Nephropathic Cystinosis: Pathogenic Roles of Inflammation and Potential for New Therapies
Abstract
:1. Introduction
2. Role of Macrophages in Cystinosis
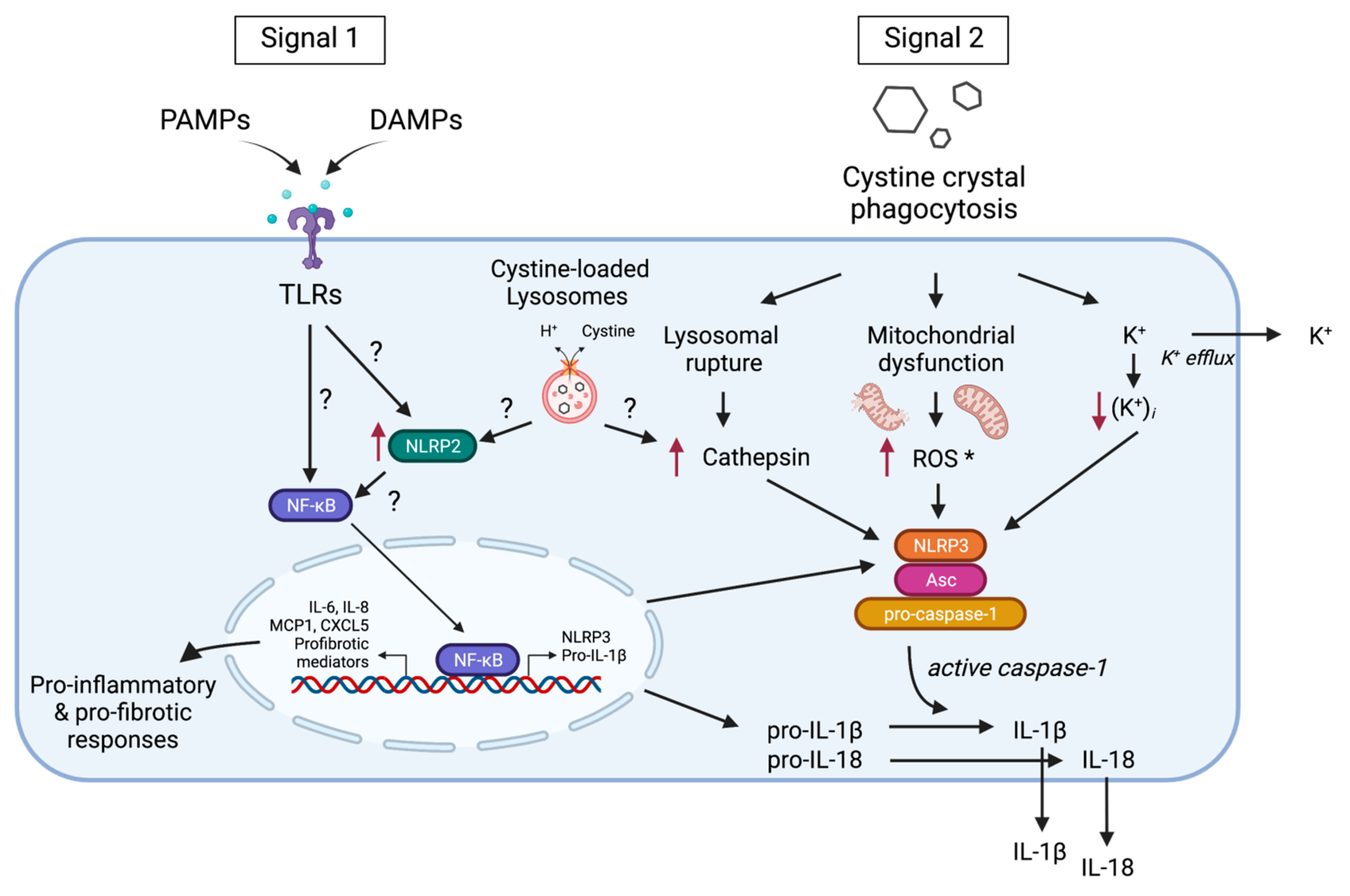
3. Inflammasome Activation in Cystinosis
4. Interplay between Inflammation, Autophagy and Apoptosis in Cystinosis
5. Response to Cysteamine Therapy in Cystinosis
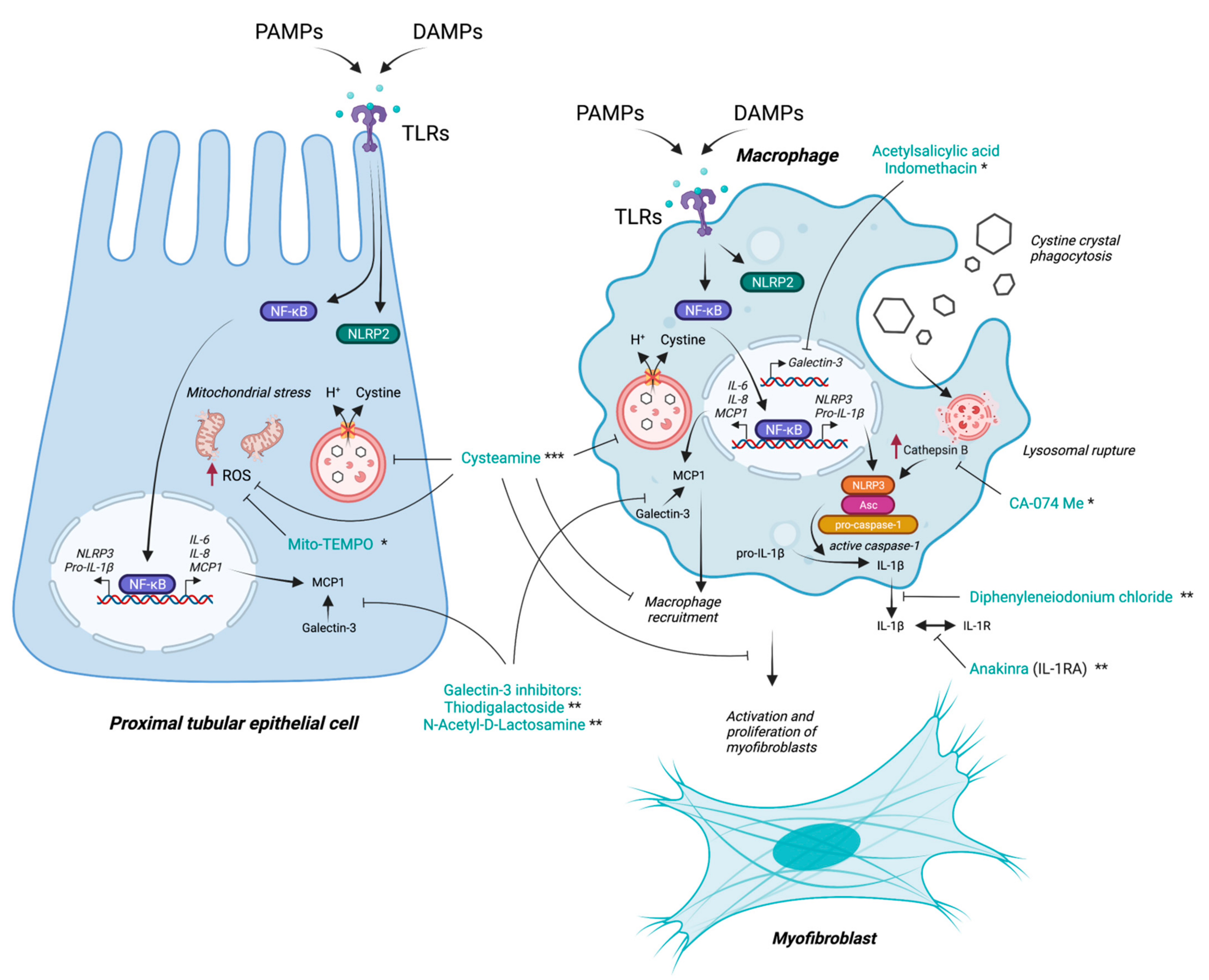
6. Targeting Inflammation as a Potential Therapeutic Option in Cystinosis
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.R.; Canales, B.K.; Dominguez-Gutierrez, P.R. Randall’s plaque and calcium oxalate stone formation: Role for immunity and inflammation. Nat. Rev. Nephrol. 2021, 17, 417–433. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.-J. Crystallopathies. N. Engl. J. Med. 2016, 374, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-H.; Luo, X.-B.; Li, Q.; Zhong, H.-Q. The systematic classification of urinary stones combine-using FTIR and SEM-EDAX. Int. J. Surg. 2017, 41, 150–161. [Google Scholar] [CrossRef]
- Elmonem, M.A.; Veys, K.R.; Soliman, N.A.; van Dyck, M.; van den Heuvel, L.P.; Levtchenko, E. Cystinosis: A review. Orphanet J. Rare Dis. 2016, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesterova, G.; Gahl, W.A. Cystinosis: The evolution of a treatable disease. Pediatr. Nephrol. 2013, 28, 51–59. [Google Scholar] [CrossRef] [Green Version]
- David, D.; Berlingerio, S.P.; Elmonem, M.A.; Arcolino, F.O.; Soliman, N.; van den Heuvel, B.; Gijsbers, R.; Levtchenko, E. Molecular Basis of Cystinosis: Geographic Distribution, Functional Consequences of Mutations in the CTNS Gene, and Potential for Repair. Nephron 2019, 141, 133–146. [Google Scholar] [CrossRef]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef]
- Park, M.A.; Pejovic, V.; Kerisit, K.G.; Junius, S.; Thoene, J.G. Increased Apoptosis in Cystinotic Fibroblasts and Renal Proximal Tubule Epithelial Cells Results from Cysteinylation of Protein Kinase Cδ. J. Am. Soc. Nephrol. 2006, 17, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Arcolino, F.O.; Elmonem, M.A.; Rastaldi, M.P.; Giardino, L.; Cornelissen, E.M.; van den Heuvel, L.P.; Levtchenko, E.N. Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int. 2016, 89, 1037–1048. [Google Scholar] [CrossRef]
- Wilmer, M.J.; van den Heuvel, L.P.; Levtchenko, E.N. The Use of CDME in Cystinosis Research. Neurochem. Res. 2008, 33, 2373–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherqui, S.; Courtoy, P.J. The renal Fanconi syndrome in cystinosis: Pathogenic insights and therapeutic perspectives. Nat. Rev. Nephrol. 2017, 13, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, F.; Signorile, A.; Tamma, G.; Ranieri, M.; Emma, F.; De Rasmo, D. Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis. Cell. Mol. Life Sci. 2018, 75, 3411–3422. [Google Scholar] [CrossRef]
