Crosstalk between Extracellular Matrix Stiffness and ROS Drives Endometrial Repair via the HIF-1α/YAP Axis during Menstruation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Mouse Model of Menstruation and Treatment Regimens
2.3. Primary Culture and Treatment
2.4. Immunofluorescence
2.5. Measurement of Intracellular ROS Generation
2.6. Cell Cycle Analysis
2.7. Total Antioxidant Ability and Antioxidant Enzyme Activity in the Uterus
2.8. qRT-PCR
2.9. Western Blots
2.10. Histological Analysis
2.11. TUNEL Assay
2.12. Statistics and Reproducibility
3. Results
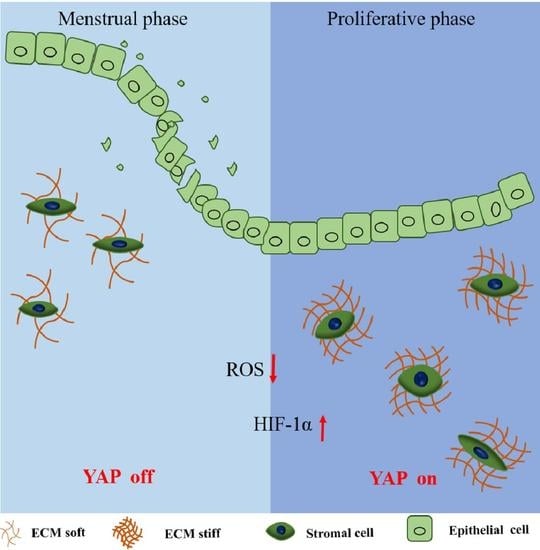
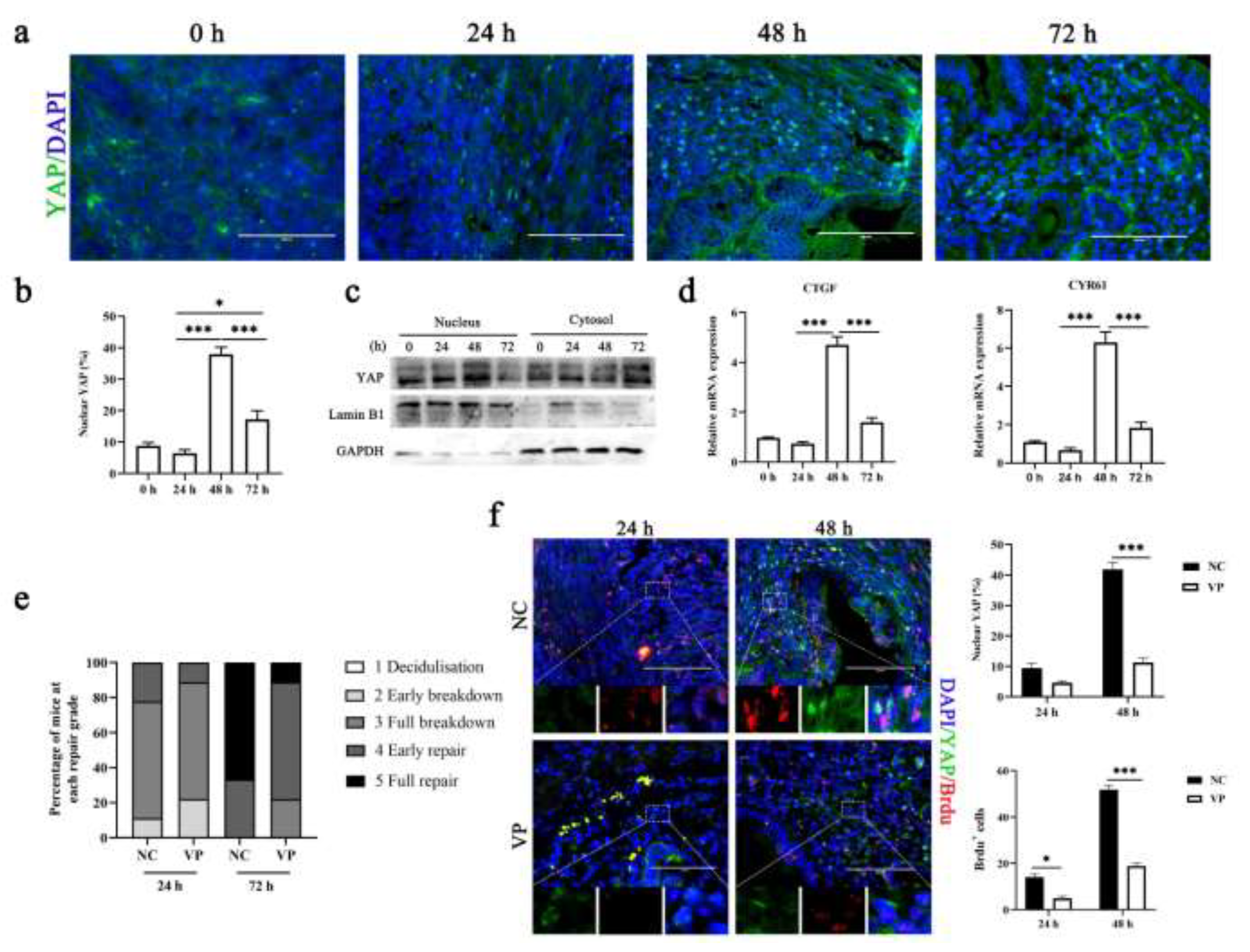
3.1. Mechanotransduction Signaling of ECM Stiffness Is Involved in Mouse Menstrual Progression

3.2. Inhibition of YAP Activation Prevents Efficient Repair
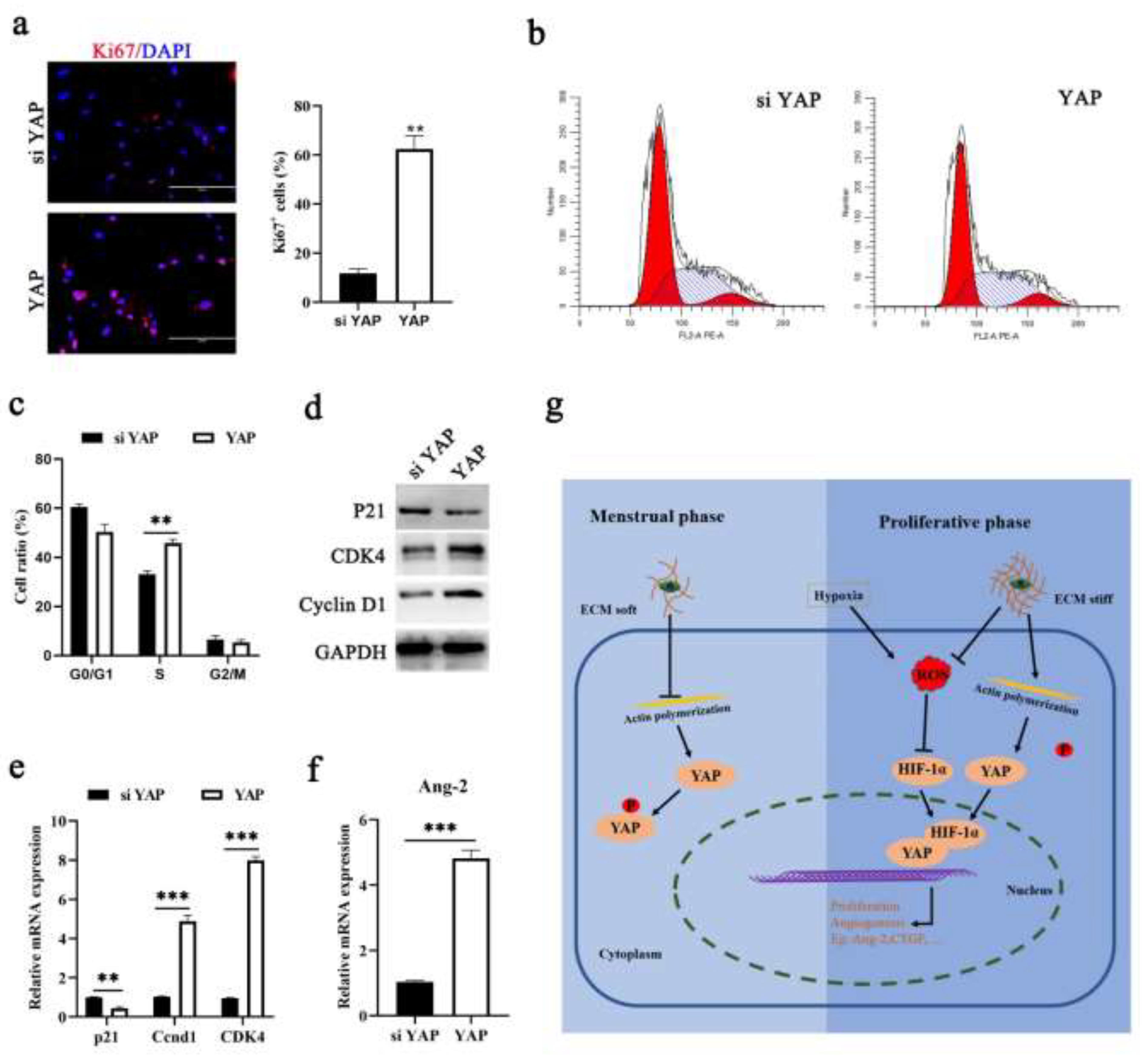
3.3. Hypoxia Can Activate YAP by Increasing HIF-1α Expression
3.4. ECM Stiffness Mediated the Activation of HIF-1α and ROS Production by Regulating YAP Activity
3.5. YAP Activation Is Required for Endometrial Regeneration during Menstruation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jain, V.; Chodankar, R.R.; Maybin, J.A.; Critchley, H.O.D. Uterine bleeding: How understanding endometrial physiology underpins menstrual health. Nat. Rev. Endocrinol. 2022, 18, 290–308. [Google Scholar] [CrossRef]
- Bellofiore, N.; Rana, S.; Dickinson, H.; Temple-Smith, P.; Evans, J. Characterization of human-like menstruation in the spiny mouse: Comparative studies with the human and induced mouse model. Hum. Reprod. 2018, 33, 1715–1726. [Google Scholar] [CrossRef]
- Bohlmann, M.K.; Rath, W. Medical prevention and treatment of postpartum hemorrhage: A comparison of different guidelines. Arch. Gynecol. Obstet. 2014, 289, 555–567. [Google Scholar] [CrossRef]
