA | NA | NA | NA | ++ | FFPE tissues | Nkp46 | ↑ NK cell infiltration | IHC | Melaniu et al., 2020 [38] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Valderrey, A.; Alvarez, M.; De Maria, A.; Margolin, K.; Melero, I.; Ascierto, M.L. Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity. Cells 2022, 11, 3147. https://doi.org/10.3390/cells11193147
Mendoza-Valderrey A, Alvarez M, De Maria A, Margolin K, Melero I, Ascierto ML. Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity. Cells. 2022; 11(19):3147. https://doi.org/10.3390/cells11193147
Chicago/Turabian StyleMendoza-Valderrey, Alberto, Maite Alvarez, Andrea De Maria, Kim Margolin, Ignacio Melero, and Maria Libera Ascierto. 2022. "Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity" Cells 11, no. 19: 3147. https://doi.org/10.3390/cells11193147
APA StyleMendoza-Valderrey, A., Alvarez, M., De Maria, A., Margolin, K., Melero, I., & Ascierto, M. L. (2022). Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity. Cells, 11(19), 3147. https://doi.org/10.3390/cells11193147







