Human Sensory Neuron-like Cells and Glycated Collagen Matrix as a Model for the Screening of Analgesic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Glycated Type I Collagen
2.2. Cell Culture and Neuronal Differentiation
2.3. Validation of the Sensory Neuron-like Cells Differentiation Process
2.4. Cell Viability
2.5. Immunofluorescence Detection of Receptors for AGEs Using HCS Equipment
2.6. c-Fos Transcription Factor Expression Using HCS Analysis
2.7. SCN9A (Nav1.7) and TACR1 (NK1) Gene Expression—Real-Time Quantitative PCR
2.8. Neurite Growth Analysis by the HCS Assay
2.9. Analysis Substance P and β-Endorphin Release—Muliplex Assay
2.10. Statistical Analysis
3. Results
3.1. Confirmation of Glycation Collagen Process
3.2. SH-SY5Y Differentiation into Sensory-like Neuron Culture
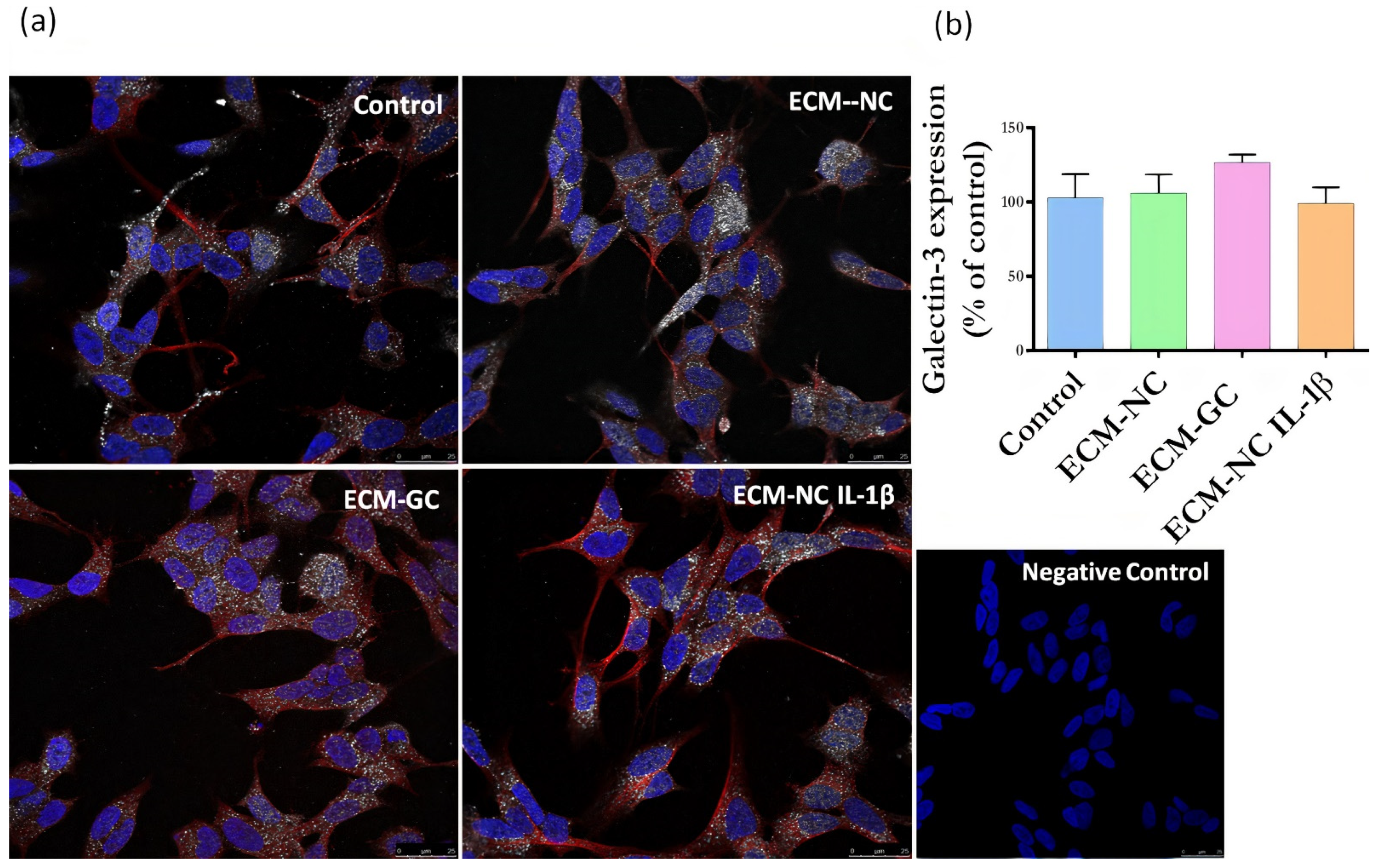
3.3. Cell Viability and AGEs Receptors (AGER (RAGE) and LGALS3 (Galectin-3) Expression
3.4. GC Matrix Activates Sensory Neurons-like Cells
3.5. Effect of GC on SCN9A (Nav1.7) and TACR1 (NK1) Genes
3.6. Neurite Growth Inhibition by GC Matrix
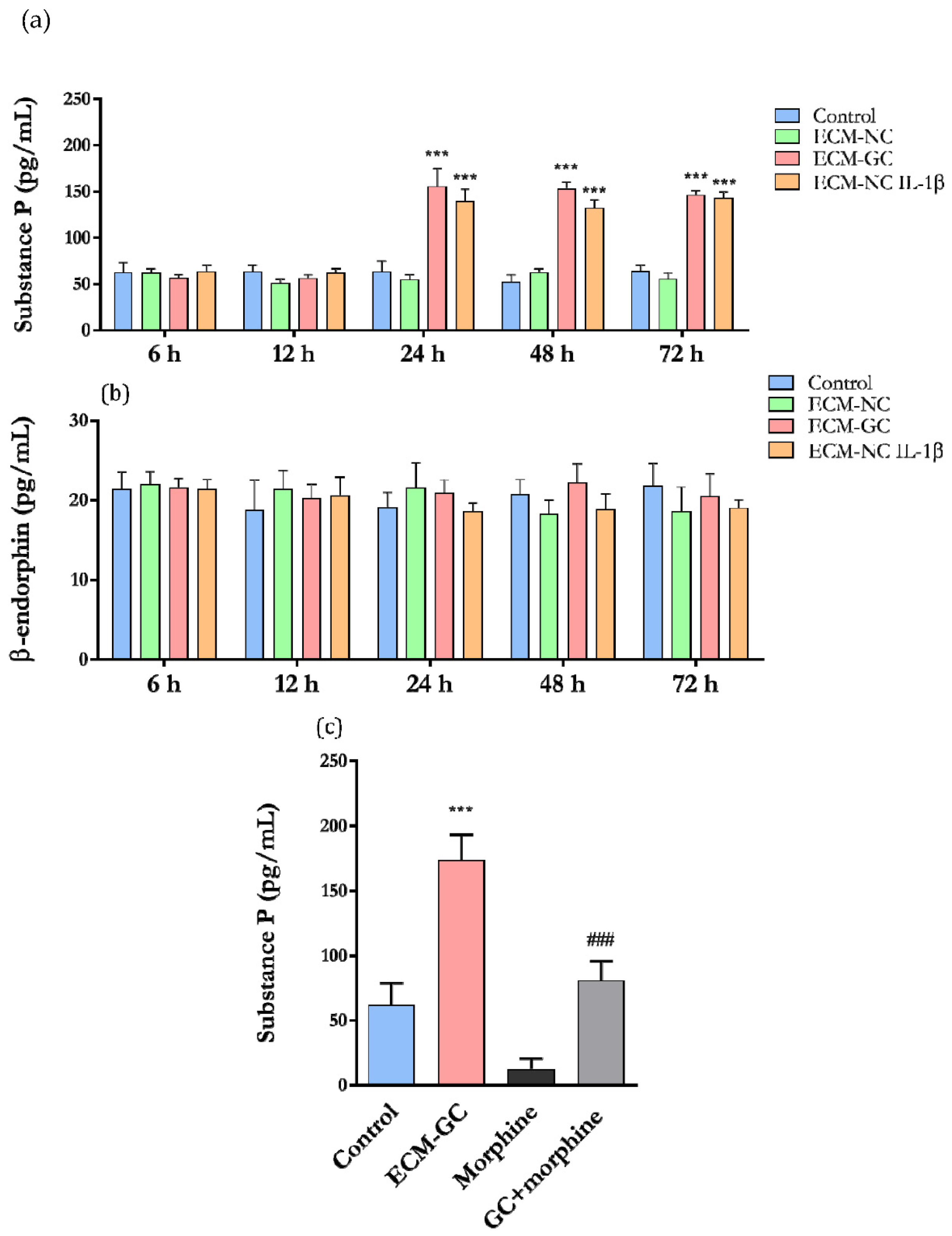
3.7. The Collagen Glycation Functionally Activates Sensory-like Neurons
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Dandia, H.; Makkad, K.; Tayalia, P. Glycated collagen—A 3D matrix system to study pathological cell behavior. Biomater. Sci. 2019, 7, 3480–3488. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes-the good, the bad, and the ugly. Muscle Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef] [PubMed]
- Aktar, R.; Peiris, M.; Fikree, A.; Cibert-Goton, V.; Walmsley, M.; Tough, I.R.; Watanabe, P.; Araujo, E.J.D.A.; Mohammed, S.D.; Delalande, J.-M.; et al. The extracellular matrix glycoprotein tenascin-X regulates peripheral sensory and motor neurones. J. Physiol. 2018, 596, 4237–4251. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.-M.; Miller, R.J. Emerging targets for the management of osteoarthritis pain. Curr. Osteoporos. Rep. 2016, 14, 260–268. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Luo, Z.J.; King, R.H.M.; Lewin, J.; Thomas, P.K. Effects of nonenzymatic glycosylation of extracellular matrix components on cell survival and sensory neurite extension in cell culture. J. Neurol. 2002, 249, 424–431. [Google Scholar] [CrossRef]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced Glycation End Products in Extracellular Matrix Proteins Contribute to the Failure of Sensory Nerve Regeneration in Diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef]