- Xholli, A.; Simoncini, G.; Vujosevic, S.; Trombetta, G.; Chiodini, A.; Ferraro, M.F.; Cagnacci, A. Menstrual Pain and Elasticity of Uterine Cervix. J. Clin. Med. 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhou, H.J.; Lin, C.; Long, L.; Yang, X.; Zhang, H.; Taylor, H.; Min, W. CD34(+)KLF4(+) Stromal Stem Cells Contribute to Endometrial Regeneration and Repair. Cell Rep. 2019, 27, 2709–2724.e2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.; Arvizu, M.; Rich-Edwards, J.W.; Stuart, J.J.; Manson, J.E.; Missmer, S.A.; Pan, A.; Chavarro, J.E. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: Prospective cohort study. BMJ Clin. Res. Ed. 2020, 371, m3464. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Middleton, L.J.; Tsourapas, A.; Lee, A.J.; Champaneria, R.; Daniels, J.P.; Roberts, T.; Hilken, N.H.; Barton, P.; Gray, R.; et al. Hysterectomy, endometrial ablation and Mirena® for heavy menstrual bleeding: A systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol. Assess. 2011, 15, iii–xvi, 1–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.; Kaitu’u-Lino, T.; Salamonsen, L.A. Extracellular matrix dynamics in scar-free endometrial repair: Perspectives from mouse in vivo and human in vitro studies. Biol. Reprod. 2011, 85, 511–523. [Google Scholar] [CrossRef]
- Yoshii, A.; Kitahara, S.; Ueta, H.; Matsuno, K.; Ezaki, T. Role of uterine contraction in regeneration of the murine postpartum endometrium. Biol. Reprod. 2014, 91, 32. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef]
- Dalrymple, A.; Mahn, K.; Poston, L.; Songu-Mize, E.; Tribe, R.M. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol. Hum. Reprod. 2007, 13, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Asbach, P.; Streitberger, K.J.; Thomas, A.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Guo, S.; Zhou, H.; Wu, Z.; Liu, J.; Qiu, C.; Deng, G. Endometrial extracellular matrix rigidity and IFNτ ensure the establishment of early pregnancy through activation of YAP. Cell Prolif. 2021, 54, e12976. [Google Scholar] [CrossRef]
- Russell, J.O.; Camargo, F.D. Hippo signalling in the liver: Role in development, regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 297–312. [Google Scholar] [CrossRef]