- Ivanova, E.A.; De Leo, M.G.; van den Heuvel, L.; Pastore, A.; Dijkman, H.; De Matteis, M.A.; Levtchenko, E.N. Endo-Lysosomal Dysfunction in Human Proximal Tubular Epithelial Cells Deficient for Lysosomal Cystine Transporter Cystinosin. PLoS ONE 2015, 10, e0120998. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; van den Heuvel, L.P.; Elmonem, M.; De Smedt, H.; Missiaen, L.; Pastore, A.; Mekahli, D.; Bultynck, G.; Levtchenko, E.N. Altered mTOR signalling in nephropathic cystinosis. J. Inherit. Metab. Dis. 2016, 39, 457–464. [Google Scholar] [CrossRef]
- Rega, L.R.; Polishchuk, E.; Montefusco, S.; Napolitano, G.; Tozzi, G.; Zhang, J.; Bellomo, F.; Taranta, A.; Pastore, A.; Polishchuk, R.; et al. Activation of the transcription factor EB rescues lysosomal abnormalities in cystinotic kidney cells. Kidney Int. 2016, 89, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Elmonem, A.M.; Makar, S.H.; van den Heuvel, L.; Abdelaziz, H.; Abdelrahman, S.M.; Bossuyt, X.; Janssen, M.C.; Cornelissen, E.A.; Lefeber, D.J.; AB Joosten, L.; et al. Clinical utility of chitotriosidase enzyme activity in nephropathic cystinosis. Orphanet J. Rare Dis. 2014, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prencipe, G.; Caiello, I.; Cherqui, S.; Whisenant, T.; Petrini, S.; Emma, F.; De Benedetti, F. Inflammasome Activation by Cystine Crystals: Implications for the Pathogenesis of Cystinosis. J. Am. Soc. Nephrol. 2014, 25, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Stokes, M.; Jernigan, S.; D’Agati, V. Infantile nephropathic cystinosis. Kidney Int. 2008, 73, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.; Walker, P.D.; Thoene, J.G. The incidence of atubular glomeruli in nephropathic cystinosis renal biopsies. Mol. Genet. Metab. 2010, 101, 417–420. [Google Scholar] [CrossRef]
- Lobry, T.; Miller, R.; Nevo, N.; Rocca, C.J.; Zhang, J.; Catz, S.D.; Moore, F.; Thomas, L.; Pouly, D.; Bailleux, A.; et al. Interaction between galectin-3 and cystinosin uncovers a pathogenic role of inflammation in kidney involvement of cystinosis. Kidney Int. 2019, 96, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okusa, M.D. Macrophages, Dendritic Cells, and Kidney Ischemia-Reperfusion Injury. Semin. Nephrol. 2010, 30, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Monier, L.; Mauvieux, L. Cystine crystals in bone marrow aspirate. Blood 2015, 126, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherqui, S.; Sevin, C.; Hamard, G.; Kalatzis, V.; Sich, M.; Pequignot, M.O.; Gogat, K.; Abitbol, M.; Broyer, M.; Gubler, M.-C.; et al. Intralysosomal Cystine Accumulation in Mice Lacking Cystinosin, the Protein Defective in Cystinosis. Mol. Cell. Biol. 2002, 22, 7622–7632. [Google Scholar] [CrossRef] [Green Version]
