Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treadmill Exercise Protocol
2.3. Eight-Arm Radial Maze Test
2.4. Golgi Staining
2.5. Western Blot
2.6. Electron Microscopy
2.7. Statistics
3. Results
3.1. Treadmill Exercise Prevented Decline in Spatial Learning and Memory in 3×Tg-AD Mice
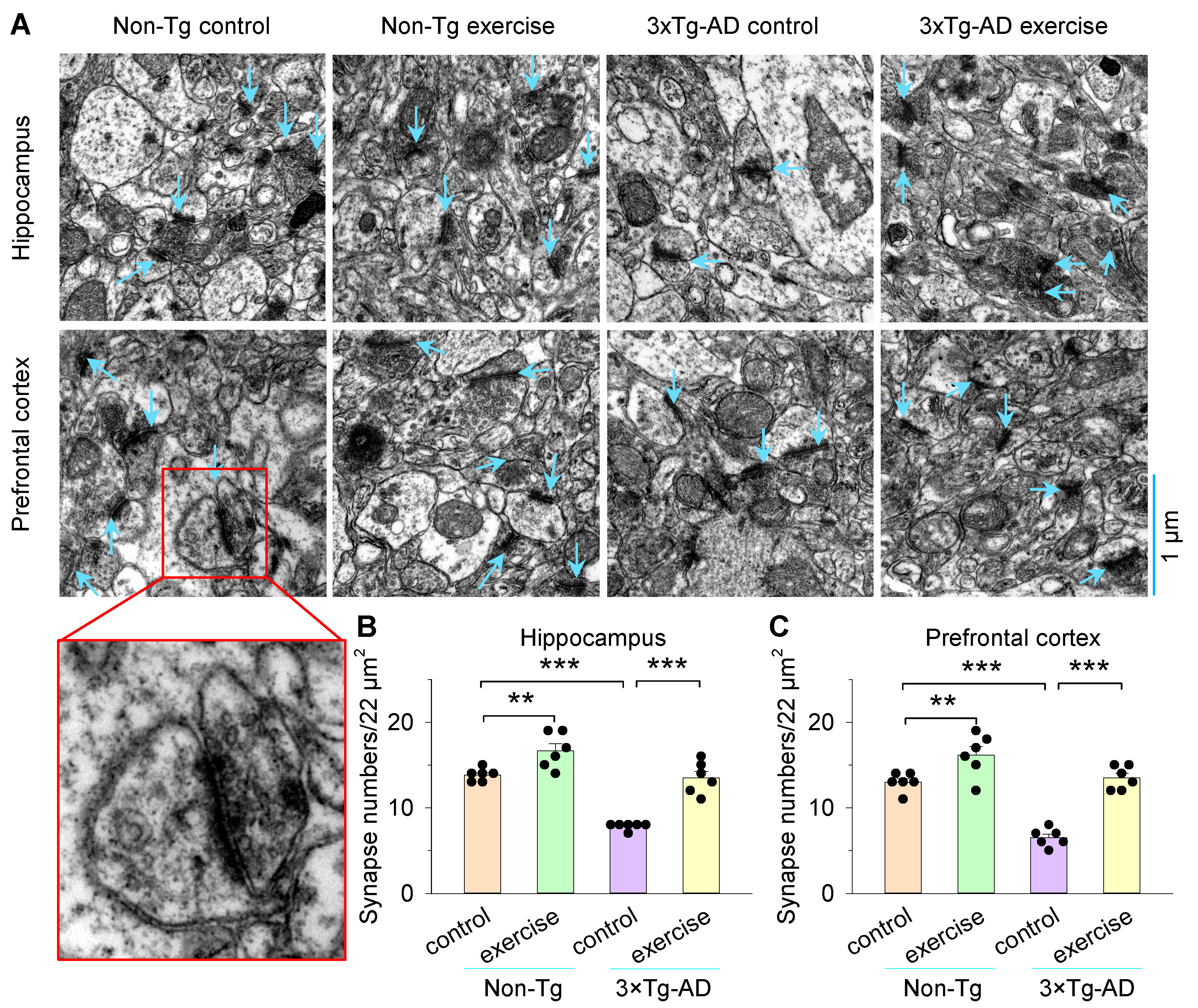
3.2. Treadmill Exercise Increased Synapse Numbers and Improved Synaptic Structural Parameters of the Hippocampus and Prefrontal Cortex in 3×Tg-AD Mice
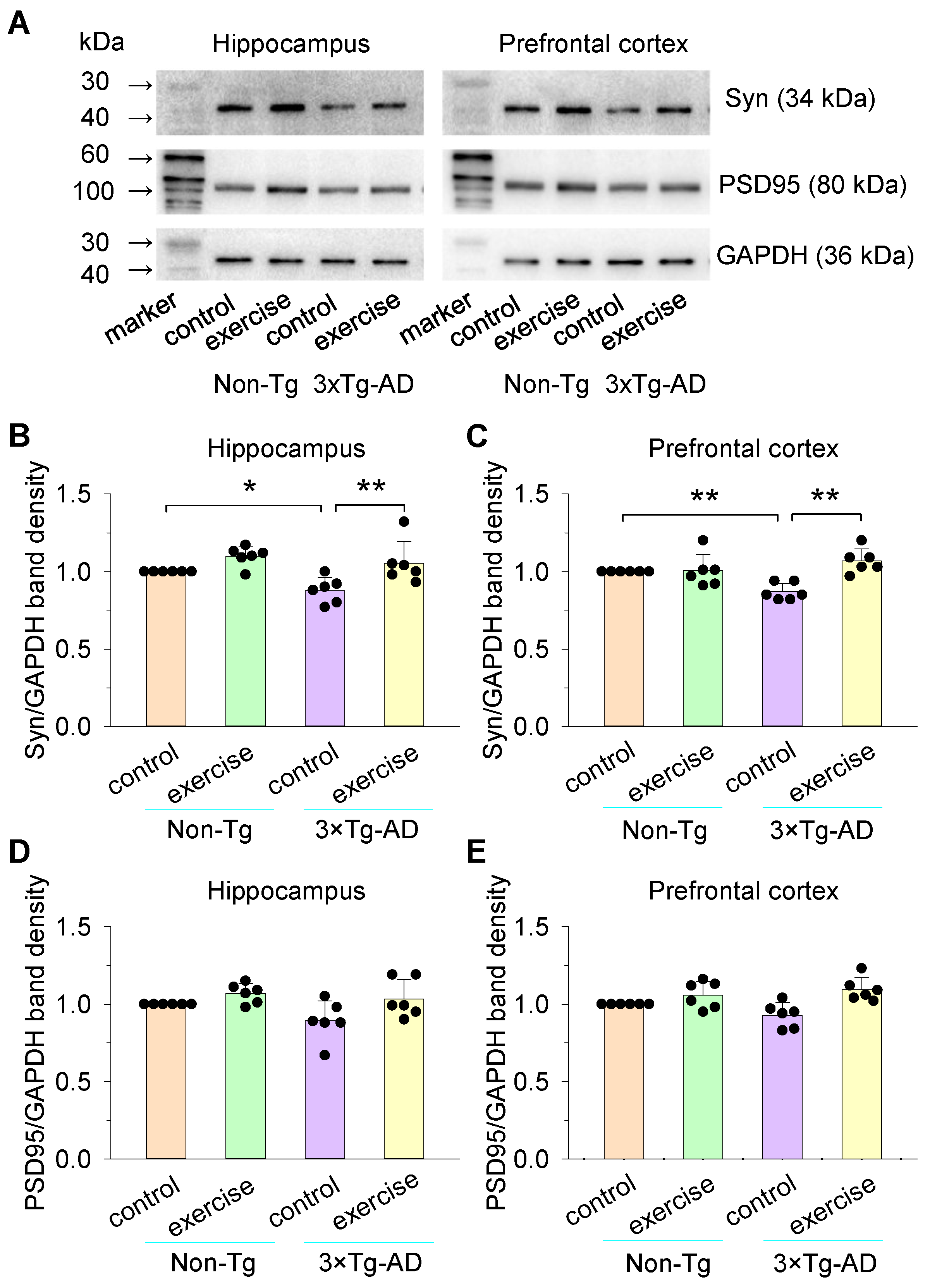
3.3. Effects of Treadmill Exercise on the Expression of Synaptophysin (Syn) and PSD95 of the Hippocampus and Prefrontal Cortex in 3×Tg-AD Mice
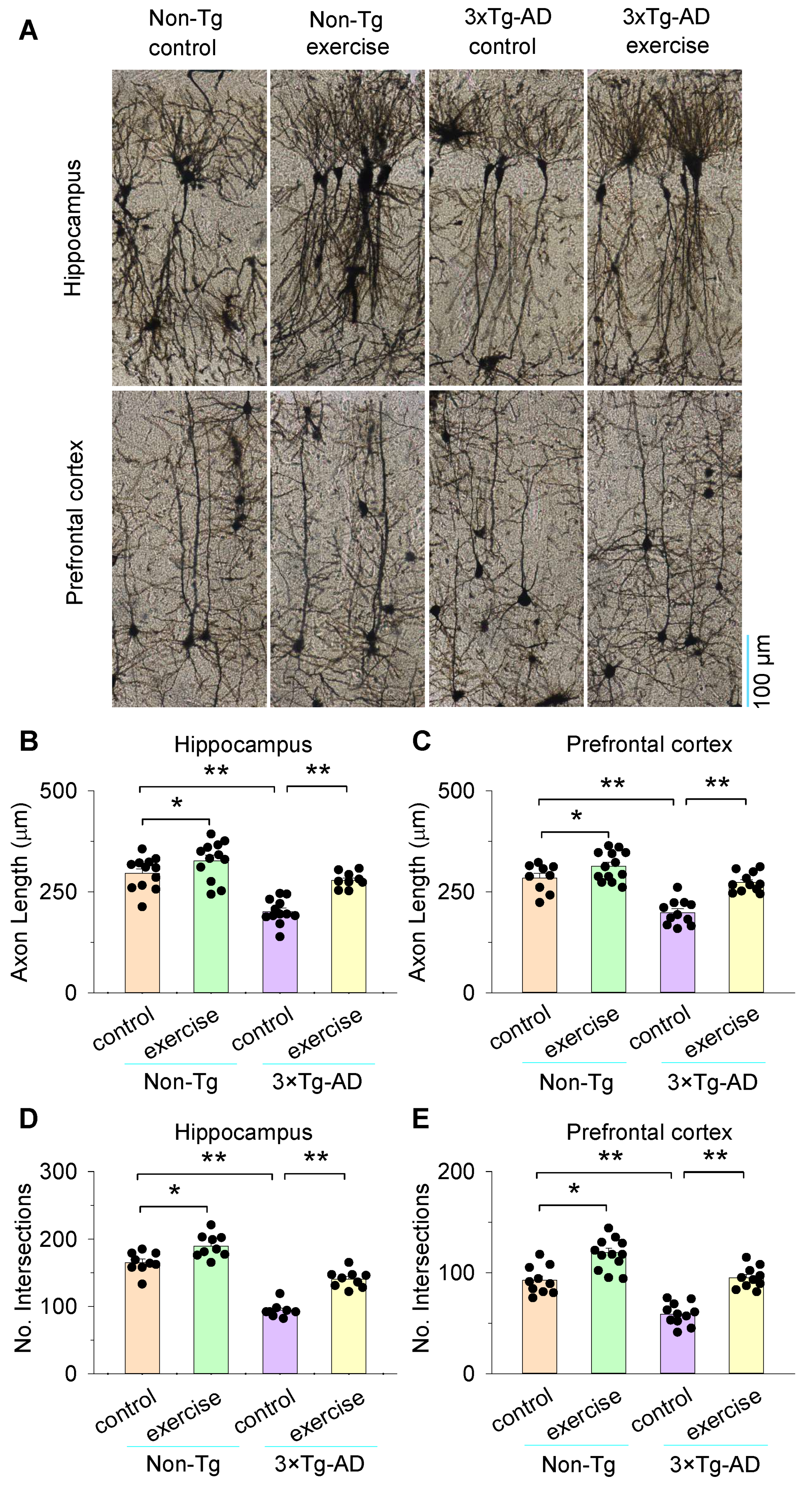
3.4. Treadmill Exercise Enhanced the Axon Length and Dendritic Complexity of the Hippocampus and Prefrontal Cortex in 3×Tg-AD Mice
3.5. Treadmill Exercise Improved the Numbers of Dendritic Spines of the Hippocampus and Prefrontal Cortex in 3×Tg-AD Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.; Okada, J.; Jung, M.W.; Kim, J.J. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn. Mem. 2008, 15, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Laroche, S.; Davis, S.; Jay, T.M. Plasticity at hippocampal to prefrontal cortex synapses: Dual roles in working memory and consolidation. Hippocampus 2000, 10, 438–446. [Google Scholar] [CrossRef]
- Puzzo, D.; Argyrousi, E.K.; Staniszewski, A.; Zhang, H.; Calcagno, E.; Zuccarello, E.; Acquarone, E.; Fa, M.; Li Puma, D.D.; Grassi, C.; et al. Tau is not necessary for amyloid-β-induced synaptic and memory impairments. J. Clin. Investig. 2020, 130, 4831–4844. [Google Scholar] [CrossRef]
- Selkoe, D.J. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008, 192, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Laurén, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Yuan, L.; Yang, D.; Han, W.N.; Li, Q.S.; Yang, W.; Liu, Q.S.; Qi, J.S. Melatonin protects against amyloid-beta-induced impairments of hippocampal LTP and spatial learning in rats. Synapse 2013, 67, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-García, L.E.; Isla, A.G.; Andrade-Talavera, Y.; Balleza-Tapia, H.; Loera-Valencia, R.; Alvarez-Jimenez, L.; Pizzirusso, G.; Tambaro, S.; Nilsson, P.; Fisahn, A. Impaired spike-gamma coupling of area CA3 fast-spiking interneurons as the earliest functional impairment in the App(NL-G-F) mouse model of Alzheimer’s disease. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Saito, T.; Matsuba, Y.; Mihira, N.; Takano, J.; Nilsson, P.; Itohara, S.; Iwata, N.; Saido, T.C. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 2014, 17, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.F.; White, G.L.; Jones, M.W.; Cooper-Blacketer, D.; Marshall, V.J.; Irizarry, M.; Younkin, L.; Good, M.A.; Bliss, T.V.; Hyman, B.T.; et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999, 2, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.S.; Edwards, G., 3rd; Levites, Y.; Kumar, A.; Myers, C.E.; Gluck, M.A.; Setlow, B.; Bizon, J.L. Deficits in hippocampal-dependent transfer generalization learning accompany synaptic dysfunction in a mouse model of amyloidosis. Hippocampus 2016, 26, 455–471. [Google Scholar] [CrossRef] [Green Version]
- Crouzin, N.; Baranger, K.; Cavalier, M.; Marchalant, Y.; Cohen-Solal, C.; Roman, F.S.; Khrestchatisky, M.; Rivera, S.; Féron, F.; Vignes, M. Area-specific alterations of synaptic plasticity in the 5XFAD mouse model of Alzheimer’s disease: Dissociation between somatosensory cortex and hippocampus. PLoS ONE 2013, 8, e74667. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, K.; Bouter, Y.; Müller, M.; Bayer, T.A. Synaptic Alterations in Mouse Models for Alzheimer Disease-A Special Focus on N-Truncated Abeta 4-42. Molecules 2018, 23, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.Y.; Toga, A.W.; Thompson, P.M. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Norfray, J.F.; Provenzale, J.M. Alzheimer’s disease: Neuropathologic findings and recent advances in imaging. AJR Am. J. Roentgenol. 2004, 182, 3–13. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Tang, J.; Sun, J.; Gao, J.; Wu, T.; Xiao, M. Voluntary exercise counteracts Aβ25-35-induced memory impairment in mice. Behav. Brain Res. 2013, 256, 618–625. [Google Scholar] [CrossRef]
- Azimi, M.; Gharakhanlou, R.; Naghdi, N.; Khodadadi, D.; Heysieattalab, S. Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/FNDC5/BDNF pathway. Peptides 2018, 102, 78–88. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; García, Y.; Buccieri, K.; Revilla, S.; Suñol, C.; Cristofol, R.; Sanfeliu, C. Gender-Specific Neuroimmunoendocrine Response to Treadmill Exercise in 3xTg-AD Mice. Int. J. Alzheimers Dis. 2010, 2010, 128354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkadhi, K.A.; Dao, A.T. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 86, 25–29. [Google Scholar] [CrossRef] [PubMed]