- Sorrentino, G.; Ruggeri, N.; Zannini, A.; Ingallina, E.; Bertolio, R.; Marotta, C.; Neri, C.; Cappuzzello, E.; Forcato, M.; Rosato, A.; et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017, 8, 14073. [Google Scholar] [CrossRef]
- Feng, X.; Degese, M.S.; Iglesias-Bartolome, R.; Vaque, J.P.; Molinolo, A.A.; Rodrigues, M.; Zaidi, M.R.; Ksander, B.R.; Merlino, G.; Sodhi, A.; et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014, 25, 831–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasted, M.; White, C.A.; Kennedy, T.G.; Salamonsen, L.A. Mimicking the events of menstruation in the murine uterus. Biol. Reprod. 2003, 69, 1273–1280. [Google Scholar] [CrossRef]
- Yu, H.F.; Yang, Z.Q.; Xu, M.Y.; Huang, J.C.; Yue, Z.P.; Guo, B. Yap is essential for uterine decidualization through Rrm2/GSH/ROS pathway in response to Bmp2. Int. J. Biol. Sci. 2022, 18, 2261–2276. [Google Scholar] [CrossRef]
- Yu, H.F.; Zheng, L.W.; Yang, Z.Q.; Wang, Y.S.; Wang, T.T.; Yue, Z.P.; Guo, B. TAZ as a novel regulator of oxidative damage in decidualization via Nrf2/ARE/Foxo1 pathway. Exp. Mol. Med. 2021, 53, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitu’u-Lino, T.J.; Morison, N.B.; Salamonsen, L.A. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007, 328, 197–206. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, S.; Zhu, X.; Qiu, J.; Deng, G.; Qiu, C. Alpinetin inhibits breast cancer growth by ROS/NF-kappa B/HIF-1 alpha axis. J. Cell. Mol. Med. 2020, 24, 8430–8440. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
- Coudyzer, P.; Lemoine, P.; Jordan, B.F.; Gallez, B.; Galant, C.; Nisolle, M.; Courtoy, P.J.; Henriet, P.; Marbaix, E. Hypoxia is not required for human endometrial breakdown or repair in a xenograft model of menstruation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 3711–3719. [Google Scholar] [CrossRef] [Green Version]