- Korn, D. Demonstration of Cystine Crystals in Peripheral White Blood Cells in a Patient with Cystinosis. N. Engl. J. Med. 1960, 262, 545–548. [Google Scholar] [CrossRef]
- Lee, H.; Fessler, M.B.; Qu, P.; Heymann, J.; Kopp, J.B. Macrophage polarization in innate immune responses contributing to pathogenesis of chronic kidney disease. BMC Nephrol. 2020, 21, 270. [Google Scholar] [CrossRef]
- Veys, K.R.; Elmonem, M.A.; van Dyck, M.; Janssen, M.C.; Cornelissen, E.A.; Hohenfellner, K.; Prencipe, G.; van den Heuvel, L.P.; Levtchenko, E. Chitotriosidase as a Novel Biomarker for Therapeutic Monitoring of Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2020, 31, 1092–1106. [Google Scholar] [CrossRef]
- Ries, M.; Schaefer, E.; Lührs, T.; Mani, L.; Kuhn, J.; Vanier, M.T.; Krummenauer, F.; Gal, A.; Beck, M.; Mengel, E. Critical assessment of chitotriosidase analysis in the rational laboratory diagnosis of children with Gaucher disease and Niemann–Pick disease type A/B and C. J. Inherit. Metab. Dis. 2006, 29, 647–652. [Google Scholar] [CrossRef]
- Elmonem, M.A.; van den Heuvel, L.P.; Levtchenko, E.N. Immunomodulatory Effects of Chitotriosidase Enzyme. Enzym. Res. 2016, 2016, 2682680. [Google Scholar] [CrossRef] [Green Version]
- Mulay, S.R.; Desai, J.; Kumar, S.V.; Eberhard, J.N.; Thomasova, D.; Romoli, S.; Grigorescu, M.; Kulkarni, O.P.; Popper, B.; Vielhauer, V.; et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat. Commun. 2016, 7, 10274. [Google Scholar] [CrossRef] [PubMed]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.-J. Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat. Rev. Nephrol. 2017, 13, 226–240. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Franklin, B.S.; Mangan, M.S.; Latz, E. Crystal Formation in Inflammation. Annu. Rev. Immunol. 2016, 34, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. Macrophage Recognition of Crystals and Nanoparticles. Front. Immunol. 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Shi, C.; Ma, X.; Anders, H.J. Novel Insights into Crystal-Induced Kidney Injury. Kidney Dis. 2018, 4, 49–57. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Cheung, W.W.; Hao, S.; Zheng, R.; Wang, Z.; Gonzalez, A.; Zhou, P.; Hoffman, H.M.; Mak, R.H. Targeting interleukin-1 for reversing fat browning and muscle wasting in infantile nephropathic cystinosis. J. Cachexia Sarcopenia Muscle 2021, 12, 1296–1311. [Google Scholar] [CrossRef]
- Leemans, J.C.; Kors, L.; Anders, H.-J.; Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 2014, 10, 398–414. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, Y.G.; Kim, D.-J.; Park, S.H.; Jeong, K.-H.; Lee, Y.H.; Lim, S.J.; Lee, S.-H.; Moon, J.-Y. Inflammasome-Independent Role of NLRP3 Mediates Mitochondrial Regulation in Renal Injury. Front. Immunol. 