- George, E.K.; Reddy, P.H. Can Healthy Diets, Regular Exercise, and Better Lifestyle Delay the Progression of Dementia in Elderly Individuals? J. Alzheimers Dis. JAD 2019, 72, S37–S58. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Rodrigues, C.M.; Mendes, B.F.; Improta-Caria, A.C.; Peixoto, M.F.D.; Cassilhas, R.C. Physical exercise protocols in animal models of Alzheimer’s disease: A systematic review. Metab. Brain Dis. 2021, 36, 85–95. [Google Scholar] [CrossRef]
- Koo, J.H.; Kang, E.B.; Oh, Y.S.; Yang, D.S.; Cho, J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 288, 142–152. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Sousa, R.A.L.; Lima, N.S.; Amorim, F.T.; Gripp, F.; Magalhães, C.; Pinto, S.H.; Dias-Peixoto, M.F.; Monteiro-Junior, R.S.; Bourbeau, K.; Cassilhas, R.C. Endurance and high-intensity interval training improve the levels of anxiety and quality of life in overweight men. Rev. Assoc. Med. Bras. (1992) 2021, 67, 1177–1181. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Gu, B.; Cai, J.; Lv, Y.; Yu, L. Aerobic exercise regulates Rho/cofilin pathways to rescue synaptic loss in aged rats. PLoS ONE 2017, 12, e0171491. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, K.H.; Lee, J. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol. Med. Rep. 2016, 13, 2981–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.M.; Dong, Z.Q.; Li, Q.; Chen, X. Treadmill training improves neurological deficits and suppresses neuronal apoptosis in cerebral ischemic stroke rats. Neural Regen. Res. 2019, 14, 1387–1393. [Google Scholar] [CrossRef]
- Preston, A.R.; Eichenbaum, H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. CB 2013, 23, R764–R773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Talavera, Y.; Rodríguez-Moreno, A. Synaptic Plasticity and Oscillations in Alzheimer’s Disease: A Complex Picture of a Multifaceted Disease. Front. Mol. Neurosci. 2021, 14, 696476. [Google Scholar] [CrossRef]