- Reavey, J.J.; Walker, C.; Nicol, M.; Murray, A.A.; Critchley, H.O.D.; Kershaw, L.E.; Maybin, J.A. Markers of human endometrial hypoxia can be detected in vivo and ex vivo during physiological menstruation. Hum. Reprod. 2021, 36, 941–950. [Google Scholar] [CrossRef]
- Laird, S.M.; Widdowson, R.; El-Sheikhi, M.; Hall, A.J.; Li, T.C. Expression of CXCL12 and CXCR4 in human endometrium; effects of CXCL12 on MMP production by human endometrial cells. Hum. Reprod. 2011, 26, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maybin, J.A.; Battersby, S.; Hirani, N.; Nikitenko, L.L.; Critchley, H.O.; Jabbour, H.N. The expression and regulation of adrenomedullin in the human endometrium: A candidate for endometrial repair. Endocrinology 2011, 152, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lan, X.; Zhao, Y.; Wang, G.; Shi, G.; Li, H.; Hu, Y.; Xu, X.; Zhang, B.; Ye, K.; et al. SDF-1/CXCR4 axis enhances the immunomodulation of human endometrial regenerative cells in alleviating experimental colitis. Stem Cell Res. Ther. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Salamonsen, L.A. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum. Reprod. 2002, 17, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoep, M.E.; Nieboer, T.E.; van der Zanden, M.; Braat, D.D.M.; Nap, A.W. The impact of menstrual symptoms on everyday life: A survey among 42,879 women. Am. J. Obstet. Gynecol. 2019, 220, 569.e1–569.e7. [Google Scholar] [CrossRef]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Smith, L.; Cho, S.; Colasurdo, M.; García, A.J.; Safran, S. Matrix Mechanosensing: From Scaling Concepts in’Omics Data to Mechanisms in the Nucleus, Regeneration, and Cancer. Annu. Rev. Biophys. 2017, 46, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between mechanotransduction and metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape—The rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- He, J.; Bao, Q.; Zhang, Y.; Liu, M.; Lv, H.; Liu, Y.; Yao, L.; Li, B.; Zhang, C.; He, S.; et al. Yes-Associated Protein Promotes Angiogenesis via Signal