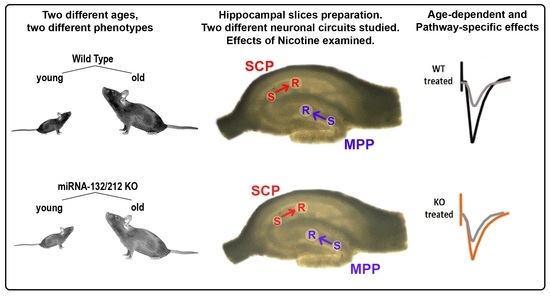
Age-Dependent and Pathway-Specific Bimodal Action of Nicotine on Synaptic Plasticity in the Hippocampus of Mice Lacking the miR-132/212 Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunoblotting
2.3. Electrophysiology
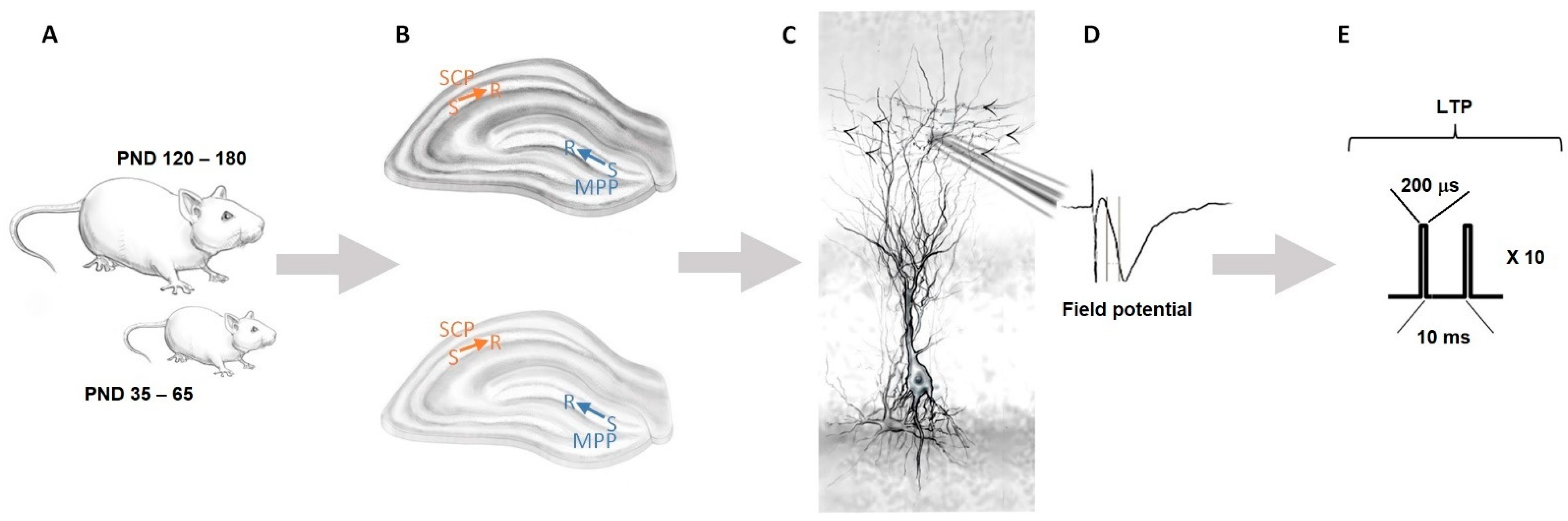
2.3.1. Hippocampal Slices Preparation
2.3.2. Extracellular Recordings
2.4. Statistical Analysis
3. Results
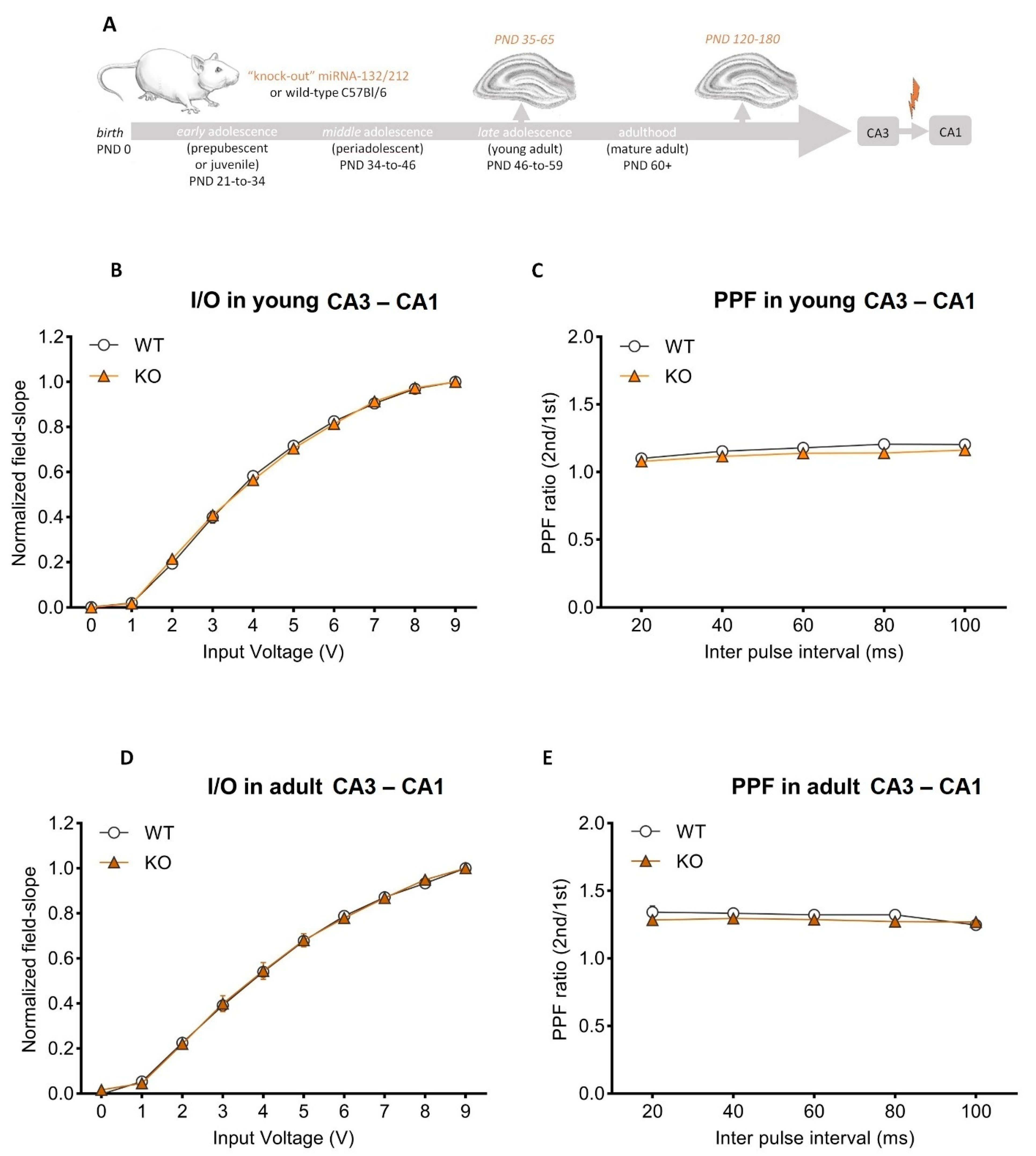
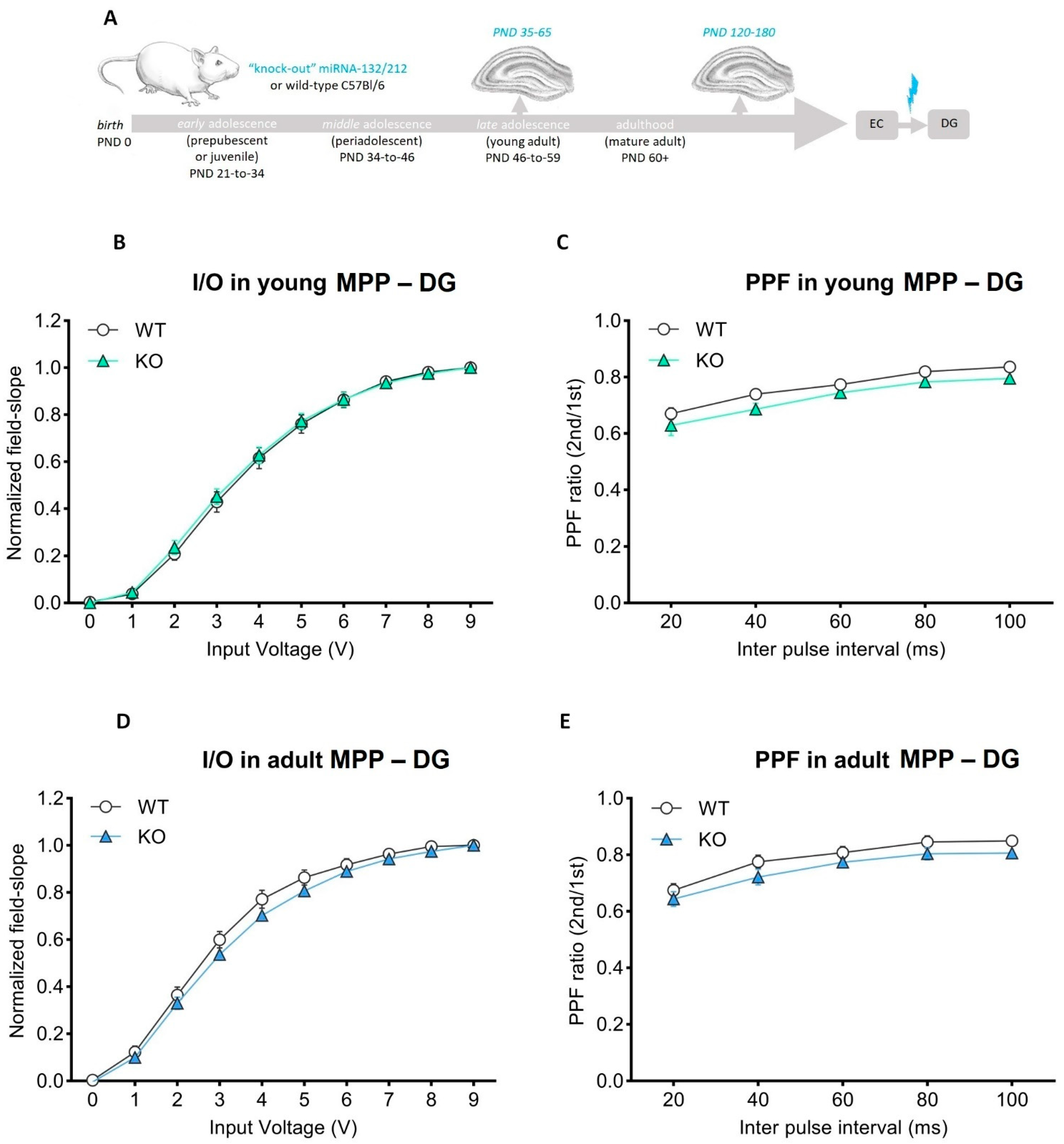
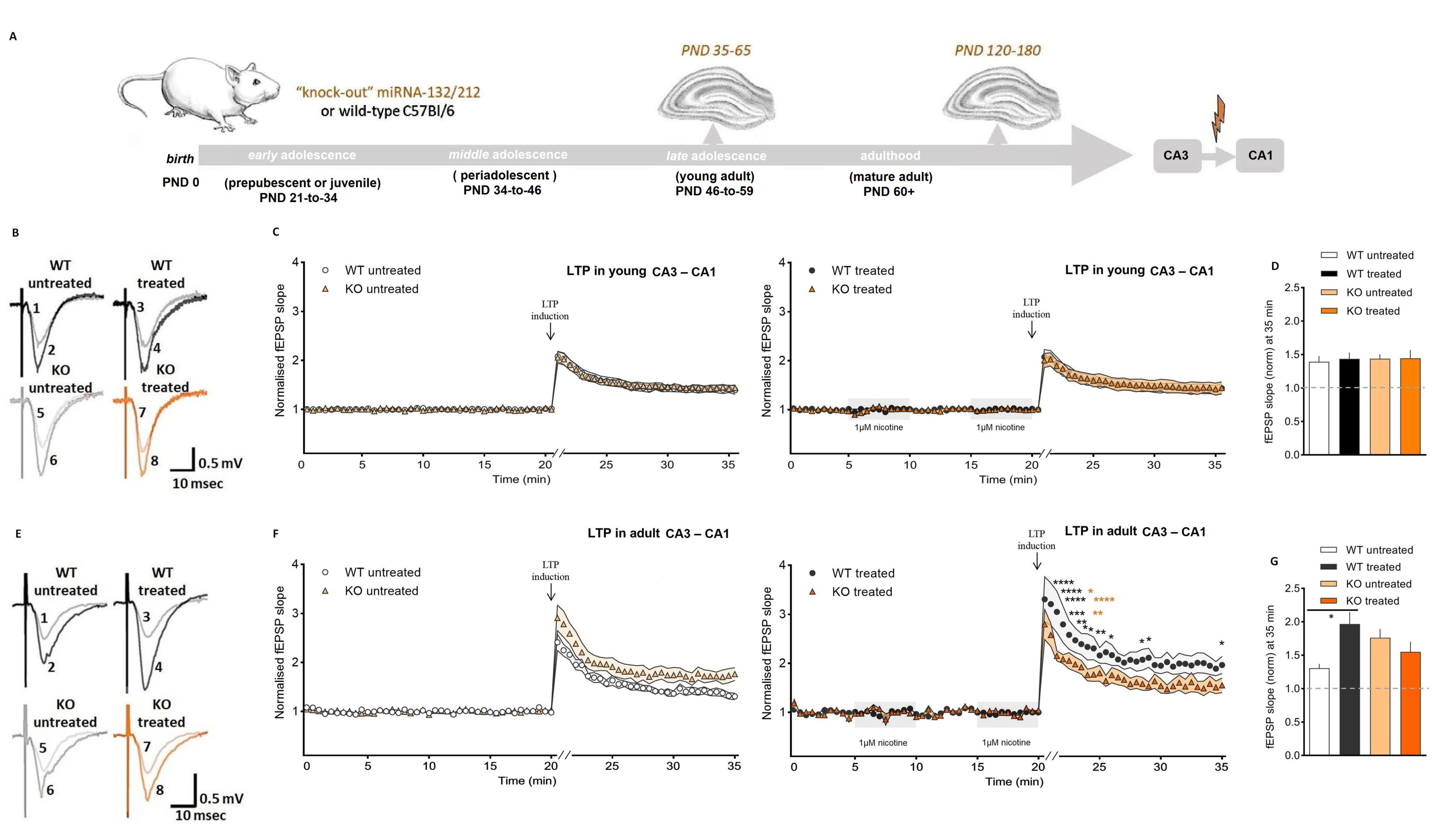
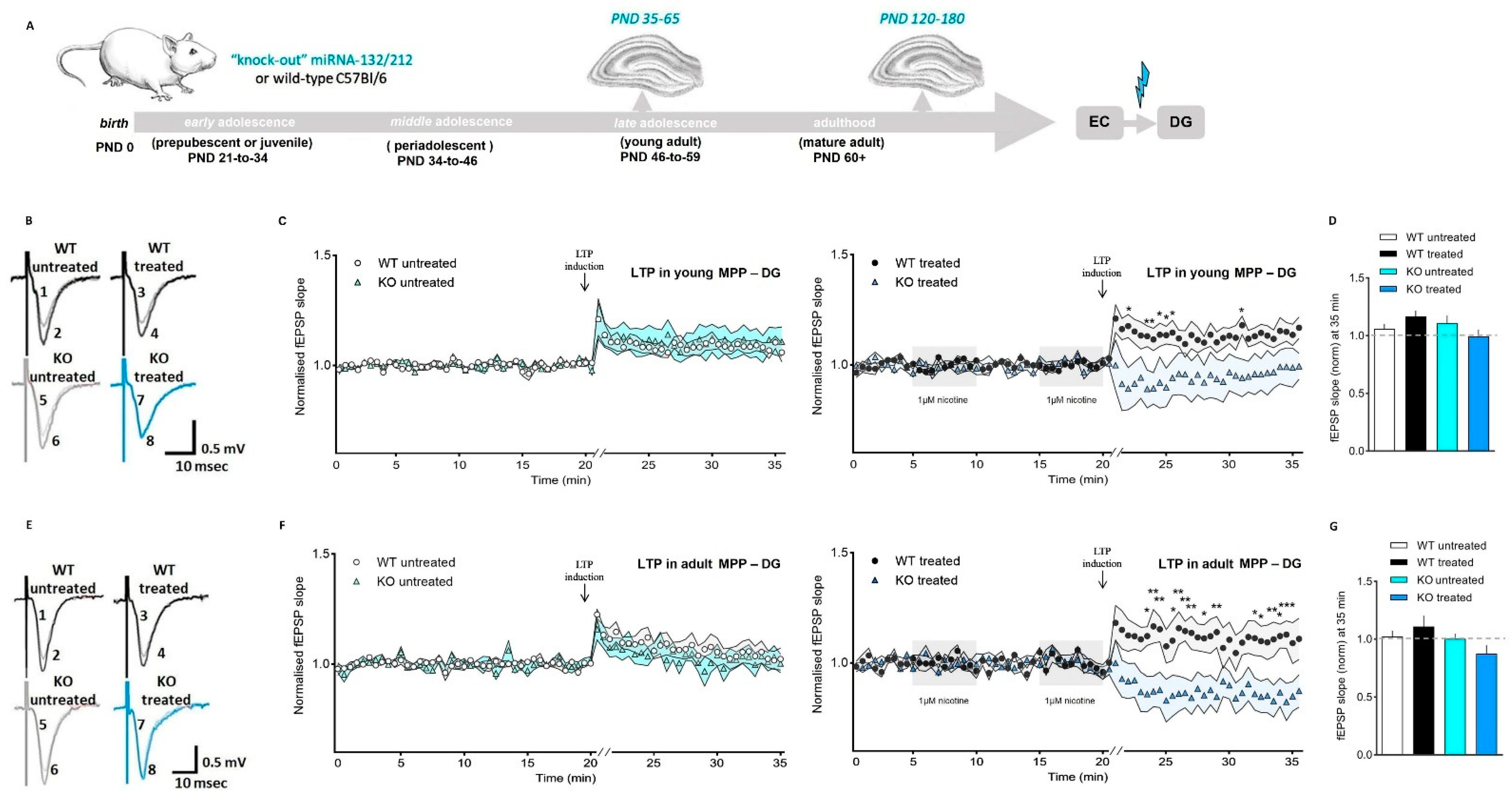
3.1. Basal Synaptic Transmission and Paired-Pulse-Induced Plasticity Is Unaltered in Adolescent and Mature Adult miRNA-132/212−/− Mouse Hippocampi
3.2. Nicotine Bolsters Synaptic Potentiation at CA3–CA1 Synapses of Mature Adult, Not Adolescent Wild-Type Mice
3.3. Nicotine Shifts from Promoter to Suppressor of Synaptic Potentiation in the Dentate Gyrus in Absence of miRNA-132/212
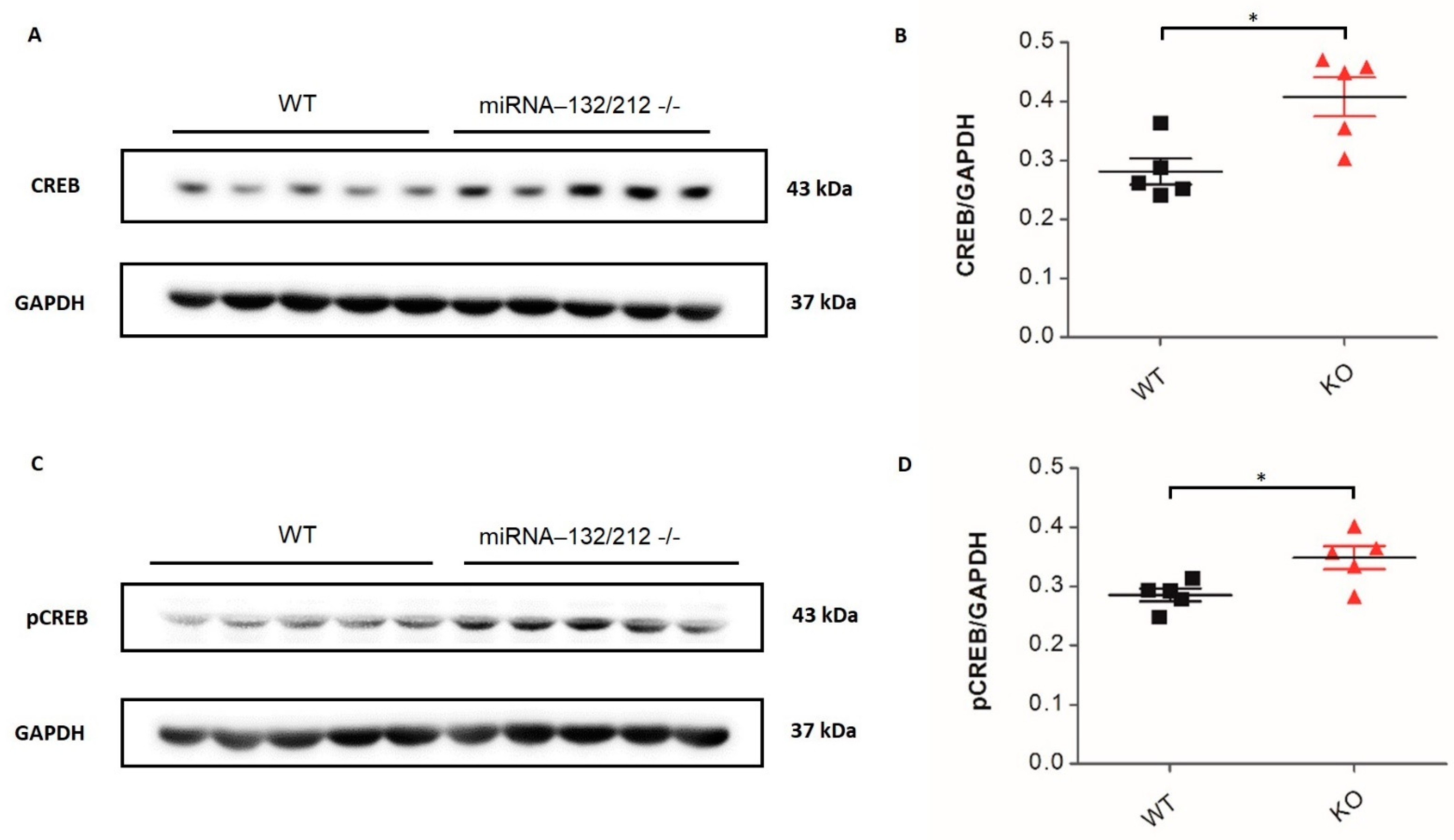
3.4. Altered Expression Levels of CREB, pCREB and AChE in the Adult Hippocampus of miRNA-132/212 Knockout Mice
4. Discussion
5. Acetylcholinergic Signaling and Hippocampal Synaptic Plasticity
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.; DeLand, K. Translating the WHO Framework Convention on Tobacco Control (FCTC): Can we use tobacco control as a model for other non-communicable disease control? Public Health 2011, 125, 847–853. [Google Scholar] [CrossRef]
- Yach, D. The origins, development, effects, and future of the WHO Framework Convention on Tobacco Control: A personal perspective. Lancet 2014, 383, 1771–1779. [Google Scholar] [CrossRef]
- Jha, P. Deaths and taxes: Stronger global tobacco control by 2025. Lancet 2015, 385, 918–920. [Google Scholar] [CrossRef]
- Ezzati, M.; Lopez, A.D. Estimates of global mortality attributable to smoking in 2000. Lancet 2003, 362, 847–852. [Google Scholar] [CrossRef]
- Makate, M.; Whetton, S.; Tait, R.J.; Dey, T.; Scollo, M.; Banks, E.; Norman, R.; Pidd, K.; Roche, A.M.; Allsop, S. Tobacco Cost of Illness Studies: A Systematic Review. Nicotine Tob. Res. 2020, 22, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Kandel, D.; Yamaguchi, K. From beer to crack: Developmental patterns of drug involvement. Am. J. Public Health 1993, 83, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Kandel, D.B.; Yamaguchi, K. Developmental patterns of the use of legal, illegal, and medically prescribed psychotropic drugs from adolescence to young adulthood. NIDA Res. Monogr. 1985, 56, 193–235. [Google Scholar]
- Resnick, M.D.; Bearman, P.S.; Blum, R.W.; Bauman, K.E.; Harris, K.M.; Jones, J.; Tabor, J.; Beuhring, T.; Sieving, R.E.; Shew, M.; et al. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA 1997, 278, 823–832. [Google Scholar] [CrossRef]
- Hanna, E.Z.; Yi, H.Y.; Dufour, M.C.; Whitmore, C.C. The relationship of early-onset regular smoking to alcohol use, depression, illicit drug use, and other risky behaviors during early adolescence: Results from the youth supplement to the third national health and nutrition examination survey. J. Subst. Abus. 2001, 13, 265–282. [Google Scholar] [CrossRef]
- Lai, S.; Lai, H.; Page, J.B.; McCoy, C.B. The association between cigarette smoking and drug abuse in the United States. J. Addict. Dis. 2000, 19, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.; White, A.; Swartzwelder, H.S.; Bell, R.L.; Rodd, Z.A.; Slawecki, C.J.; Ehlers, C.L.; Levin, E.D.; Rezvani, A.H.; Spear, L.P. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: Findings from rodent models. Alcohol. Clin. Exp. Res. 2005, 29, 1720–1725. [Google Scholar] [CrossRef]
- Biederman, J.; Monuteaux, M.C.; Mick, E.; Wilens, T.E.; Fontanella, J.A.; Poetzl, K.M.; Kirk, T.; Masse, J.; Faraone, S.V. Is cigarette smoking a gateway to alcohol and illicit drug use disorders? A study of youths with and without attention deficit hyperactivity disorder. Biol. Psychiatry 2006, 59, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Spear, L. Modeling adolescent development and alcohol use in animals. Alcohol. Health 2000, 24, 115–123. [Google Scholar]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hagino, H.; Nohara, S.; Zhou, S.Y.; Kawasaki, Y.; Takahashi, T.; Matsui, M.; Seto, H.; Ono, T.; Kurachi, M. Male-specific volume expansion of the human hippocampus during adolescence. Cereb. Cortex 2005, 15, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Wierenga, L.; Langen, M.; Ambrosino, S.; van Dijk, S.; Oranje, B.; Durston, S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage 2014, 96, 67–72. [Google Scholar] [CrossRef]
