Endogenous pH 6.0 β-Galactosidase Activity Is Linked to Neuronal Differentiation in the Olfactory Epithelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Tissue Processing
2.2. Detection of β-Galactosidase Activity
2.3. Immunohistochemistry
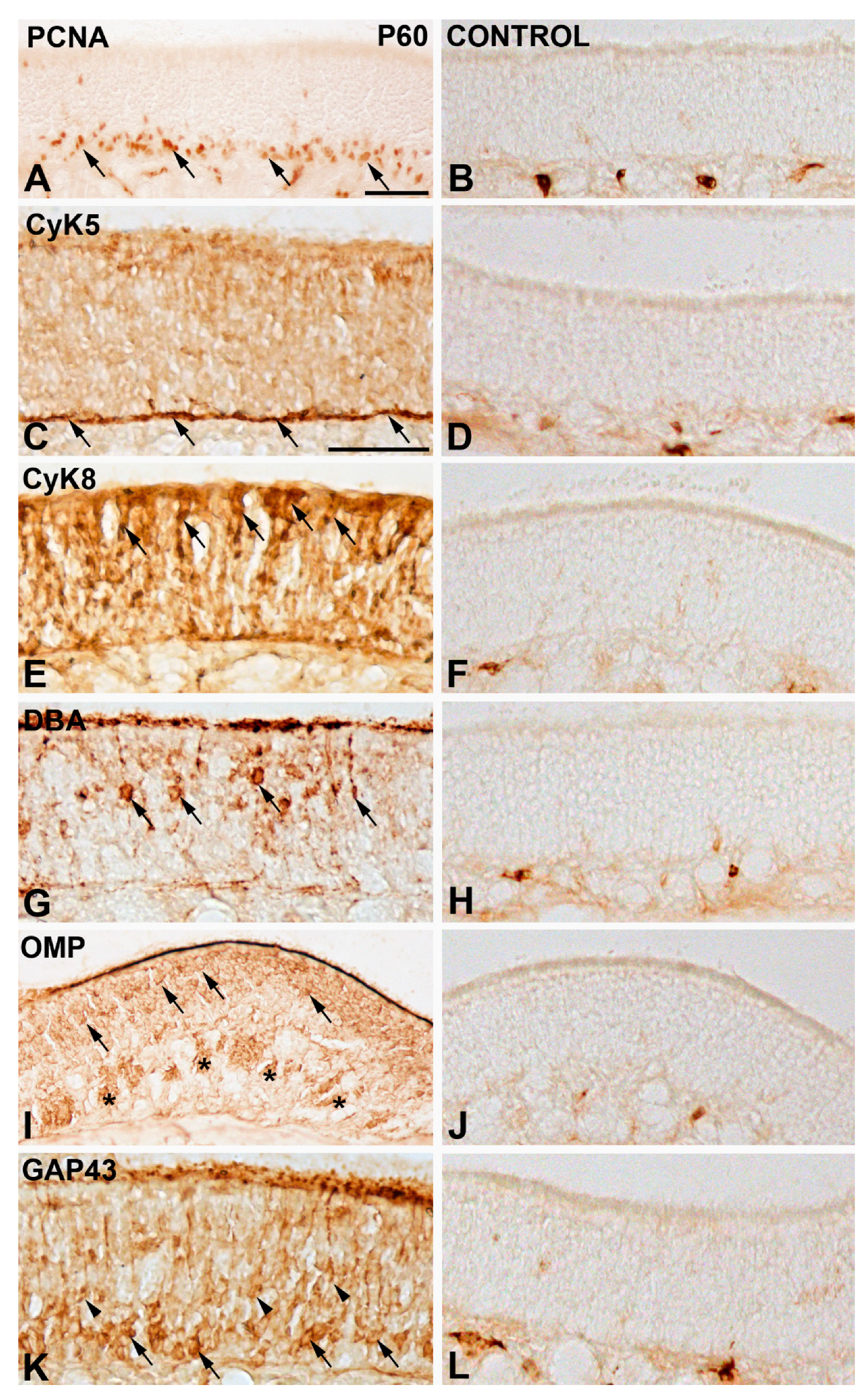
- Goat anti-olfactory-marker-protein (OMP) polyclonal antibody, used at 1:100 (Wako Chemicals, Neuss, Germany; Ref. 544-10001/IUP1001), which identifies mature olfactory sensory neurons [33].
