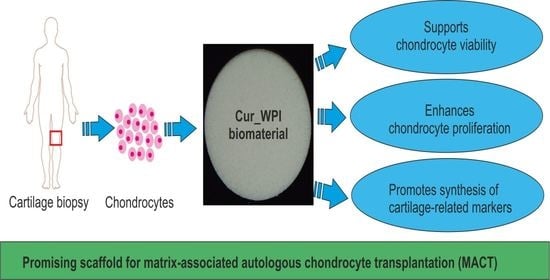
Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation—A Pilot In-Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Freeze-Dried Curdlan/Whey Protein Isolate-Based Scaffold
2.2. Macro- and Microstructural Characterization
2.3. Evaluation of Mechanical Properties
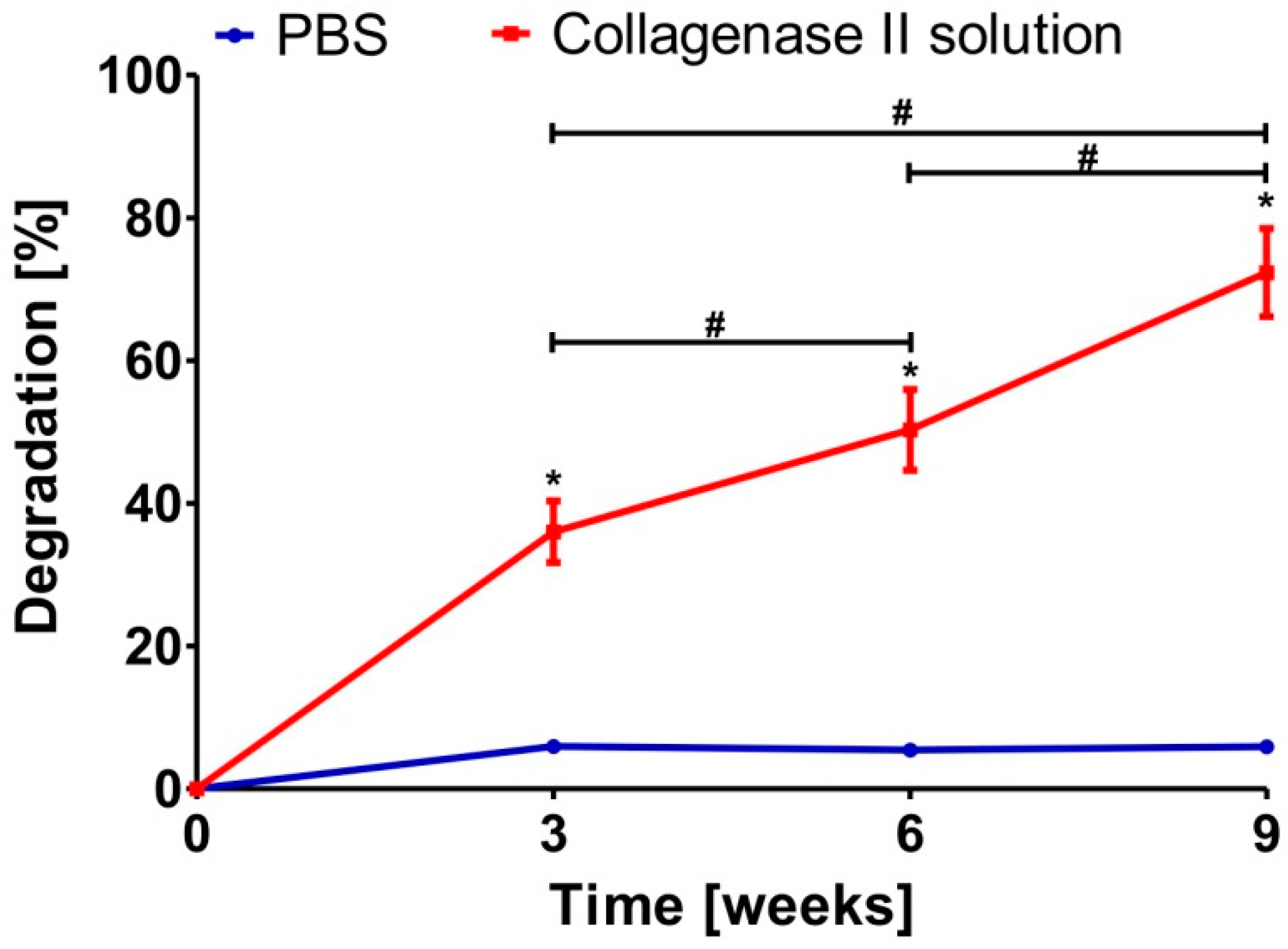
2.4. Evaluation of Susceptibility of Biomaterial to Enzymatic Degradation
2.5. Evaluation of Chondrocyte–Biomaterial Interactions In Vitro
2.5.1. Isolation and Identification of Primary Human Chondrocytes
2.5.2. Assessment of Chondrocyte Viability
2.5.3. Assessment of Chondrocyte Proliferation
2.5.4. Assessment of Expression of Cartilage-Specific Genes
2.5.5. Microscope Observations of Cartilage-Related Markers
2.6. Statistical Analysis
3. Results and Discussion
3.1. Macro- and Microstructures of Scaffold
3.2. Mechanical Properties of Scaffold
3.3. Enzymatic Biodegradation of Scaffold
3.4. Characterization of Primary Human Chondrocytes
3.5. Cytocompatibility of Scaffold
3.5.1. Cell Viability
3.5.2. Cell Proliferation
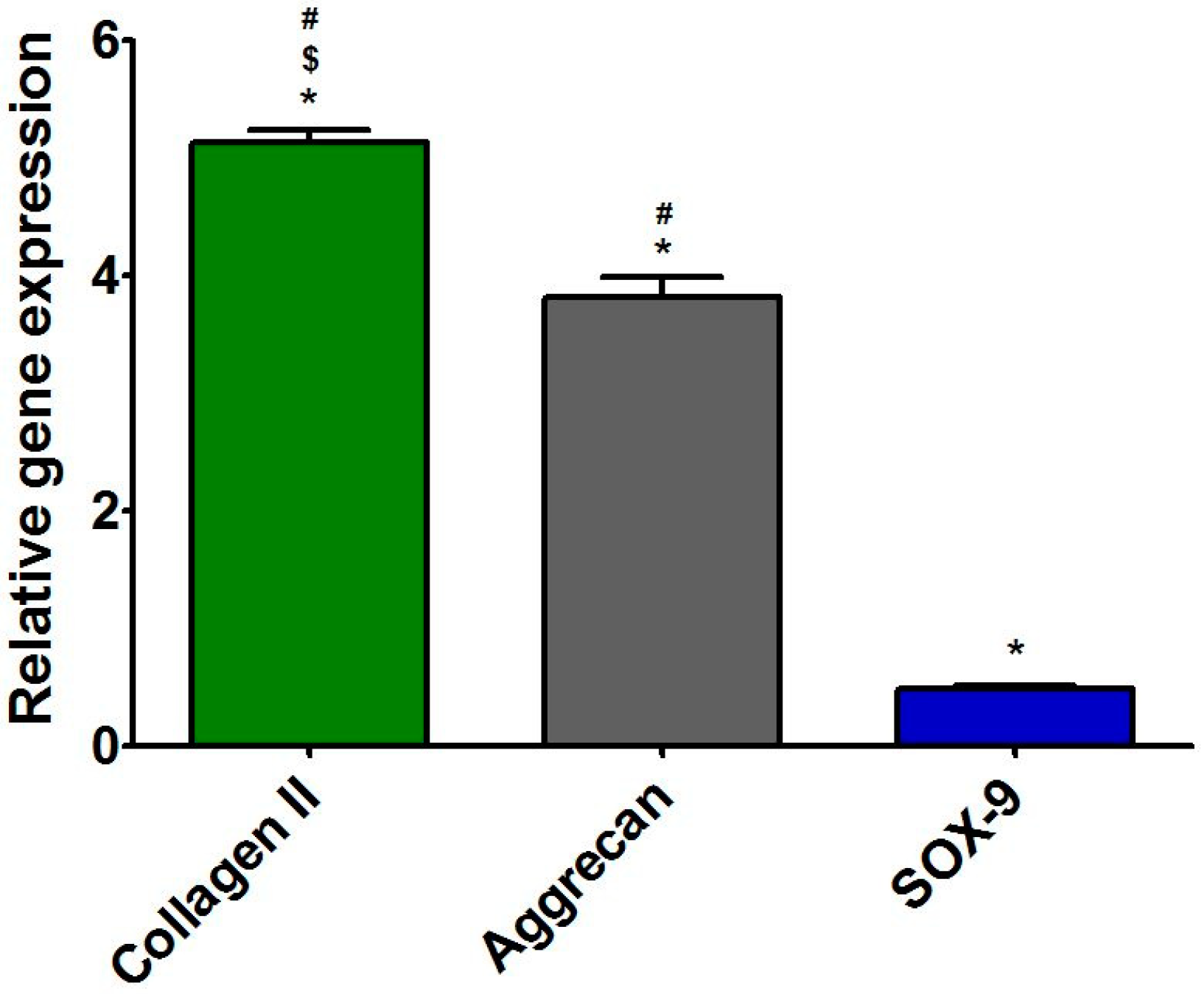
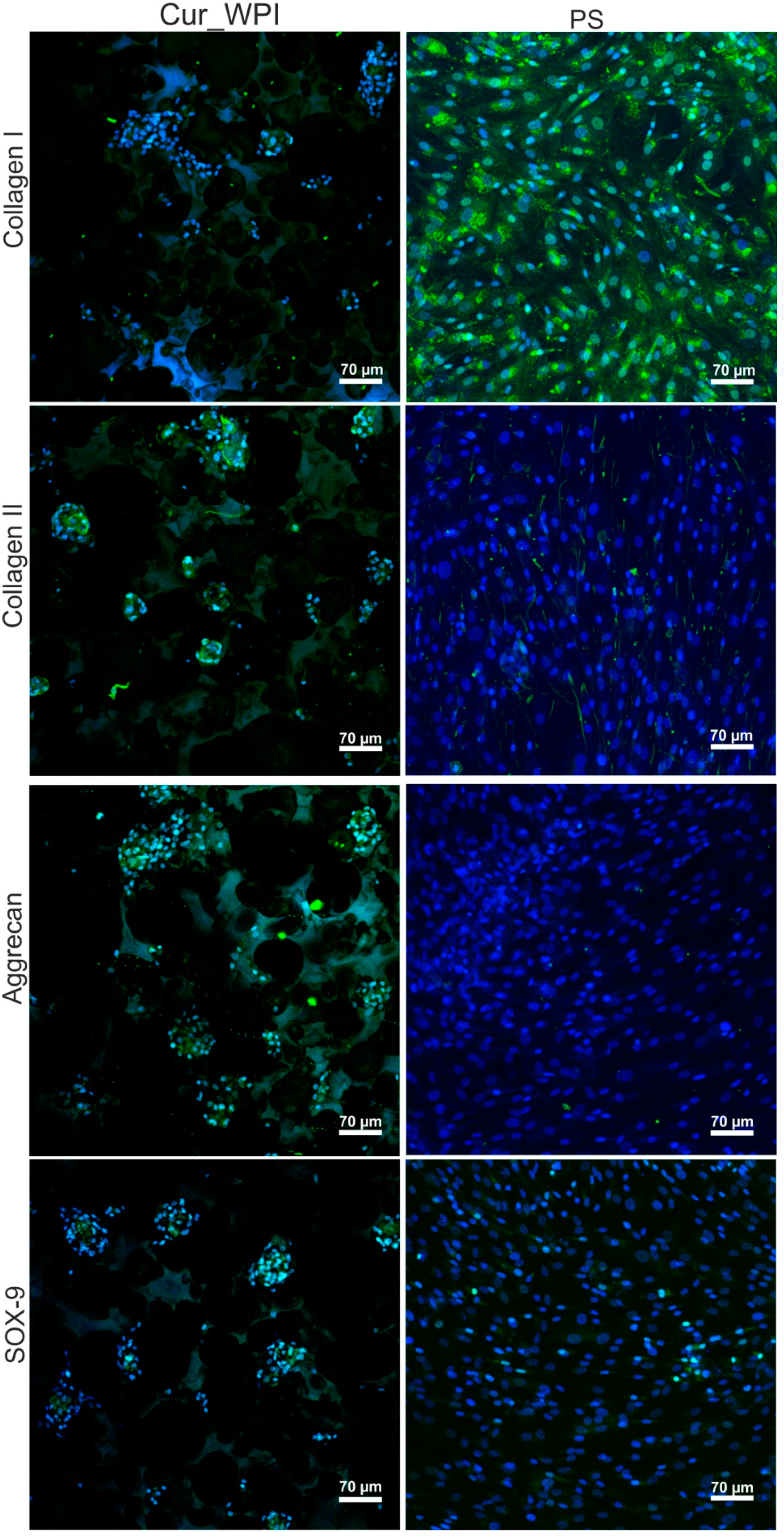
3.5.3. Presence of Cartilage-Specific Markers—RT-qPCR Analysis and Immunofluorescence Staining
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Deng, C.; Xu, C.; Zhou, Q.; Cheng, Y. Advances of nanotechnology in osteochondral regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1576. [Google Scholar] [CrossRef]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Cassar-Gheiti, A.J.; Burke, N.G.; Cassar-Gheiti, T.M.; Mulhall, K.J. Charper 6- Chondral Lesion in the Hip Joint and Current Chondral Repair Techniques. In Cartilage Repair and Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; IntechOpen: London, UK, 2018; pp. 103–122. [Google Scholar]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Cao, Y. Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer Fiber Scaffolds for Bone and Cartilage Tissue Engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar] [CrossRef]