- Marrone, D.F.; Petit, T.L. The role of synaptic morphology in neural plasticity: Structural interactions underlying synaptic power. Brain Res. Brain Res. Rev. 2002, 38, 291–308. [Google Scholar] [CrossRef]
- Savtchenko, L.P.; Rusakov, D.A. The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. USA 2007, 104, 1823–1828. [Google Scholar] [CrossRef] [Green Version]
- Wahl, L.M.; Pouzat, C.; Stratford, K.J. Monte Carlo simulation of fast excitatory synaptic transmission at a hippocampal synapse. J. Neurophysiol. 1996, 75, 597–608. [Google Scholar] [CrossRef]
- Coba, M.P.; Pocklington, A.J.; Collins, M.O.; Kopanitsa, M.V.; Uren, R.T.; Swamy, S.; Croning, M.D.; Choudhary, J.S.; Grant, S.G. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci. Signal. 2009, 2, ra19. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.L.; Huang, K.P.; Wu, J.; Boucheron, C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 6230–6237. [Google Scholar] [CrossRef]
- Ren, W.J.; Liu, Y.; Zhou, L.J.; Li, W.; Zhong, Y.; Pang, R.P.; Xin, W.J.; Wei, X.H.; Wang, J.; Zhu, H.Q.; et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Son, K.; Palmore, G.T. Neuron Image Analyzer: Automated and Accurate Extraction of Neuronal Data from Low Quality Images. Sci. Rep. 2015, 5, 17062. [Google Scholar] [CrossRef] [Green Version]
- Alcantara-Gonzalez, F.; Juarez, I.; Solis, O.; Martinez-Tellez, I.; Camacho-Abrego, I.; Masliah, E.; Mena, R.; Flores, G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse 2010, 64, 786–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, F.; Gómez-Villalobos Mde, J.; Juarez, I.; Quevedo, L.; Flores, G. Dendritic morphology of neurons in medial prefrontal cortex, hippocampus, and nucleus accumbens in adult SH rats. Synapse 2011, 65, 198–206. [Google Scholar] [CrossRef]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar] [PubMed]
- Ma, X.M.; Huang, J.; Wang, Y.; Eipper, B.A.; Mains, R.E. Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 10593–10603. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Trinh, V.N.; Sears-Kraxberger, I.; Li, K.W.; Steward, O.; Luo, Z.D. Synaptic ultrastructure changes in trigeminocervical complex posttrigeminal nerve injury. J. Comp. Neurol. 2016, 524, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Güldner, F.H. Sexual dimorphisms of axo-spine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neurosci. Lett. 1982, 28, 145–150. [Google Scholar] [CrossRef]