- Gould, T.J. Nicotine and hippocampus-dependent learning: Implications for addiction. Mol. Neurobiol. 2006, 34, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Soleiman, M.T.; Talia, R.; Koob, G.F.; George, O.; Mandyam, C.D. Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology 2015, 232, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.L.; LeMalefant, R.M.; Zhou, L.; Huang, G.; Turner, J.R. Distinct Roles of CREB Within the Ventral and Dorsal Hippocampus in Mediating Nicotine Withdrawal Phenotypes. Neuropsychopharmacology 2017, 42, 1599–1609. [Google Scholar] [CrossRef] [Green Version]
- Kenney, J.W.; Gould, T.J. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008, 38, 101–121. [Google Scholar] [CrossRef] [Green Version]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, R.J.; Mandyam, C.D. Regulation of Adult Neurogenesis by Non-coding RNAs: Implications for Substance Use Disorders. Front. Neurosci. 2018, 12, 849. [Google Scholar] [CrossRef]
- Stojanovic, T.; Benes, H.; Awad, A.; Bormann, D.; Monje, F.J. Nicotine abolishes memory-related synaptic strengthening and promotes synaptic depression in the neurogenic dentate gyrus of miR-132/212 knockout mice. Addict. Biol. 2020, 26, e12905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Abeta(1-42)-induced model of Alzheimer’s disease. Aging Cell 2020, 19, e13046. [Google Scholar] [CrossRef] [Green Version]
- Berentsen, B.; Patil, S.; Ronnestad, K.; Goff, K.M.; Pajak, M.; Simpson, T.I.; Wibrand, K.; Bramham, C.R. MicroRNA-34a Acutely Regulates Synaptic Efficacy in the Adult Dentate Gyrus In Vivo. Mol. Neurobiol. 2020, 57, 1432–1445. [Google Scholar] [CrossRef]
- Zhang, H.P.; Liu, X.L.; Chen, J.J.; Cheng, K.; Bai, S.J.; Zheng, P.; Zhou, C.J.; Wang, W.; Wang, H.Y.; Zhong, L.M.; et al. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Transl. Psychiatry 2020, 10, 95. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Dreyer, J.L. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol. Cell Neurosci. 2009, 42, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.L.; Liu, H.; Guan, X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J. Biomed. Sci. 2013, 20, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Rapp, J.; Smith, P.Y.; Filali, M.; Goupil, C.; Planel, E.; Magill, S.T.; Goodman, R.H.; Hebert, S.S. Memory formation and retention are affected in adult miR-132/212 knockout mice. Behav. Brain Res. 2015, 287, 15–26. [Google Scholar] [CrossRef]
- Remenyi, J.; Hunter, C.J.; Cole, C.; Ando, H.; Impey, S.; Monk, C.E.; Martin, K.J.; Barton, G.J.; Hutvagner, G.; Arthur, J.S. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem. J. 2010, 428, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, A.S.; DiRocco, D.P.; Lambert, T.J.; Garelick, M.G.; Le, J.; Nathanson, N.M.; Storm, D.R. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 2010, 20, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Richards, M.; Adachi, N.; Kishi, S.; Kunugi, H.; Hashido, K. MicroRNA function and neurotrophin BDNF. Neurochem. Int. 2011, 59, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Barco, A.; Patterson, S.; Alarcon, J.M.; Gromova, P.; Mata-Roig, M.; Morozov, A.; Kandel, E.R. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 2005, 48, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.L.; Pittenger, C.; Morozov, A.; Martin, K.C.; Scanlin, H.; Drake, C.; Kandel, E.R. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 2001, 32, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Bormann, D.; Stojanovic, T.; Cicvaric, A.; Schuld, G.J.; Cabatic, M.; Ankersmit, H.J.; Monje, F.J. miRNA-132/212 Gene-Deletion Aggravates the Effect of Oxygen-Glucose Deprivation on Synaptic Functions in the Female Mouse Hippocampus. Cells 2021, 10, 1709. [Google Scholar] [CrossRef]