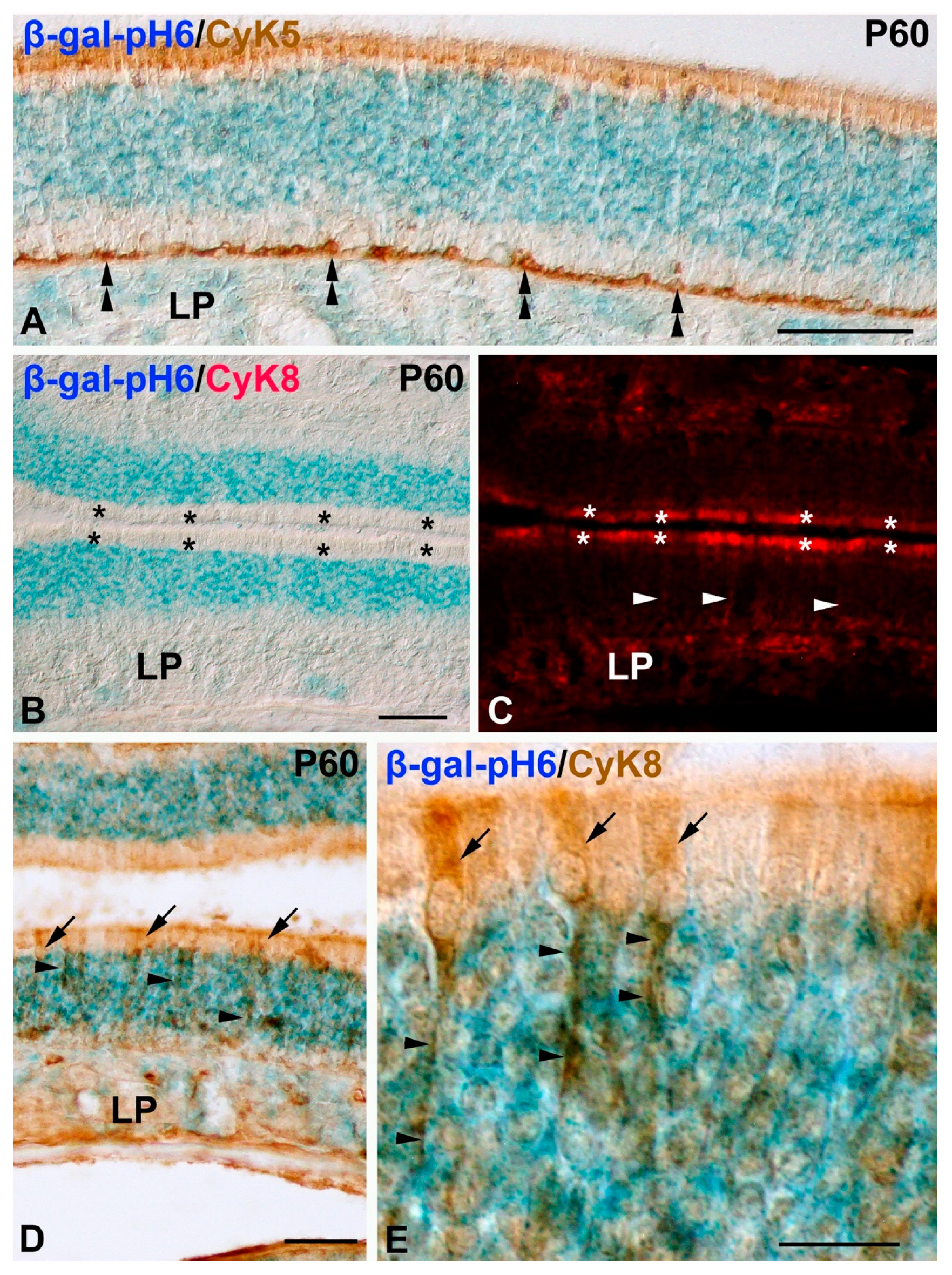
- Mouse anti-cytokeratin 8 (CyK8) monoclonal antibody, used at 1:50 (Novusbio, Centennial, CO, USA, Ref. NB120-9287), which stains supporting cells in the olfactory epithelium [34].