2018, 9, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, J.P.Y.; Duncan, J.A.; Lei, Y. How the Noninflammasome NLRs Function in the Innate Immune System. Science 2010, 327, 286–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.N.; Pascarella, A.; Licursi, V.; Caiello, I.; Taranta, A.; Rega, L.R.; Levtchenko, E.; Emma, F.; De Benedetti, F.; Prencipe, G. NLRP2 Regulates Proinflammatory and Antiapoptotic Responses in Proximal Tubular Epithelial Cells. Front. Cell Dev. Biol. 2019, 7, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagoo, P.; Chan, G.; Larkin, D.F.P.; George, A.J.T. Inflammatory Cytokines Induce Apoptosis of Corneal Endothelium through Nitric Oxide. Investig. Opthalmol. Vis. Sci. 2004, 45, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Størling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; Mandrup-Poulsen, T. Proinflammatory Cytokines Activate the Intrinsic Apoptotic Pathway in β-Cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.K.; Cha, D.R.; Cho, W.Y.; Kim, H.K.; Chang, K.H.; Yun, S.Y.; Won, N.H. Inflammatory Cytokines and Lipopolysaccharide Induce Fas-Mediated Apoptosis in Renal Tubular Cells. Nephron 2002, 91, 406–415. [Google Scholar] [CrossRef]
- Collier, J.J.; Burke, S.J.; Eisenhauer, M.E.; Lu, D.; Sapp, R.C.; Frydman, C.J.; Campagna, S. Pancreatic β-Cell Death in Response to Pro-Inflammatory Cytokines Is Distinct from Genuine Apoptosis. PLoS ONE 2011, 6, e22485. [Google Scholar] [CrossRef]
- Messer, J.S. The cellular autophagy/apoptosis checkpoint during inflammation. Cell. Mol. Life Sci. 2017, 74, 1281–1296. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Ding, Z.; Khaidakov, M.; Mercanti, F.; Mehta, J.L. Regulation of autophagy and apoptosis in response to angiotensin II in HL-1 cardiomyocytes. Biochem. Biophys. Res. Commun. 2013, 440, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Lambelet, M.; Terra, L.F.; Fukaya, M.; Meyerovich, K.; Labriola, L.; Cardozo, A.K.; Allagnat, F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis. 2018, 9, 92. [Google Scholar] [CrossRef]
- Sansanwal, P.; Yen, B.; Gahl, W.A.; Ma, Y.; Ying, L.; Wong, L.-J.C.; Sarwal, M.M. Mitochondrial Autophagy Promotes Cellular Injury in Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2010, 21, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansanwal, P.; Sarwal, M.M. Abnormal mitochondrial autophagy in nephropathic cystinosis. Autophagy 2010, 6, 971–973. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Kim, S.-J.; Zhang, Z.; Tsai, P.-C.; Wisniewski, K.E.; Mukherjee, A.B. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum. Mol. Genet. 2008, 17, 469–477. [Google Scholar] [CrossRef]
- Chevronnay, H.P.G.; Janssens, V.; van der Smissen, P.; Liao, X.H.; Abid, Y.; Nevo, N.; Antignac, C.; Refetoff, S.; Cherqui, S.; Pierreux, C.; et al. A Mouse Model Suggests Two Mechanisms for Thyroid Alterations in Infantile Cystinosis: Decreased Thyroglobulin Synthesis Due to Endoplasmic Reticulum Stress/Unfolded Protein Response and Impaired Lysosomal Processing. Endocrinology 2015, 156, 2349–2364. [Google Scholar] [CrossRef] [Green Version]