Transducer and Activator of Transcription 3 in Endothelial Cells. Circ. Res. 2018, 122, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Rehman, J.; Chan, M.; Fu, P.; Dudek, S.M.; Natarajan, V.; Malik, A.B.; Liu, Y. Angiocrine Sphingosine-1-Phosphate Activation of S1PR2-YAP Signaling Axis in Alveolar Type II Cells Is Essential for Lung Repair. Cell Rep. 2020, 31, 107828. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhu, X.Y.; Jin, L.P.; Duan, Z.L.; Li, D.J.; Li, M.Q. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum. Reprod. 2015, 30, 1677–1689. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, J.; He, B.; Li, Y.; Liu, S.; Wu, B.; Wang, S.; Zhang, S.; Xu, X.; Wang, J. Vascular endothelial growth factor (VEGF) regulation by hypoxia inducible factor-1 alpha (HIF1A) starts and peaks during endometrial breakdown, not repair, in a mouse menstrual-like model. Hum. Reprod. 2015, 30, 2160–2170. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.L. Self-Sustained Regulation or Self-Perpetuating Dysregulation: ROS-dependent HIF-YAP-Notch Signaling as a Double-Edged Sword on Stem Cell Physiology and Tumorigenesis. Front. Cell Dev. Biol. 2022, 10, 862791. [Google Scholar] [CrossRef]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, Y.; Wang, Y.; Liu, C.; Han, C. Crosstalk between Extracellular Matrix Stiffness and ROS Drives Endometrial Repair via the HIF-1α/YAP Axis during Menstruation. Cells 2022, 11, 3162. https://doi.org/10.3390/cells11193162
Zhang T, Wang Y, Wang Y, Liu C, Han C. Crosstalk between Extracellular Matrix Stiffness and ROS Drives Endometrial Repair via the HIF-1α/YAP Axis during Menstruation. Cells. 2022; 11(19):3162. https://doi.org/10.3390/cells11193162
Chicago/Turabian StyleZhang, Tao, Yan Wang, Yingnan Wang, Cuiyan Liu, and Chunyang Han. 2022. "Crosstalk between Extracellular Matrix Stiffness and ROS Drives Endometrial Repair via the HIF-1α/YAP Axis during Menstruation" Cells 11, no. 19: 3162. https://doi.org/10.3390/cells11193162