- Rabbit anti-Growth associated protein-43 (GAP43) polyclonal antibody, used at 1:200 (Millipore, Darmstadt, Germany; Ref. AB5220), a marker of immature neurons in the olfactory system [38].
2.4. Dolichos Biflorus Agglutinin (DBA) Histochemistry
2.5. TUNEL Technique
2.6. Image Acquisition and Processing
3. Results
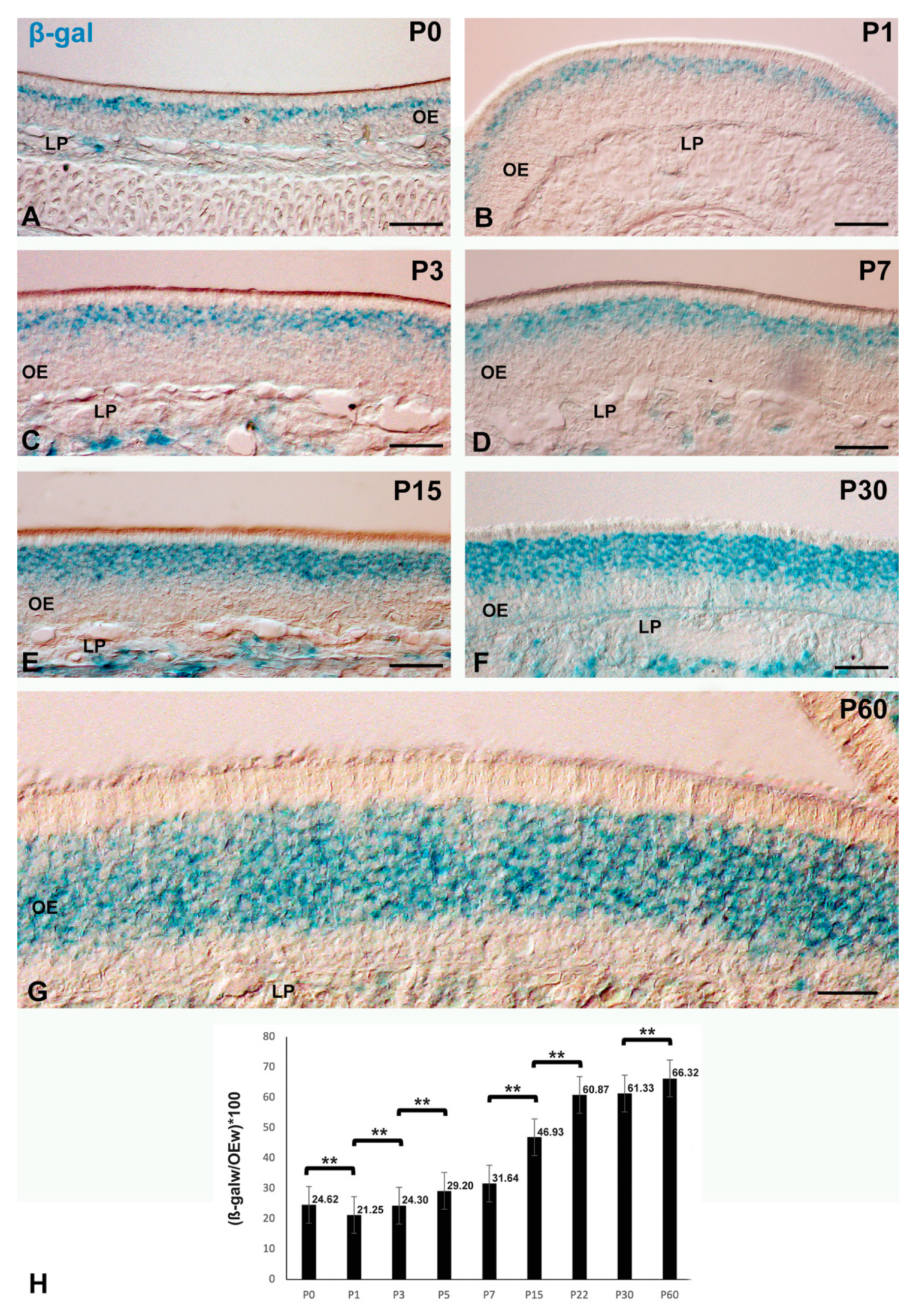
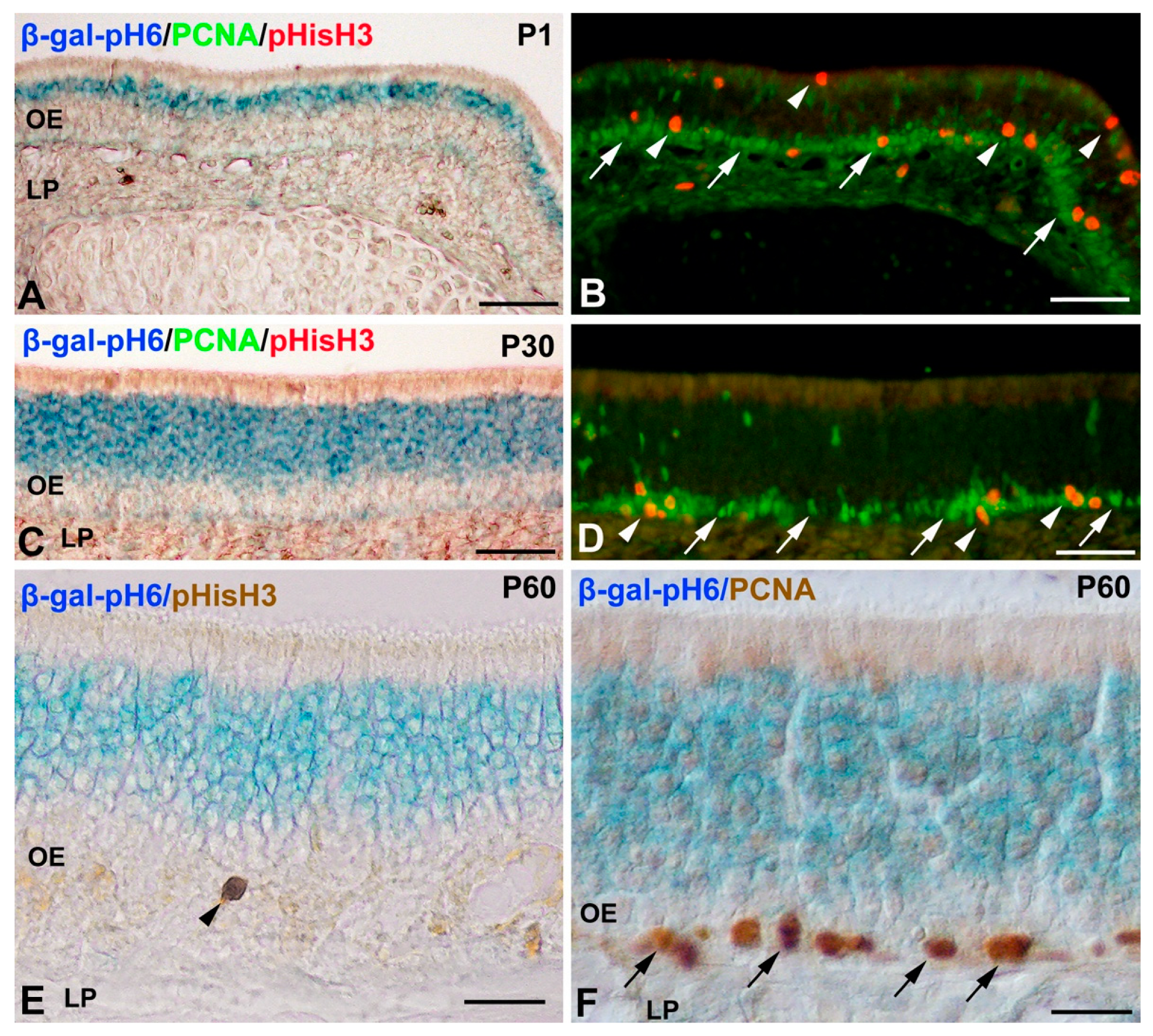
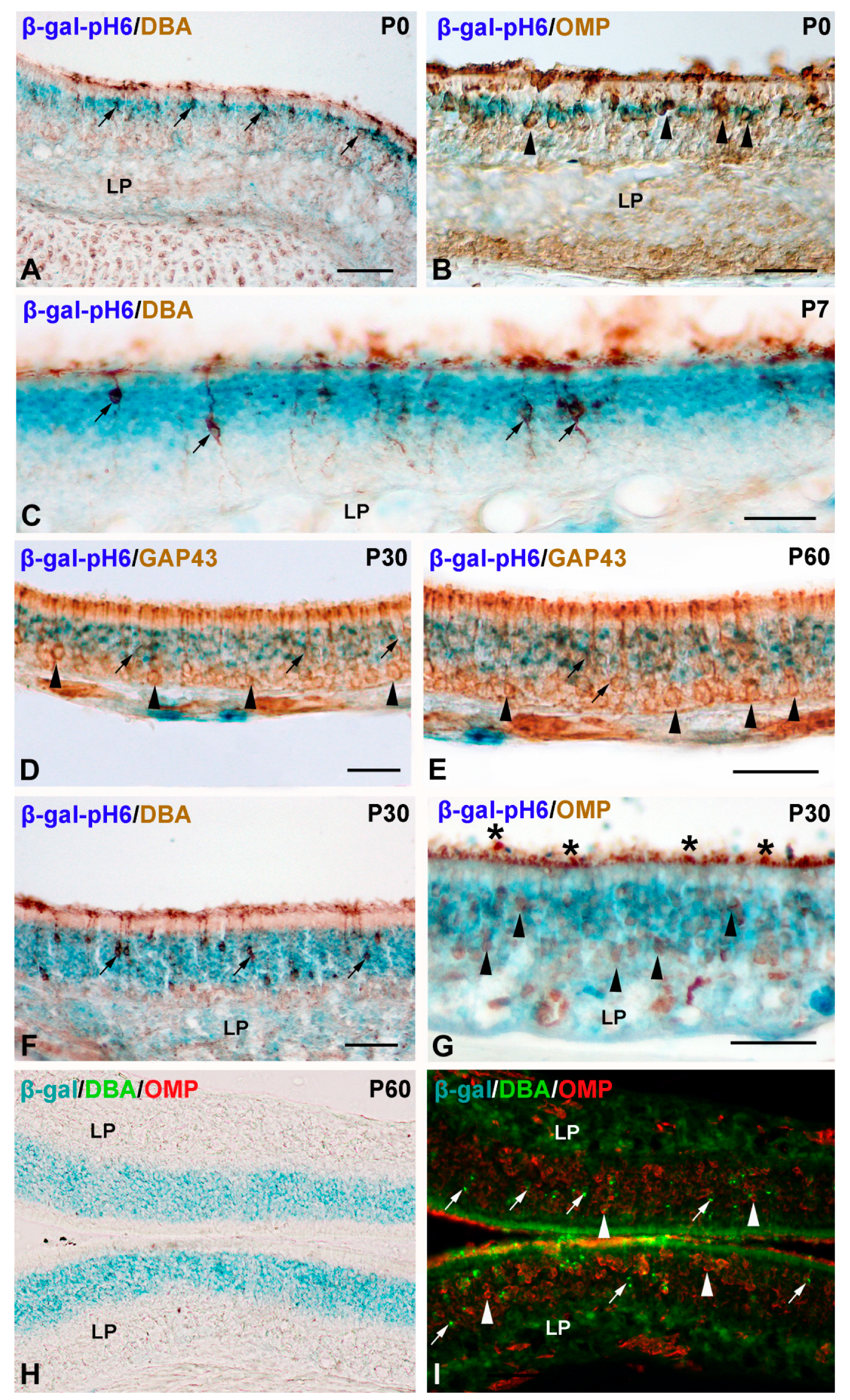
Histochemistry of β-gal-pH6 in the Developing Postnatal Mouse Olfactory Epithelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A Biomarker That Identifies Senescent Human Cells in Culture and in Aging Skin in Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Βgal) Activity, a Biomarker of Senescent Cells in Culture and In Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Campisi, J. Senescent Cells, Tumor Suppression, and Organismal Aging: Good Citizens, Bad Neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Chakraborty, S.; Bhattacharya, R.; Chowdhury, G. Senescence-Induced Chemoresistance in Triple Negative Breast Cancer and Evolution-Based Treatment Strategies. Front. Oncol. 2021, 11, 674354. [Google Scholar] [CrossRef]
- Banito, A.; Lowe, S.W. XA New Development in Senescence. Cell 2013, 155, 977–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [Green Version]
- Lorda-Díez, C.I.; Montero, J.A.; García-Porrero, J.A.; Hurlé, J.M. Interdigital Tissue Regression in the Developing Limb of Vertebrates. Int. J. Dev. Biol. 2015, 59, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorda-Díez, C.I.; Solis-Mancilla, M.E.; Sánchez-Fernández, C.; García-Porrero, J.A.; Hurlé, J.M.; Montero, J.A. Cell Senescence, Apoptosis and DNA Damage Cooperate in the Remodeling Processes Accounting for Heart Morphogenesis. J. Anat. 2019, 234, 815–829. [Google Scholar] [CrossRef]