- Federoff, H.J.; Lawrence, N.; Brownlee, M. Nonenzymatic glycosylation of laminin and the laminin peptide CIKVAVS inhibits neurite outgrowth. Diabetes 1993, 42, 509–513. [Google Scholar] [CrossRef]
- Bufalo, M.C.; Almeida, M.E.; Franca, I.A.; Zambelli, V.O.; Sant’Anna, M.; Kimura, L.F.; Giardini, A.C.; Cury, Y.; Sampaio, S.C. Advanced glycation endproducts produced by in vitro glycation of type I collagen modulate the functional and secretory behavior of dorsal root ganglion cells cultivated in two-dimensional system. Exp. Cell Res. 2019, 382, 111475. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Baillie, G.; Vetter, I. Neuronal cell lines as model dorsal root ganglion neurons: A transcriptomic comparison. Mol. Pain 2016, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chen, J.-C. Dorsal root ganglia isolation and primary culture to study neurotransmitter release. J. Vis. Exp. 2018, 140, e57569. [Google Scholar] [CrossRef] [PubMed]
- Pennacchi, P.; De Almeida, M.E.S.; Gomes, O.L.A.; Faião-Flores, F.; Crepaldi, M.C.D.A.; Santos, M.; Barros, S.B.D.M.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.; Buck, L.; Balling, R.; Antony, P.M.A. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef]
- Yang, S.-H.; Liao, C.-C.; Chen, Y.; Syu, J.-P.; Jeng, C.-J.; Wang, S.-M. Daidzein induces neuritogenesis in DRG neuronal cultures. J. Biomed. Sci. 2012, 19, 80. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Simões, R.F.; Ferrão, R.; Silva, M.R.; Pinho, S.L.; Ferreira, L.; Oliveira, P.J.; Cunha-Oliveira, T. Refinement of a differentiation protocol using neuroblastoma SH-SY5Y cells for use in neurotoxicology research. Food Chem. Toxicol. 2021, 149, 111967. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. c-Fos or pERK, Which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef]