- Pichler, S.; Gu, W.; Hartl, D.; Gasparoni, G.; Leidinger, P.; Keller, A.; Meese, E.; Mayhaus, M.; Hampel, H.; Riemenschneider, M. The miRNome of Alzheimer’s disease: Consistent downregulation of the miR-132/212 cluster. Neurobiol. Aging 2017, 50, 167.e1–167.e10. [Google Scholar] [CrossRef] [PubMed]
- Remenyi, J.; van den Bosch, M.W.; Palygin, O.; Mistry, R.B.; McKenzie, C.; Macdonald, A.; Hutvagner, G.; Arthur, J.S.; Frenguelli, B.G.; Pankratov, Y. miR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLoS ONE 2013, 8, e62509. [Google Scholar] [CrossRef]
- Ronovsky, M.; Zambon, A.; Cicvaric, A.; Boehm, V.; Hoesel, B.; Moser, B.A.; Yang, J.; Schmid, J.A.; Haubensak, W.E.; Monje, F.J.; et al. A role for miR-132 in learned safety. Sci. Rep. 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aten, S.; Hansen, K.F.; Hoyt, K.R.; Obrietan, K. The miR-132/212 locus: A complex regulator of neuronal plasticity, gene expression and cognition. RNA Dis. 2016, 3, e1375. [Google Scholar]
- Hansen, K.F.; Karelina, K.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. miRNA-132: A dynamic regulator of cognitive capacity. Brain Struct. Funct. 2013, 218, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, K.F.; Sakamoto, K.; Aten, S.; Snider, K.H.; Loeser, J.; Hesse, A.M.; Page, C.E.; Pelz, C.; Arthur, J.S.; Impey, S.; et al. Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learn. Mem. 2016, 23, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.D.; McClernon, F.J.; Rezvani, A.H. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 2006, 184, 523–539. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Fote, G.M.; Blakeman, S.; Cahuzac, E.L.; Newbold, S.A.; Picciotto, M.R. Multiple Nicotinic Acetylcholine Receptor Subtypes in the Mouse Amygdala Regulate Affective Behaviors and Response to Social Stress. Neuropsychopharmacology 2016, 41, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Dehkordi, O.; Rose, J.E.; Asadi, S.; Manaye, K.F.; Millis, R.M.; Jayam-Trouth, A. Neuroanatomical circuitry mediating the sensory impact of nicotine in the central nervous system. J. Neurosci. Res. 2015, 93, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, L.E.; Hodgkinson, C.A.; Yang, Y.; Sampath, H.; Ross, T.J.; Buchholz, B.; Salmeron, B.J.; Srivastava, V.; Thaker, G.K.; Goldman, D.; et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. USA 2010, 107, 13509–13514. [Google Scholar] [CrossRef] [Green Version]
- Zarrindast, M.R.; Khakpai, F. The modulatory role of nicotine on cognitive and non-cognitive functions. Brain Res. 2019, 1710, 92–101. [Google Scholar] [CrossRef]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykhmus, O.; Mishra, N.; Koval, L.; Kalashnyk, O.; Gergalova, G.; Uspenska, K.; Komisarenko, S.; Soreq, H.; Skok, M. Molecular Mechanisms Regulating LPS-Induced Inflammation in the Brain. Front. Mol. Neurosci. 2016, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Friedson, L.; Hanin, G.; Bekenstein, U.; Volovich, M.; Bennett, E.R.; Greenberg, D.S.; Soreq, H. Antisense miR-132 blockade via the AChE-R splice variant mitigates cortical inflammation. Sci. Rep. 2017, 7, 42755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wibrand, K.; Panja, D.; Tiron, A.; Ofte, M.L.; Skaftnesmo, K.O.; Lee, C.S.; Pena, J.T.; Tuschl, T.; Bramham, C.R. Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. Eur. J. Neurosci. 2010, 31, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Rapp, J.; Rainone, S.; Goupil, C.; Dorval, V.; Smith, P.Y.; Saint-Pierre, M.; Vallee, M.; Planel, E.; Droit, A.; Calon, F.; et al. microRNA-132/212 deficiency enhances Abeta production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci. Rep. 2016, 6, 30953. [Google Scholar] [CrossRef] [Green Version]
- Nagy, V.; Hollstein, R.; Pai, T.P.; Herde, M.K.; Buphamalai, P.; Moeseneder, P.; Lenartowicz, E.; Kavirayani, A.; Korenke, G.C.; Kozieradzki, I.; et al. HACE1 deficiency leads to structural and functional neurodevelopmental defects. Neurol. Genet. 2019, 5, e330. [Google Scholar] [CrossRef] [Green Version]
- Monje, F.J.; Kim, E.J.; Pollak, D.D.; Cabatic, M.; Li, L.; Baston, A.; Lubec, G. Focal adhesion kinase regulates neuronal growth, synaptic plasticity and hippocampus-dependent spatial learning and memory. Neuro-Signals 2012, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cicvaric, A.; Yang, J.; Bulat, T.; Zambon, A.; Dominguez-Rodriguez, M.; Kuhn, R.; Sadowicz, M.G.; Siwert, A.; Egea, J.; Pollak, D.D.; et al. Enhanced synaptic plasticity and spatial memory in female but not male FLRT2-haplodeficient mice. Sci. Rep. 2018, 8, 3703. [Google Scholar] [CrossRef]