- Montero, J.A.; Lorda-Díez, C.I.; Hurlé, J.M. Confluence of Cellular Degradation Pathways During Interdigital Tissue Remodeling in Embryonic Tetrapods. Front. Cell Dev. Biol. 2020, 8, 1217. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ibeas, P.; Gimeno, I.; Cañón-Beltrán, K.; Gutiérrez-Adán, A.; Rizos, D.; Gómez, E. Senescence and Apoptosis During in Vitro Embryo Development in a Bovine Model. Front. Cell Dev. Biol. 2020, 8, 1646. [Google Scholar] [CrossRef] [PubMed]
- Antelo-Iglesias, L.; Picallos-Rabina, P.; Estévez-Souto, V.; Da Silva-Álvarez, S.; Collado, M. The Role of Cellular Senescence in Tissue Repair and Regeneration. Mech. Ageing Dev. 2021, 198, 111528. [Google Scholar] [CrossRef]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is Senescence-Associated β-Galactosidase a Reliable in Vivo Marker of Cellular Senescence During Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence Is a Developmental Mechanism That Contributes to Embryonic Growth and Patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Rivera-Pérez, J.A. Senescence-Associated β-Galactosidase Activity Marks the Visceral Endoderm of Mouse Embryos but Is Not Indicative of Senescence. Genesis 2014, 52, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapieha, P.; Mallette, F.A. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol. 2018, 28, 595–607. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Wan, T.; Miwa, S. Senescence in Post-Mitotic Cells: A Driver of Aging? Antioxid. Redox Signal. 