- Dewan, A.K.; Gibson, M.A.; Elisseeff, J.H.; Trice, M.E. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed. Res. Int. 2014, 2014, 272481. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonk, L.A.; Roël, G.; Hernigou, J.; Kaps, C.; Hernigou, P. Role of matrix-associated autologous chondrocyte implantation with spheroids in the treatment of large chondral defects in the knee: A systematic review. Int. J. Mol. Sci. 2021, 22, 7149. [Google Scholar] [CrossRef]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)-5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef]
- Anders, S.; Schaumburger, J.; Schubert, T.; Grifka, J.; Behrens, P. Matrix-associated autologous chondrocyte transplantation (MACT). Minimally invasive technique in the knee. Oper. Orthop. Traumatol. 2008, 20, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.; Smith, R.C.; Robertson, B.; Wood, D.; Zheng, M.H. Matrix-associated autologous chondrocyte transplantation and implantation for an osteochondral defect after cement treatment of a giant cell tumor. Curr. Orthop. Pract. 2009, 20, 575–578. [Google Scholar] [CrossRef]
- Zellner, J.; Krutsch, W.; Pfeifer, C.G.; Koch, M.; Nerlich, M.; Angele, P. Autologous chondrocyte implantation for cartilage repair: Current perspectives. Orthop. Res. Rev. 2015, 7, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Binder, H.; Hoffman, L.; Zak, L.; Tiefenboeck, T.; Aldrian, S.; Albrecht, C. Clinical evaluation after matrix-associated autologous chondrocyte transplantation: A comparison of four different graft types. Bone Jt. Res. 2021, 10, 370–379. [Google Scholar] [CrossRef]
- Marlovits, S.; Zeller, P.; Singer, P.; Resinger, C.; Vécsei, V. Cartilage repair: Generations of autologous chondrocyte transplantation. Eur. J. Radiol. 2006, 57, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tognana, E.; Borrione, A.; De Luca, C.; Pavesio, A. Hyalograft® C: Hyaluronan-based scaffolds in tissue-engineered cartilage. Cells Tissues Organs 2007, 186, 97–103. [Google Scholar] [CrossRef]
- Gille, J.; Behrens, P.; Schulz, A.P.; Oheim, R.; Kienast, B. Matrix-Associated Autologous Chondrocyte Implantation: A Clinical Follow-Up at 15 Years. Cartilage 2016, 7, 309–315. [Google Scholar] [CrossRef]