- Cicvaric, A.; Yang, J.; Krieger, S.; Khan, D.; Kim, E.J.; Dominguez-Rodriguez, M.; Cabatic, M.; Molz, B.; Acevedo Aguilar, J.P.; Milicevic, R.; et al. The brain-tumor related protein podoplanin regulates synaptic plasticity and hippocampus-dependent learning and memory. Ann. Med. 2016, 48, 652–668. [Google Scholar] [CrossRef] [Green Version]
- Halff, A.W.; Gomez-Varela, D.; John, D.; Berg, D.K. A novel mechanism for nicotinic potentiation of glutamatergic synapses. J. Neurosci. 2014, 34, 2051–2064. [Google Scholar] [CrossRef] [Green Version]
- Szabo, S.I.; Zelles, T.; Vizi, E.S.; Lendvai, B. The effect of nicotine on spiking activity and Ca2+ dynamics of dendritic spines in rat CA1 pyramidal neurons. Hippocampus 2008, 18, 376–385. [Google Scholar] [CrossRef]
- Galvez, B.; Gross, N.; Sumikawa, K. Activation of alpha7 nicotinic acetylcholine receptors protects potentiated synapses from depotentiation during theta pattern stimulation in the hippocampal CA1 region of rats. Neuropharmacology 2016, 105, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Prestori, F.; Bonardi, C.; Mapelli, L.; Lombardo, P.; Goselink, R.; De Stefano, M.E.; Gandolfi, D.; Mapelli, J.; Bertrand, D.; Schonewille, M.; et al. Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS ONE 2013, 8, e64828. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Udo, H.; Li, H.L.; Youn, T.Y.; Chen, M.; Kandel, E.R.; Bailey, C.H. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron 2003, 40, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Abraham, W.C. Long-term depression in hippocampus. Annu. Neurosci. 1996, 19, 437–462. [Google Scholar] [CrossRef] [PubMed]
- Lomo, T. The discovery of long-term potentiation. Philos. Trans. R. Soc. Lond. 2003, 358, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Lomo, T. Control of hippocampal output by afferent volley frequency. Prog. Brain Res. 1967, 27, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Asaka, Y.; Jugloff, D.G.; Zhang, L.; Eubanks, J.H.; Fitzsimonds, R.M. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 2006, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.B.; Bronzino, J.D.; Morgane, P.J. Paired-pulse facilitation and inhibition in the dentate gyrus is dependent on behavioral state. Exp. Brain Res. 1989, 77, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, J.W.; Lothman, E.W. Electrophysiological characterization of associational pathway terminating on dentate gyrus granule cells in the rat. Hippocampus 1991, 1, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, U.; Voronin, L.L. Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea-pig hippocampal slices: Application of quantal analysis. Neuroscience 1994, 62, 391–397. [Google Scholar] [CrossRef]
- Gruart, A.; Benito, E.; Delgado-Garcia, J.M.; Barco, A. Enhanced cAMP response element-binding protein activity increases neuronal excitability, hippocampal long-term potentiation, and classical eyeblink conditioning in alert behaving mice. J. Neurosci. 2012, 32, 17431–17441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, S.A.; Wang, Y.T. A Place at the Table: LTD as a Mediator of Memory Genesis. Neuroscientist 2016, 22, 359–371. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Lessmann, V.; Heumann, R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: Presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience 1998, 86, 399–413. [Google Scholar] [CrossRef]
- Capron, B.; Sindic, C.; Godaux, E.; Ris, L. The characteristics of LTP induced in hippocampal slices are dependent on slice-recovery conditions. Learn. Mem. 2006, 13, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.V.; Kandel, E.R. Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn. Mem. 1997, 4, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.V.; Navarro, M.M.; Rodriguez, W.A.; Martinez, J.L., Jr.; LeBaron, R.G. Differences in the magnitude of long-term potentiation produced by theta burst and high frequency stimulation protocols matched in stimulus number. Brain Res. Brain Res. Protoc. 2005, 15, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajikumar, S.; Frey, J.U. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol. Learn. Mem. 2004, 82, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, J.D. Chapter 6—The Biochemistry of LTP Induction. In Mechanisms of Memory; Academic Press: San Diego, CA, USA, 2003; pp. 147–188. [Google Scholar] [CrossRef]