2020, 1, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, H.; Huang, X.; Tang, J.; Zhang, S.; Li, Y.; Liu, X.; He, L.; Ju, Z.; Lui, K.O.; et al. Embryonic Senescent Cells Re-Enter Cell Cycle and Contribute to Tissues after Birth. Cell Res. 2018, 28, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Jill Saffrey, M.; Cameron, K.; et al. Postmitotic Neurons Develop a P21-Dependent Senescence-like Phenotype Driven by a DNA Damage Response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piechota, M.; Sunderland, P.; Wysocka, A.; Nalberczak, M.; Sliwinska, M.A.; Radwanska, K.; Sikora, E. Is Senescence-Associated β-Galactosidase a Marker of Neuronal Senescence? Oncotarget 2016, 7, 81099–81109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau Protein Aggregation Is Associated with Cellular Senescence in the Brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, M.; Kovacovicova, K.; Bonomini, F.; Rezzani, R.; Frohlich, J.; Vinciguerra, M. Senescence-like Phenotype in Post-Mitotic Cells of Mice Entering Middle Age. Aging (Albany. NY) 2020, 12, 13979–13990. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Bautista, J.A.; Acevo-Rodríguez, P.S.; Castro-Obregón, S. Programmed Cell Senescence in the Mouse Developing Spinal Cord and Notochord. Front. Cell Dev. Biol. 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Senescence-Associated β-Galactosidase Activity in the Developing Avian Retina. Dev. Dyn. 2019, 248, 850–865. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernán, G.; De Mera-Rodríguez, J.; Gañán, Y.; Solana-Fajardo, J.