- Sansanwal, P.; Kambham, N.; Sarwal, M.M. Caspase-4 may play a role in loss of proximal tubules and renal injury in nephropathic cystinosis. Pediatr. Nephrol. 2010, 25, 105–109. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Plantinga, T.; van de Veerdonk, F.L.; Smit, J.W.; Netea, M.G. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy 2016, 12, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukseree, S.; Bakiri, L.; Palomo-Irigoyen, M.; Uluçkan, Ö; Petzelbauer, P.; Wagner, E.F. Sequestosome 1/p62 enhances chronic skin inflammation. J. Allergy Clin. Immunol. 2021, 147, 2386–2393.e4. [Google Scholar] [CrossRef]
- Lappas, M. The Adaptor Protein p62 Mediates Nuclear Factor κB Activation in Response to Inflammation and Facilitates the Formation of Prolabor Mediators in Human Myometrium. Reprod. Sci. 2017, 24, 762–772. [Google Scholar] [CrossRef]
- Sansanwal, P.; Sarwal, M.M. p62/SQSTM1 prominently accumulates in renal proximal tubules in nephropathic cystinosis. Pediatr. Nephrol. 2012, 27, 2137–2144. [Google Scholar] [CrossRef]
- Festa, B.P.; Chen, Z.; Berquez, M.; Debaix, H.; Tokonami, N.; Prange, J.A.; van de Hoek, G.; Alessio, C.; Raimondi, A.; Nevo, N.; et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 2018, 9, 161. [Google Scholar] [CrossRef]
- De Leo, E.; Elmonem, M.A.; Berlingerio, S.P.; Berquez, M.; Festa, B.P.; Raso, R.; Bellomo, F.; Starborg, T.; Janssen, M.J.; Abbaszadeh, Z.; et al. Cell-Based Phenotypic Drug Screening Identifies Luteolin as Candidate Therapeutic for Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2020, 31, 1522–1537. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscat, J.; Diaz-Meco, M.T. p62 at the Crossroads of Autophagy, Apoptosis, and Cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veys, K.R.; Elmonem, M.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E. Nephropathic cystinosis: An update. Curr. Opin. Pediatr. 2017, 29, 168–178. [Google Scholar] [CrossRef]
- Cherqui, S. Cysteamine therapy: A treatment for cystinosis, not a cure. Kidney Int. 2012, 81, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Gahl, W.A.; Balog, J.Z.; Kleta, R. Nephropathic Cystinosis in Adults: Natural History and Effects of Oral Cysteamine Therapy. Ann. Intern. Med. 2007, 147, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Van Stralen, K.J.; Emma, F.; Jager, K.J.; Verrina, E.E.; Schaefer, F.; Laube, G.F.; Lewis, M.A.; Levtchenko, E.N. Improvement in the Renal Prognosis in Nephropathic Cystinosis. Clin. J. Am. Soc. Nephrol. 2011, 6, 2485–2491. [Google Scholar] [CrossRef] [Green Version]