- Gruart, A.; Leal-Campanario, R.; Lopez-Ramos, J.C.; Delgado-Garcia, J.M. Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 2015, 124, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruart, A.; Munoz, M.D.; Delgado-Garcia, J.M. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 2006, 26, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Gruart, A.; Sanchez-Campusano, R.; Fernandez-Guizan, A.; Delgado-Garcia, J.M. A Differential and Timed Contribution of Identified Hippocampal Synapses to Associative Learning in Mice. Cereb. Cortex 2015, 25, 2542–2555. [Google Scholar] [CrossRef] [Green Version]
- Fukazawa, Y.; Saitoh, Y.; Ozawa, F.; Ohta, Y.; Mizuno, K.; Inokuchi, K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 2003, 38, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Stepan, J.; Dine, J.; Fenzl, T.; Polta, S.A.; von Wolff, G.; Wotjak, C.T.; Eder, M. Entorhinal theta-frequency input to the dentate gyrus trisynaptically evokes hippocampal CA1 LTP. Front. Neural Circuits 2012, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.L.; Flynn, J.R.; Smith, D.W.; Dayas, C.V. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int. J. Neuropsychopharmacol. 2011, 14, 1099–1110. [Google Scholar] [CrossRef] [Green Version]
- Christie, M.J. Cellular neuroadaptations to chronic opioids: Tolerance, withdrawal and addiction. Br. J. Pharmacol. 2008, 154, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.; Bonci, A. Synaptic plasticity and drug addiction. Curr. Opin. Pharm. 2005, 5, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kauer, J.A.; Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. 2007, 8, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Common molecular and cellular substrates of addiction and memory. Neurobiol. Learn. Mem. 2002, 78, 637–647. [Google Scholar] [CrossRef]
- Thomas, M.J.; Kalivas, P.W.; Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008, 154, 327–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, M.E. Addiction: Making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol. Interv. 2002, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Wen, Z.; Song, H.; Christian, K.M.; Ming, G.L. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb. Perspect. Biol. 2016, 8, a019026. [Google Scholar] [CrossRef] [Green Version]
- Abrous, D.N.; Adriani, W.; Montaron, M.F.; Aurousseau, C.; Rougon, G.; Le Moal, M.; Piazza, P.V. Nicotine self-administration impairs hippocampal plasticity. J. Neurosci. 2002, 22, 3656–3662. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.A.; Maramara, L.A.; Lisman, J. A single brief burst induces GluR1-dependent associative short-term potentiation: A potential mechanism for short-term memory. J. Cogn. Neurosci. 2010, 22, 2530–2540. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Wong, D.; Lynch, G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaarani, B.; Kan, K.J.; Mackey, S.; Spechler, P.A.; Potter, A.; Orr, C.; D’Alberto, N.; Hudson, K.E.; Banaschewski, T.; Bokde, A.L.W.; et al. Low Smoking Exposure, the Adolescent Brain, and the Modulating Role of CHRNA5 Polymorphisms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Treur, J.L.; Willemsen, G.; Bartels, M.; Geels, L.M.; van Beek, J.H.; Huppertz, C.; van Beijsterveldt, C.E.; Boomsma, D.I.; Vink, J.M. Smoking During Adolescence as a Risk Factor for Attention Problems. Biol. Psychiatry 2015, 78, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.F.; McDonald, C.G.; Bergstrom, H.C.; Ehlinger, D.G.; Brielmaier, J.M. Adolescent nicotine induces persisting changes in development of neural connectivity. Neurosci. Biobehav. Rev. 2015, 55, 432–443. [Google Scholar] [CrossRef]
- Soderstrom, K.; Qin, W.; Williams, H.; Taylor, D.A.; McMillen, B.A. Nicotine increases FosB expression within a subset of reward- and memory-related brain regions during both peri- and post-adolescence. Psychopharmacology 2007, 191, 891–897. [Google Scholar] [CrossRef]