- Gursoy, S.; Akkaya, M.; Simsek, M.E.; Gursoy, M.; Dogan, M.; Bozkurt, M. Factors Influencing the Results in Matrix-Associated Autologous Chondrocyte Implantation: A 2–5 Year Follow-Up Study. J. Clin. Med. Res. 2019, 11, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Hoburg, A.; Löer, I.; Körsmeier, K.; Siebold, R.; Niemeyer, P.; Fickert, S.; Ruhnau, K. Matrix-Associated Autologous Chondrocyte Implantation Is an Effective Treatment at Midterm Follow-up in Adolescents and Young Adults. Orthop. J. Sport. Med. 2019, 7, 2325967119841077. [Google Scholar] [CrossRef] [Green Version]
- Pinker, K.; Szomolanyi, P.; Welsch, G.C.; Mamisch, T.C.; Marlovits, S.; Stadlbauer, A.; Trattnig, S. Longitudinal evaluation of cartilage composition of matrix-associated autologous chondrocyte transplants with 3-T delayed gadolinium-enhanced MRI of cartilage. Am. J. Roentgenol. 2008, 191, 1391–1396. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Boffa, A.; Andriolo, L.; Reale, D.; Busacca, M.; Di Martino, A.; Filardo, G. Mosaicplasty versus matrix-assisted autologous chondrocyte transplantation for knee cartilage defects: A long-term clinical and imaging evaluation. Appl. Sci. 2020, 10, 4615. [Google Scholar] [CrossRef]
- Mamisch, T.C.; Menzel, M.I.; Welsch, G.H.; Bittersohl, B.; Salomonowitz, E.; Szomolanyi, P.; Kordelle, J.; Marlovits, S.; Trattnig, S. Steady-state diffusion imaging for MR in-vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3 tesla-Preliminary results. Eur. J. Radiol. 2008, 65, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Laute, V.; Zinser, W.; John, T.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Oseni, A.O.; Butler, P.E.; Seifalian, A.M. Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J. Surg. Res. 2013, 181, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Naranda, J.; Gradišnik, L.; Gorenjak, M.; Vogrin, M.; Maver, U. Isolation and characterization of human articular chondrocytes from surgical waste after total knee arthroplasty (TKA). PeerJ 2017, 2017, e3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Tan, S.W.; Fakurazi, S. Optimization of Protocol for Isolation of Chondrocytes from Human Articular Cartilage. Cartilage 2019, 13, 872S–884S. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 2013, 3, 71–85. [Google Scholar]