- Okamura, D.M.; Bahrami, N.M.; Ren, S.; Pasichnyk, K.; Williams, J.M.; Gangoiti, J.A.; Lopez-Guisa, J.M.; Yamaguchi, I.; Barshop, B.A.; Duffield, J.S.; et al. Cysteamine Modulates Oxidative Stress and Blocks Myofibroblast Activity in CKD. J. Am. Soc. Nephrol. 2014, 25, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Revesz, L.; Modig, H. Cysteamine-induced Increase of Cellular Glutathione-level: A New Hypothesis of the Radioprotective Mechanism. Nat. Cell Biol. 1965, 207, 430–431. [Google Scholar] [CrossRef]
- Besouw, M.; Masereeuw, R.; van den Heuvel, L.; Levtchenko, E. Cysteamine: An old drug with new potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, M.J.; Kluijtmans, L.A.; van der Velden, T.J.; Willems, P.H.; Scheffer, P.G.; Masereeuw, R.; Monnens, L.A.; van den Heuvel, L.P.; Levtchenko, E.N. Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Elmonem, M.; Khalil, R.; Khodaparast, L.; Khodaparast, L.; Arcolino, F.O.; Morgan, J.; Pastore, A.; Tylzanowski, P.; Ny, A.; Lowe, M.; et al. Cystinosis (ctns) zebrafish mutant shows pronephric glomerular and tubular dysfunction. Sci. Rep. 2017, 7, 42583. [Google Scholar] [CrossRef] [Green Version]
- Elmonem, M.A.; Berlingerio, S.P.; Morgan, J.; Khodaparast, L.; Khodaparast, L.; Lowe, M.; van DenHeuvel, L.P.; Levtchenko, E. Cysteamine improves proximal tubular reabsorption of low molecular weight compounds in cystinotic zebrafish, but has no effect on the defective megalin expression. Pediatr. Nephrol. 2018, 33, 1821. [Google Scholar]
- Haycock, G.B.; Al-Dahhan, J.; Mak, R.H.; Chantler, C. Effect of indomethacin on clinical progress and renal function in cystinosis. Arch. Dis. Child. 1982, 57, 934–939. [Google Scholar] [CrossRef]
- Emma, F.; Nesterova, G.; Langman, C.; Labbé, A.; Cherqui, S.; Goodyer, P.; Janssen, M.C.; Greco, M.; Topaloglu, R.; Elenberg, E.; et al. Nephropathic cystinosis: An international consensus document. Nephrol. Dial. Transplant. 2014, 429, iv87–iv94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emma, F.; Hoff, W.V.; Hohenfellner, K.; Topaloglu, R.; Greco, M.; Ariceta, G.; Bettini, C.; Bockenhauer, D.; Veys, K.; Pape, L.; et al. An international cohort study spanning five decades assessed outcomes of nephropathic cystinosis. Kidney Int. 2021, 100, 1112–1123. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cheung, W.W.; Perens, E.A.; Oliveira, E.A.; Gertler, A.; Mak, R.H. A Leptin Receptor Antagonist Attenuates Adipose Tissue Browning and Muscle Wasting in Infantile Nephropathic Cystinosis-Associated Cachexia. Cells 2021, 10, 1954. [Google Scholar] [CrossRef]
- Dabelic, S.; Flogel, M.; Dumic, J. Effects of aspirin and indomethacin on galectin-3. Croat. Chem. Acta 2005, 78, 433–440. [Google Scholar]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; da Silva, G.B., Jr. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. Braz. J. Nephrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.A.; Marks, E.S.; Deuster, P.A.; O’Connor, F.G.; Kurina, L.M. Association of Nonsteroidal Anti-inflammatory Drug Prescriptions With Kidney Disease Among Active Young and Middle-aged Adults. JAMA Netw. Open 2019, 2, e187896. [Google Scholar] [CrossRef] [Green Version]