- Trauth, J.A.; Seidler, F.J.; McCook, E.C.; Slotkin, T.A. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain 1999, 851, 9–19. [Google Scholar] [CrossRef]
- Le Foll, B.; Goldberg, S.R. Effects of nicotine in experimental animals and humans: An update on addictive properties. Handb. Exp. Pharmacol. 2009, 192, 335–367. [Google Scholar] [CrossRef] [Green Version]
- Lynch, W.J.; Nicholson, K.L.; Dance, M.E.; Morgan, R.W.; Foley, P.L. Animal models of substance abuse and addiction: Implications for science, animal welfare, and society. Comp. Med. 2010, 60, 177–188. [Google Scholar]
- O’Dell, L.E.; Khroyan, T.V. Rodent models of nicotine reward: What do they tell us about tobacco abuse in humans? Pharmacol. Biochem. Behav. 2009, 91, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Ijomone, O.M.; Nwoha, P.U. Nicotine inhibits hippocampal and striatal acetylcholinesterase activities, and demonstrates dual action on adult neuronal proliferation and maturation. Pathophysiology 2015, 22, 231–239. [Google Scholar] [CrossRef]
- Fujii, S.; Jia, Y.; Yang, A.; Sumikawa, K. Nicotine reverses GABAergic inhibition of long-term potentiation induction in the hippocampal CA1 region. Brain Res. 2000, 863, 259–265. [Google Scholar] [CrossRef]
- Fujii, S.; Sumikawa, K. Nicotine accelerates reversal of long-term potentiation and enhances long-term depression in the rat hippocampal CA1 region. Brain Res. 2001, 894, 340–346. [Google Scholar] [CrossRef]
- Fujii, S.; Sumikawa, K. Acute and chronic nicotine exposure reverse age-related declines in the induction of long-term potentiation in the rat hippocampus. Brain 2001, 894, 347–353. [Google Scholar] [CrossRef]
- Ji, D.; Lape, R.; Dani, J.A. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 2001, 31, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Mann, E.O.; Greenfield, S.A. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J. Physiol. 2003, 551, 539–550. [Google Scholar] [CrossRef]
- Nakauchi, S.; Brennan, R.J.; Boulter, J.; Sumikawa, K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur. J. Neurosci. 2007, 25, 2666–2681. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, S.; Sumikawa, K. Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice. Eur. J. Neurosci. 2012, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Sawada, S.; Ohno-Shosaku, T.; Yamamoto, C. Augmenting action of nicotine on population spikes in the dentate gyrus of the guinea pig. Neurosci. Res. 1994, 20, 317–322. [Google Scholar] [CrossRef]
- Sawada, S.; Yamamoto, C.; Ohno-Shosaku, T. Long-term potentiation and depression in the dentate gyrus, and effects of nicotine. Neurosci. Res. 1994, 20, 323–329. [Google Scholar] [CrossRef]
- Gray, R.; Rajan, A.S.; Radcliffe, K.A.; Yakehiro, M.; Dani, J.A. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 1996, 383, 713–716. [Google Scholar] [CrossRef]
- Matsuyama, S.; Matsumoto, A.; Enomoto, T.; Nishizaki, T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur. J. Neurosci. 2000, 12, 3741–3747. [Google Scholar] [CrossRef]
- Fujii, S.; Ji, Z.; Morita, N.; Sumikawa, K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999, 846, 137–143. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Srivareerat, M.; Tran, T.T.; Alkadhi, K.A. Role of alpha7- and alpha4beta2-nAChRs in the neuroprotective effect of nicotine in stress-induced impairment of hippocampus-dependent memory. Int. J. Neuropsychopharmacol. 2013, 16, 1105–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.; Yakel, J.L. The effect of alpha7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem. Pharm. 2015, 97, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenney, J.W.; Raybuck, J.D.; Gould, T.J. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus 2012, 22, 1681–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.B.; Henderson, Z. Nicotine induction of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience 2010, 166, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Melichercik, A.M.; Elliott, K.S.; Bianchi, C.; Ernst, S.M.; Winters, B.D. Nicotinic receptor activation in perirhinal cortex and hippocampus enhances object memory in rats. Neuropharmacology 2012, 62, 2096–2105. [Google Scholar] [CrossRef]