- Orduz, D.; Boom, A.; Gall, D.; Brion, J.P.; Schiffmann, S.N.; Schwaller, B. Subcellular structural plasticity caused by the absence of the fast Ca(2+) buffer calbindin D-28k in recurrent collaterals of cerebellar Purkinje neurons. Front. Cell. Neurosci. 2014, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.G.; Devon, R.M. An ultrastructural study into the effects of pentobarbitone on synaptic organization. Brain Res. 1978, 147, 47–63. [Google Scholar] [CrossRef]
- Cooke, C.T.; Nolan, T.M.; Dyson, S.E.; Jones, D.G. Pentobarbital-induced configurational changes at the synapse. Brain Res. 1974, 76, 330–335. [Google Scholar] [CrossRef]
- Ehrlich, I.; Klein, M.; Rumpel, S.; Malinow, R. PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. USA 2007, 104, 4176–4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokotos, A.C.; Harper, C.B.; Marland, J.R.K.; Smillie, K.J.; Cousin, M.A.; Gordon, S.L. Synaptophysin sustains presynaptic performance by preserving vesicular synaptobrevin-II levels. J. Neurochem. 2019, 151, 28–37. [Google Scholar] [CrossRef]
- Berry, K.P.; Nedivi, E. Spine Dynamics: Are They All the Same? Neuron 2017, 96, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, J.; Harris, K.M. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007, 17, 381–386. [Google Scholar] [CrossRef]
- Holtmaat, A.J.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Lin, A.; Chang, P.; Gan, W.B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 2005, 46, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.M.; Jensen, F.E.; Tsao, B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 2685–2705. [Google Scholar] [CrossRef] [Green Version]
- Bello-Medina, P.C.; Prado-Alcalá, R.A.; Rivas-Arancibia, S. Effect of Ozone Exposure on Dendritic Spines of CA1 Pyramidal Neurons of the Dorsal Hippocampus and on Object-place Recognition Memory in Rats. Neuroscience 2019, 402, 1–10. [Google Scholar] [CrossRef]