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Development and Postnatal Neurogenesis in the Retina: A Comparison between Altricial and Precocial Bird Species. Neural Regen. Res. 2021, 16, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Sokpor, G.; Abbas, E.; Rosenbusch, J.; Staiger, J.F.; Tuoc, T. Transcriptional and Epigenetic Control of Mammalian Olfactory Epithelium Development. Mol. Neurobiol. 2018, 55, 8306–8327. [Google Scholar] [CrossRef] [PubMed]
- Calof, A.L.; Hagiwara, N.; Holcomb, J.D.; Mumm, J.S.; Shou, J. Neurogenesis and Cell Death in Olfactory Epithelium. J. Neurobiol. 1996, 30, 67–81. [Google Scholar] [CrossRef]
- Kondo, K.; Suzukawa, K.; Sakamoto, T.; Watanabe, K.; Kanaya, K.; Ushio, M.; Yamaguchi, T.; Nibu, K.I.; Kaga, K.; Yamasoba, T. Age-Related Changes in Cell Dynamics of the Postnatal Mouse Olfactory Neuroepithelium: Cell Proliferation, Neuronal Differentiation, and Cell Death. J. Comp. Neurol. 2010, 518, 1962–1975. [Google Scholar] [CrossRef]
- Caggiano, M.; Kauer, J.S.; Hunter, D.D. Globose Basal Cells Are Neuronal Progenitors in the Olfactory Epithelium: A Lineage Analysis Using a Replication-Incompetent Retrovirus. Neuron 1994, 13, 339–352. [Google Scholar] [CrossRef]
- Schwob, J.E.; Huard, J.M.T.; Luskin, M.B.; Youngentob, S.L. Retroviral Lineage Studies of the Rat Olfactory Epithelium. Chem. Senses 1994, 19, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Carr, V.M.; Farbman, A.I. The dynamics of cell death in the olfactory epithelium. Exp. Neurol. 1993, 124, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, T.J. Apparent Apoptotic Cell Death in the Olfactory Epithelium of Adult Rodents: Death Occurs at Different Developmental Stages. J. Comp. Neurol. 1996, 372, 457–464. [Google Scholar] [CrossRef]
- Voyron, S.; Giacobini, P.; Tarozzo, G.; Cappello, P.; Perroteau, I.; Fasolo, A. Apoptosis in the Development of the Mouse Olfactory Epithelium. Dev. Brain Res. 1999, 115, 49–55. [Google Scholar] [CrossRef]