- Parameshwaran, K.; Buabeid, M.A.; Karuppagounder, S.S.; Uthayathas, S.; Thiruchelvam, K.; Shonesy, B.; Dityatev, A.; Escobar, M.C.; Dhanasekaran, M.; Suppiramaniam, V. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cell Life Sci. 2012, 69, 829–841. [Google Scholar] [CrossRef]
- Birthelmer, A.; Stemmelin, J.; Jackisch, R.; Cassel, J.C. Presynaptic modulation of acetylcholine, noradrenaline, and serotonin release in the hippocampus of aged rats with various levels of memory impairments. Brain Res. Bull. 2003, 60, 283–296. [Google Scholar] [CrossRef]
- Sharp, B.M.; Yatsula, M.; Fu, Y. Effects of galantamine, a nicotinic allosteric potentiating ligand, on nicotine-induced catecholamine release in hippocampus and nucleus accumbens of rats. J. Pharm. Exp. 2004, 309, 1116–1123. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Dechkovskaia, A.; Mehta-Simmons, H.; Guan, X.; Khan, W.; Abou-Donia, M. Increased expression of glial fibrillary acidic protein in cerebellum and hippocampus: Differential effects on neonatal brain regional acetylcholinesterase following maternal exposure to combined chlorpyrifos and nicotine. J. Toxicol. Environ. Health Part A 2003, 66, 2047–2066. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ameno, K.; Miki, T.; Tanaka, N.; Ohkubo, E.; Kinoshita, H. Effects of systemic nicotine, alcohol or their combination on cholinergic markers in the frontal cortex and hippocampus of rat. Neurochem. Res. 2010, 35, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Court, J.A.; Johnson, M.; Smith, C.J.; James, V.; Cheng, A.V.; Kerwin, J.M.; Morris, C.M.; Piggott, M.A.; Edwardson, J.A.; et al. Autoradiographic comparison of cholinergic and other transmitter receptors in the normal human hippocampus. Hippocampus 1993, 3, 307–315. [Google Scholar] [CrossRef]
- Simchovitz, A.; Heneka, M.T.; Soreq, H. Personalized genetics of the cholinergic blockade of neuroinflammation. J. Neurochem. 2017, 142 (Suppl. 2), 178–187. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, J.M.; Malleret, G.; Touzani, K.; Vronskaya, S.; Ishii, S.; Kandel, E.R.; Barco, A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004, 42, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Sun, L.D.; Atkins, C.M.; Soderling, T.R.; Wilson, M.A.; Tonegawa, S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 2001, 106, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Alkadhi, K.A.; Alzoubi, K.H.; Srivareerat, M.; Tran, T.T. Chronic psychosocial stress exacerbates impairment of synaptic plasticity in beta-amyloid rat model of Alzheimer’s disease: Prevention by nicotine. Curr. Alzheimer Res. 2011, 8, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Haghparast, A.; Taslimi, Z.; Ramin, M.; Azizi, P.; Khodagholi, F.; Hassanpour-Ezatti, M. Changes in phosphorylation of CREB, ERK, and c-fos induction in rat ventral tegmental area, hippocampus and prefrontal cortex after conditioned place preference induced by chemical stimulation of lateral hypothalamus. Behav. Brain Res. 2011, 220, 112–118. [Google Scholar] [CrossRef]
- Kenney, J.W.; Poole, R.L.; Adoff, M.D.; Logue, S.F.; Gould, T.J. Learning and nicotine interact to increase CREB phosphorylation at the jnk1 promoter in the hippocampus. PLoS ONE 2012, 7, e39939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.F.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE 2010, 5, e15497. [Google Scholar] [CrossRef] [Green Version]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Zhu, J.; Zhong, Q.; Shi, C.; Dang, Y.; Han, W.; Liu, X.; Xu, M.; Chen, T. Distinct roles of methamphetamine in modulating spatial memory consolidation, retrieval, reconsolidation and the accompanying changes of ERK and CREB activation in hippocampus and prefrontal cortex. Neuropharmacology 2013, 67, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.H.; Kwon, S.H.; Lee, S.Y.; Jang, C.G. Liquiritigenin ameliorates memory and cognitive impairment through cholinergic and BDNF pathways in the mouse hippocampus. Arch. Pharm. Res. 2017, 40, 1209–1217. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.; Zhou, L.; Wan, L. RACK1 promotes maintenance of morphine-associated memory via activation of an ERK-CREB dependent pathway in hippocampus. Sci. Rep. 2016, 6, 20183. [Google Scholar] [CrossRef] [Green Version]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagaar, M.; Dao, A.; Levine, A.; Alhaider, I.; Alkadhi, K. Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampus. Sleep 2013, 36, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Lambert, T.J.; Storm, D.R.; Sullivan, J.M. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS ONE 2010, 5, e15182. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.L.; Tamagnini, F.; Narduzzo, K.E.; Howarth, J.L.; Lee, Y.B.; Wong, L.F.; Brown, M.W.; Warburton, E.C.; Bashir, Z.I.; Uney, J.B. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur. J. Neurosci. 2012, 36, 2941–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balazsfi, D.; Farkas, L.; Csikota, P.; Fodor, A.; Zsebok, S.; Haller, J.; Zelena, D. Sex-dependent role of vesicular glutamate transporter 3 in stress-regulation and related anxiety phenotype during the early postnatal period. Stress 2016, 19, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.J.; Crowley, N.A.; Reiss, D.E.; Caulfield, J.I.; Luscher, B.; Cavigelli, S.A.; Kamens, H.M. Adolescent Social Stress Increases Anxiety-like Behavior and Alters Synaptic Transmission, Without Influencing Nicotine Responses, in a Sex-Dependent Manner. Neuroscience 2018, 373, 182–198. [Google Scholar] [CrossRef]
- Chmielarz, P.; Kreiner, G.; Nalepa, I. Selective ablation of glucocorticoid receptors in the noradrenergic system affects evening corticosterone levels in a sex-dependent manner. Pharm. Rep. 2015, 67, 1201–1203. [Google Scholar] [CrossRef]
- Grech, A.M.; Ratnayake, U.; Hannan, A.J.; van den Buuse, M.; Hill, R.A. Sex-Dependent Effects of Environmental Enrichment on Spatial Memory and Brain-Derived Neurotrophic Factor (BDNF) Signaling in a Developmental “Two-Hit” Mouse Model Combining BDNF Haploinsufficiency and Chronic Glucocorticoid Stimulation. Front. Behav. Neurosci. 2018, 12, 227. [Google Scholar] [CrossRef]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocr. 2014, 35, 370–384. [Google Scholar] [CrossRef] [Green Version]
- Gillies, G.E.; Virdee, K.; McArthur, S.; Dalley, J.W. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 2014, 282, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, O.O.F.; Coppolino, M.; George, S.R.; Perreault, M.L. Sex Differences in Dopamine Receptors and Relevance to Neuropsychiatric Disorders. Brain Sci. 2021, 11, 1199. [Google Scholar] [CrossRef]
- Pollak, D.D.; John, J.; Bubna-Littitz, H.; Schneider, A.; Hoeger, H.; Lubec, G. Components of the protein quality control system are expressed in a strain-dependent manner in the mouse hippocampus. Neurochem. Int. 2006, 49, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; John, J.; Scharl, T.; Leisch, F.; Schneider, A.; Hoeger, H.; Lubec, G. Strain-dependent regulation of neurotransmission and actin-remodelling proteins in the mouse hippocampus. Genes Brain Behav. 2006, 5, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; John, J.; Schneider, A.; Hoeger, H.; Lubec, G. Strain-dependent expression of signaling proteins in the mouse hippocampus. Neuroscience 2006, 138, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; Scharl, T.; Leisch, F.; Herkner, K.; Villar, S.R.; Hoeger, H.; Lubec, G. Strain-dependent regulation of plasticity-related proteins in the mouse hippocampus. Behav. Brain Res. 2005, 165, 240–246. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, T.; Velarde Gamez, D.; Schuld, G.J.; Bormann, D.; Cabatic, M.; Uhrin, P.; Lubec, G.; Monje, F.J. Age-Dependent and Pathway-Specific Bimodal Action of Nicotine on Synaptic Plasticity in the Hippocampus of Mice Lacking the miR-132/212 Genes. Cells 2022, 11, 261. https://doi.org/10.3390/cells11020261
Stojanovic T, Velarde Gamez D, Schuld GJ, Bormann D, Cabatic M, Uhrin P, Lubec G, Monje FJ. Age-Dependent and Pathway-Specific Bimodal Action of Nicotine on Synaptic Plasticity in the Hippocampus of Mice Lacking the miR-132/212 Genes. Cells. 2022; 11(2):261. https://doi.org/10.3390/cells11020261
Chicago/Turabian StyleStojanovic, Tamara, David Velarde Gamez, Gabor Jorrid Schuld, Daniel Bormann, Maureen Cabatic, Pavel Uhrin, Gert Lubec, and Francisco J. Monje. 2022. "Age-Dependent and Pathway-Specific Bimodal Action of Nicotine on Synaptic Plasticity in the Hippocampus of Mice Lacking the miR-132/212 Genes" Cells 11, no. 2: 261. https://doi.org/10.3390/cells11020261