- Soiza-Reilly, M.; Saggau, P.; Arenkiel, B.R. Neural circuits revealed. Front. Neural Circuits 2015, 9, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Suter, D.M. An integrated cytoskeletal model of neurite outgrowth. Front. Cell Neurosci. 2018, 12, 447–466. [Google Scholar] [CrossRef]
- Van Ooyen, A.; Van Pelt, J.; Corner, M. Implications of activity dependent neurite outgrowth for neuronal morphology and network development. J. Theor. Biol. 1995, 172, 63–82. [Google Scholar] [CrossRef]
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Nelson, T.A.; Driscoll, D.; Manzardo, A. Evaluation of plasma substance p and beta-endorphin levels in children with prader-willi syndrome. J. Rare Disord. 2015, 3, 1–14. [Google Scholar]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S.; White, M.K. General overview of neuronal cell culture. Methods Mol. Biol. 2013, 1078, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; van Kesteren, R.; Blaas, E.; Spitzer, S.O.; Kamstra, J.; Smit, A.B.; Swaab, D.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. 2016, 108, 53193. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Duan, G.; Liu, Y.; Guo, S.; Wang, C.; Zhu, C.; Zhang, X. Increased Nav1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats. Mol. Pain 2018, 14, 1–8. [Google Scholar] [CrossRef]
- He, S.-J.; Cheng, J.; Feng, X.; Yu, Y.; Tian, L.; Huang, Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget 2017, 8, 64534–64550. [Google Scholar] [CrossRef]
- Ohashi, S.; Abe, H.; Takahashi, T.; Yamamoto, Y.; Takeuchi, M.; Arai, H.; Nagata, K.; Kita, T.; Okamoto, H.; Yamamoto, H.; et al. Advanced Glycation End Products Increase Collagen-specific Chaperone Protein in Mouse Diabetic Nephropathy. J. Biol. Chem. 2004, 279, 19816–19823. [Google Scholar] [CrossRef]
- Henssen, A.; Odersky, A.; Szymansky, A.K.; Seiler, M.; Althoff, K.; Beckers, A.; Speleman, F.; Schäfers, S.; De Preter, K.; Astrahanseff, K.; et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget 2016, 8, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Starchenko, A.; Williams, R.; Bonassar, L.J.; Reinhart-King, C.A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9, 4635–4644. [Google Scholar] [CrossRef]