- Coppo, R. Corticosteroids in IgA Nephropathy: Lessons from Recent Studies. J. Am. Soc. Nephrol. 2017, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Vilet, J.M.; Ayoub, I. The Use of Glucocorticoids in Lupus Nephritis: New Pathways for an Old Drug. Front. Med. 2021, 8, 622225. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Tanna, A.; Salama, A.D.; Tam, F.W.K.; Cairns, T.; Taube, D.; Cook, H.T.; Ashby, D.; Duncan, N.; Pusey, C.D. Long-term outcome in biopsy-proven acute interstitial nephritis treated with steroids. Clin. Kidney J. 2016, 10, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Koralewski, R.; Dymek, B.; Mazur, M.; Sklepkiewicz, P.; Olejniczak, S.; Czestkowski, W.; Matyszewski, K.; Andryianau, G.; Niedziejko, P.; Kowalski, M.; et al. Discovery of OATD-01, a First-in-Class Chitinase Inhibitor as Potential New Therapeutics for Idiopathic Pulmonary Fibrosis. J. Med. Chem. 2020, 63, 15527–15540. [Google Scholar] [CrossRef]
- Mazur, M.; Zielińska, A.; Grzybowski, M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6966. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.K.; Morita, R.; Kobayashi, Y.; Eisenbarth, S.; Lee, C.G.; Elias, J.; Eynon, E.E.; Flavell, R.A. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc. Natl. Acad. Sci. USA 2015, 112, E2891–E2899. [Google Scholar] [CrossRef] [Green Version]
- Mazur, M.; Dymek, B.; Koralewski, R.; Sklepkiewicz, P.; Olejniczak, S.; Mazurkiewicz, M.; Piotrowicz, M.; Salamon, M.; Jędrzejczak, K.; Zagozdzon, A.; et al. Development of Dual Chitinase Inhibitors as Potential New Treatment for Respiratory System Diseases. J. Med. Chem. 2019, 62, 7126–7145. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmonem, M.A.; Veys, K.R.P.; Prencipe, G. Nephropathic Cystinosis: Pathogenic Roles of Inflammation and Potential for New Therapies. Cells 2022, 11, 190. https://doi.org/10.3390/cells11020190
Elmonem MA, Veys KRP, Prencipe G. Nephropathic Cystinosis: Pathogenic Roles of Inflammation and Potential for New Therapies. Cells. 2022; 11(2):190. https://doi.org/10.3390/cells11020190
Chicago/Turabian StyleElmonem, Mohamed A., Koenraad R. P. Veys, and Giusi Prencipe. 2022. "Nephropathic Cystinosis: Pathogenic Roles of Inflammation and Potential for New Therapies" Cells 11, no. 2: 190. https://doi.org/10.3390/cells11020190
APA StyleElmonem, M. A., Veys, K. R. P., & Prencipe, G. (2022). Nephropathic Cystinosis: Pathogenic Roles of Inflammation and Potential for New Therapies. Cells, 11(2), 190. https://doi.org/10.3390/cells11020190