- Brown, M.F.; Farley, R.F.; Lorek, E.J. Remembrance of places you passed: Social spatial working memory in rats. J. Exp. Psychol. Anim. Behav. Process. 2007, 33, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008, 169, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizon, J.L.; Foster, T.C.; Alexander, G.E.; Glisky, E.L. Characterizing cognitive aging of working memory and executive function in animal models. Front. Aging Neurosci. 2012, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Cho, J.; Kang, H. Protective effect of exercise training against the progression of Alzheimer’s disease in 3xTg-AD mice. Behav. Brain Res. 2019, 374, 112105. [Google Scholar] [CrossRef]
- Cho, J.; Shin, M.K.; Kim, D.; Lee, I.; Kim, S.; Kang, H. Treadmill Running Reverses Cognitive Declines due to Alzheimer Disease. Med. Sci. Sports Exerc. 2015, 47, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. JoVE 2011, 53, 2920. [Google Scholar] [CrossRef]
- Bailey, C.H.; Kandel, E.R.; Harris, K.M. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021758. [Google Scholar] [CrossRef] [Green Version]
- Weyhersmüller, A.; Hallermann, S.; Wagner, N.; Eilers, J. Rapid active zone remodeling during synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6041–6052. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.; Chen, X.; Guan, D.; Xu, J.; Wu, H.; Tong, P.; Zhang, M. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 2018, 174, 1172–1187.e16. [Google Scholar] [CrossRef] [Green Version]
- Kolos, Y.A.; Grigoriyev, I.P.; Korzhevskyi, D.E. A synaptic marker synaptophysin. Morfologiia 2015, 147, 78–82. [Google Scholar]
- Jeanneret, V.; Ospina, J.P.; Diaz, A.; Manrique, L.G.; Merino, P.; Gutierrez, L.; Torre, E.; Wu, F.; Cheng, L.; Yepes, M. Tissue-type plasminogen activator protects the postsynaptic density in the ischemic brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Revilla, S.; Suñol, C.; García-Mesa, Y.; Giménez-Llort, L.; Sanfeliu, C.; Cristòfol, R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014, 81, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Grutzendler, J.; Duff, K.; Gan, W.B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004, 7, 1181–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; He, Z.; Chen, J.; Wang, X.; Jiang, L. Dendritic complexity change in the triple transgenic mouse model of Alzheimer’s disease. PeerJ 2020, 8, e8178. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Campanac, E.; Bialowas, A.; Carlier, E.; Alcaraz, G. Axon physiology. Physiol. Rev. 2011, 91, 555–602. [Google Scholar] [CrossRef] [Green Version]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [Green Version]