- Yoo, H.I.; Kim, E.-G.; Lee, E.-J.; Hong, S.-Y.; Yoon, C.-S.; Hong, M.-J.; Park, S.-J.; Woo, R.-S.; Baik, T.-K.; Song, D.-Y. Neuroanatomical distribution of galectin-3 in the adult rat brain. J. Mol. Histol. 2017, 48, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yamasoba, D.; Tsubota, M.; Domoto, R.; Sekiguchi, F.; Nishikawa, H.; Liu, K.; Nishibori, M.; Ishikura, H.; Yamamoto, T.; Taga, A.; et al. Peripheral HMGB1-induced hyperalgesia in mice: Redox state-dependent distinct roles of RAGE and TLR4. J. Pharmacol. Sci. 2016, 130, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Chung, L. A Brief Introduction to the Transduction of Neural Activity into Fos Signal. Dev. Reprod. 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Clarkson, B.D.S.; Kahoud, R.J.; McCarthy, C.B.; Howe, C.L. Inflammatory cytokine-induced changes in neural network activity measured by waveform analysis of high-content calcium imaging in murine cortical neurons. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, P.A.; Huh, B.Y.; Terrando, P.N.; Maixner, D.W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Jacobsen, T.D.; Chahine, N. Effects of Inflammation on Multiscale Biomechanical Properties of Cartilaginous Cells and Tissues. ACS Biomater. Sci. Eng. 2017, 3, 2644–2656. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Moreno-Flores, M.T.; Avila, J.; Wandosell, F. The Neurite Retraction Induced by Lysophosphatidic Acid Increases Alzheimer’s Disease-like Tau Phosphorylation. J. Biol. Chem. 1999, 274, 37046–37052. [Google Scholar] [CrossRef]
- Baynes, J.W. The role of AGEs in aging: Causation or correlation. Exp. Gerontol. 2001, 36, 1527–1537. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ogata, A.; Shinpo, K.; Moriwaka, F.; Fujii, J.; Taniguchi, N.; Tashiro, K. Detection of an Amadori product, 1-hexitol-lysine, in the anterior horn of the amyotrophic lateral sclerosis and spinobulbar muscular atrophy spinal cord: Evidence for early involvement of glycation in motoneuron diseases. Acta Neuropathol. 2000, 99, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Graefe, S.; Mohiuddin, S.S. Biochemistry; Substante, P., Ed.; StatPearles Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sprouse-Blum, A.S.; Smith, G.; Sugai, D.; Parsa, F.D. Understanding endorphins and their importance in pain manage-ment. Hawaii Med. J. 2010, 69, 70–71. [Google Scholar]