- Keller, A.; Margolis, F.L. Immunological Studies of the Rat Olfactory. J. Neurochem. 1975, 24, 1101–1106. [Google Scholar] [CrossRef]
- Maurya, D.K.; Henriques, T.; Marini, M.; Pedemonte, N.; Galietta, L.J.V.; Rock, J.R.; Harfe, B.D.; Menini, A.M. Development of the Olfactory Epithelium and Nasal Glands in TMEM16A-/-and TMEM16A+/+Mice. PLoS ONE 2015, 10, e0129171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana-Urzainqui, I.; Rodríguez-Moldes, I.; Candal, E. Developmental, Tract-Tracing and Immunohistochemical Study of the Peripheral Olfactory System in a Basal Vertebrate: Insights on Pax6 Neurons Migrating along the Olfactory Nerve. Brain Struct. Funct. 2014, 219, 85–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejarano-Escobar, R.; Blasco, M.; Martín-Partido, G.; Francisco-Morcillo, J. Molecular Characterization of Cell Types in the Developing, Mature, and Regenerating Fish Retina. Rev. Fish Biol. Fish. 2014, 24, 127–158. [Google Scholar] [CrossRef]
- Álvarez-Hernán, G.; Hernández-Núñez, I.; Rico-Leo, E.M.; Marzal, A.; de Mera-Rodríguez, J.A.; Rodríguez-León, J.; Martín-Partido, G.; Francisco-Morcillo, J. Retinal Differentiation in an Altricial Bird Species, Taeniopygia Guttata: An Immunohistochemical Study. Exp. Eye Res. 2020, 190, 107869. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, E.H.; Szumowski, K.E.M.; Schwob, J.E. An Immunochemical, Ultrastructural, and Developmental Characterization of the Horizontal Basal Cells of Rat Olfactory Epithelium. J. Comp. Neurol. 1995, 363, 129–146. [Google Scholar] [CrossRef]