- Alobuia, W.M.; Xia, W.; Vohra, B.P. Axon degeneration is key component of neuronal death in amyloid-β toxicity. Neurochem. Int. 2013, 63, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Talavera, Y.; Chen, G.; Kurudenkandy, F.R.; Johansson, J.; Fisahn, A. Bri2 BRICHOS chaperone rescues impaired fast-spiking interneuron behavior and neuronal network dynamics in an AD mouse model in vitro. Neurobiol. Dis. 2021, 159, 105514. [Google Scholar] [CrossRef]
- Pchitskaya, E.; Bezprozvanny, I. Dendritic Spines Shape Analysis-Classification or Clusterization? Perspective. Front. Synaptic Neurosci. 2020, 12, 31. [Google Scholar] [CrossRef]
- Kasai, H.; Fukuda, M.; Watanabe, S.; Hayashi-Takagi, A.; Noguchi, J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010, 33, 121–129. [Google Scholar] [CrossRef]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, L.K.; Wako, K.; Maruo, S.; Kakuta, S.; Taguchi, T.; Ikuno, M.; Yamakado, H.; Takahashi, R.; Koike, M. Developmental Changes in Dendritic Spine Morphology in the Striatum and Their Alteration in an A53T α-Synuclein Transgenic Mouse Model of Parkinson’s Disease. eNeuro 2020, 7, ENEURO.0072-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.K.; Gibson, E.S.; Potter, H.; Dell’Acqua, M.L. Inhibition of the Motor Protein Eg5/Kinesin-5 in Amyloid β-Mediated Impairment of Hippocampal Long-Term Potentiation and Dendritic Spine Loss. Mol. Pharmacol. 2016, 89, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankfurt, M.; Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav. 2015, 74, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Cai, J.; Gu, B.; Yu, L.; Li, C.; Liu, Q.-S.; Zhao, L. Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex. Cells 2022, 11, 244. https://doi.org/10.3390/cells11020244
Mu L, Cai J, Gu B, Yu L, Li C, Liu Q-S, Zhao L. Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex. Cells. 2022; 11(2):244. https://doi.org/10.3390/cells11020244
Chicago/Turabian StyleMu, Lianwei, Jiajia Cai, Boya Gu, Laikang Yu, Cui Li, Qing-Song Liu, and Li Zhao. 2022. "Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3×Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex" Cells 11, no. 2: 244. https://doi.org/10.3390/cells11020244