- Gallagher, R.M. Neuropathic pain: The global challenge. Pain Med. 2004, 5, S1–S2. [Google Scholar] [CrossRef]
- Al-Nimer, M.S.; Ali, F.S.; Al-Ani, F.S. Dyslipidemia as a contributory factor in etiopathogenesis of diabetic neuropathy. Indian J. Endocrinol. Metab. 2011, 15, 110–114. [Google Scholar] [CrossRef]
- Papachristou, S.; Pafili, K.; Papanas, N. Skin AGEs and diabetic neuropathy. BMC Endocr. Disord. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef]









| Genes | Primer Forward Sequence | Primer Forward Sequence |
|---|---|---|
| HPRT1 | CCTGGCGTCGTGATTAGTGAT | AGACGTTCAGTCCTGTCCATAA |
| PPIA | GCCGAGGAAAACCGTGTACT | TGTCTGCAAACAGCTCAAAGGA |
| SCN9A | GTCTCCCTGGTTGATGGACG | TGATTGGTCGTGCCCTCTGG |
| TACR1 | ATGACAGGTTCCGTCTGGGC | TACACACTGCCCTGGGTCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bufalo, M.C.; Almeida, M.E.S.d.; Jensen, J.R.; DeOcesano-Pereira, C.; Lichtenstein, F.; Picolo, G.; Chudzinski-Tavassi, A.M.; Sampaio, S.C.; Cury, Y.; Zambelli, V.O. Human Sensory Neuron-like Cells and Glycated Collagen Matrix as a Model for the Screening of Analgesic Compounds. Cells 2022, 11, 247. https://doi.org/10.3390/cells11020247
Bufalo MC, Almeida MESd, Jensen JR, DeOcesano-Pereira C, Lichtenstein F, Picolo G, Chudzinski-Tavassi AM, Sampaio SC, Cury Y, Zambelli VO. Human Sensory Neuron-like Cells and Glycated Collagen Matrix as a Model for the Screening of Analgesic Compounds. Cells. 2022; 11(2):247. https://doi.org/10.3390/cells11020247
Chicago/Turabian StyleBufalo, Michelle Cristiane, Maíra Estanislau Soares de Almeida, José Ricardo Jensen, Carlos DeOcesano-Pereira, Flavio Lichtenstein, Gisele Picolo, Ana Marisa Chudzinski-Tavassi, Sandra Coccuzzo Sampaio, Yara Cury, and Vanessa Olzon Zambelli. 2022. "Human Sensory Neuron-like Cells and Glycated Collagen Matrix as a Model for the Screening of Analgesic Compounds" Cells 11, no. 2: 247. https://doi.org/10.3390/cells11020247
APA StyleBufalo, M. C., Almeida, M. E. S. d., Jensen, J. R., DeOcesano-Pereira, C., Lichtenstein, F., Picolo, G., Chudzinski-Tavassi, A. M., Sampaio, S. C., Cury, Y., & Zambelli, V. O. (2022). Human Sensory Neuron-like Cells and Glycated Collagen Matrix as a Model for the Screening of Analgesic Compounds. Cells, 11(2), 247. https://doi.org/10.3390/cells11020247