- Xu, J.; Wang, W.; Ludeman, M.; Cheng, K.; Hayami, T.; Lotz, J.C.; Kapila, S. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng. Part A 2008, 14, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Primer-BLAST Tool. Available online: http://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 12 April 2021).
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Seo, S.J.; Mahapatra, C.; Singh, R.K.; Knowles, J.C.; Kim, H.W. Strategies for osteochondral repair: Focus on scaffolds. J. Tissue Eng. 2014, 5, 2041731414541850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Huang, J.; Narayan, R.J. Gradient scaffolds for osteochondral tissue engineering and regeneration. J. Mater. Chem. B 2020, 8, 8149–8170. [Google Scholar] [CrossRef]
- Nanda, H.S.; Chen, S.; Zhang, Q.; Kawazoe, N.; Chen, G. Collagen scaffolds with controlled insulin release and controlled pore structure for cartilage tissue engineering. Biomed. Res. Int. 2014, 2014, 623805. [Google Scholar] [CrossRef]
- Rogan, H.; Ilagan, F.; Tong, X.; Chu, C.R.; Yang, F. Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes. Biomaterials 2020, 228, 119579. [Google Scholar] [CrossRef] [PubMed]
- Horan, R.L.; Antle, K.; Collette, A.L.; Wang, Y.; Huang, J.; Moreau, J.E.; Volloch, V.; Kaplan, D.L.; Altman, G.H. In vitro degradation of silk fibroin. Biomaterials 2005, 26, 3385–3393. [Google Scholar] [CrossRef]
- Ahn, G.; Kim, Y.; Lee, S.W.; Jeong, Y.J.; Son, H.; Lee, D. Effect of heterogeneous multi-layered gelatin scaffolds on the diffusion characteristics and cellular activities of preosteoblasts. Macromol. Res. 2014, 22, 99–107. [Google Scholar] [CrossRef]
- Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; van Donkelaar, C.C.; Thies, J.C.; van Rhijn, L.W.; Emans, P.J. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Chen, Y.; Dong, S.; Chen, X.; Zhou, Y. Poly(lactic-co-glycolic) acid/nanohydroxyapatite scaffold containing chitosan microspheres with adrenomedullin delivery for modulation activity of osteoblasts and vascular endothelial cells. Biomed. Res. Int. 2013, 2013, 530712. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, S.; Garreau, H.; Vert, M. Selective enzymatic degradations of poly(L-lactide) and poly(∈-caprolactone) blend films. Biomacromolecules 2000, 1, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Sca ff olds for Tissue Engineering. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomater. Silver Jubil. Compend. 2000, 21, 175–189. [Google Scholar]
- Xu, F.; Xu, L.; Wang, Q.; Ye, Z.; Zhou, Y.; Tan, W.S. 3D dynamic culture of rabbit articular chondrocytes encapsulated in alginate gel beads using spinner flasks for cartilage tissue regeneration. Biomed. Res. Int. 2014, 2014, 539789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, M.; Seidel, J.; Vunjak-Novakovic, G.; Freed, L.E. Growth factors for sequential cellular de- and re-differentation in tissue engineering. Biochem. Biophys. Res. Commun. 2002, 294, 149–154. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan / agarose / hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Homicz, M.R.; Chia, S.H.; Schumacher, B.L.; Masuda, K.; Thonar, E.J.; Sah, R.L.; Watson, D. Human septal chondrocyte redifferentiation in alginate, polyglycolic acid scaffold, and monolayer culture. Laryngoscope 2003, 113, 25–32. [Google Scholar] [CrossRef]
- Malda, J.; Kreijveld, E.; Temenoff, J.S.; Van Blitterswijk, C.A.; Riesle, J. Expansion of human nasal chondrocytes on macroporous microcarriers enhances redifferentiation. Biomaterials 2003, 24, 5153–5161. [Google Scholar] [CrossRef]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef]
- Miot, S.; Woodfield, T.; Daniels, A.U.; Suetterlin, R.; Peterschmitt, I.; Heberer, M.; Van Blitterswijk, C.A.; Riesle, J.; Martin, I. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials 2005, 26, 2479–2489. [Google Scholar] [CrossRef]
- Wang, Y.; Blasioli, D.J.; Kim, H.J.; Kim, H.S.; Kaplan, D.L. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Pati, F.; Chameettachal, S.; Pahwa, S.; Ray, A.R.; Dhara, S.; Ghosh, S. Enhanced Redifferentiation of Chondrocytes on Microperiodic Silk/Gelatin Scaffolds: Toward Tailor-Made Tissue Engineering. Biomacromolecules 2013, 14, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Giralt, N.; Izquierdo, R.; Nogués, X.; Perez-Olmedilla, M.; Benito, P.; Gómez-Ribelles, J.L.; Checa, M.A.; Suay, J.; Caceres, E.; Monllau, J.C. A porous PCL scaffold promotes the human chondrocytes redifferentiation and hyaline-specific extracellular matrix protein synthesis. J. Biomed. Mater. Res. Part A 2008, 85, 1082–1089. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Li, X.; Zhong, D.; Li, Y.; Li, J.; Jin, R. Strategies to Modulate the Redifferentiation of Chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 764193. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Nürnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: A comparative study. Osteoarthr. Cartil. 2011, 19, 1219–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Kolettas, E.; Muir, H.I.; Barrett, J.C.; Hardingham, T.E. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology 2001, 40, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| collagen type I (COL1A1) | F: GGCCCAGAAGAACTGGTACA R: AATCCATCGGTCATGCTCTC | 81 |
| collagen type II (COL2A1) | F: GGCAATAGCAGGTTCACGTACA R: CGATAACAGTCTTGCCCCACTTA | 79 |
| aggrecan (ACAN) | F: AGCCTGCGCTCCAATGACT R: TAATGGAACACGATGCCTTTCA | 107 |
| SRY-box transcription factor 9 (SOX-9) | F: GAGACTTCTGAACGAGAGCGA R: CGTTCTTCACCGACTTCCTCC | 125 |
| glyceraldehyde-3-Phosphate dehydrogenase (GAPDH) | F: CACCACACTGAATCTCCCCT R: TGGTTGAGCACAGGGTACTT | 115 |
| Young’s Modulus (MPa) ± SD |
|---|
| 0.849 ± 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, K.; Tarczynska, M.; Truszkiewicz, W.; Gaweda, K.; Douglas, T.E.L.; Ginalska, G. Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation—A Pilot In-Vitro Study. Cells 2022, 11, 282. https://doi.org/10.3390/cells11020282
Klimek K, Tarczynska M, Truszkiewicz W, Gaweda K, Douglas TEL, Ginalska G. Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation—A Pilot In-Vitro Study. Cells. 2022; 11(2):282. https://doi.org/10.3390/cells11020282
Chicago/Turabian StyleKlimek, Katarzyna, Marta Tarczynska, Wieslaw Truszkiewicz, Krzysztof Gaweda, Timothy E. L. Douglas, and Grazyna Ginalska. 2022. "Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation—A Pilot In-Vitro Study" Cells 11, no. 2: 282. https://doi.org/10.3390/cells11020282