- Leung, C.T.; Coulombe, P.A.; Reed, R.R. Contribution of Olfactory Neural Stem Cells to Tissue Maintenance and Regeneration. Nat. Neurosci. 2007, 10, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, W.; Iulianella, A.; Gunhaga, L. Cux2 Acts as a Critical Regulator for Neurogenesis in the Olfactory Epithelium of Vertebrates. Dev. Biol. 2014, 388, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavón-Muñoz, T.; Bejarano-Escobar, R.; Blasco, M.; Martín-Partido, G.; Francisco-Morcillo, J. Retinal Development in the Gilthead Seabream Sparus Aurata. J. Fish Biol. 2016, 88, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Bejarano-Escobar, R.; Holguín-Arévalo, M.S.; Montero, J.A.; Francisco-Morcillo, J.; Martín-Partido, G. Macrophage and Microglia Ontogeny in the Mouse Visual System Can Be Traced by the Expression of Cathepsins B and D. Dev. Dyn. 2011, 240, 1841–1855. [Google Scholar] [CrossRef]
- Bejarano-Escobar, R.; Blasco, M.; Durán, A.C.; Martín-Partido, G.; Francisco-Morcillo, J. Chronotopographical Distribution Patterns of Cell Death and of Lectin-Positive Macrophages/Microglial Cells during the Visual System Ontogeny of the Small-Spotted Catshark Scyliorhinus Canicula. J. Anat. 2013, 223, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Plendl, J.; Schmahl, W. Dolichos Biflorus Agglutinin: A Marker of the Developing Olfactory System in the NMRI-Mouse Strain. Anat. Embryol. 1988, 177, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Morcillo, J.; Bejarano-Escobar, R.; Rodríguez-León, J.; Navascués, J.; Martín-Partido, G. Ontogenetic Cell Death and Phagocytosis in the Visual System of Vertebrates. Dev. Dyn. 2014, 243, 1203–1225. [Google Scholar] [CrossRef]
- Francisco-Morcillo, J.; Hidalgo-Sánchez, M.; Martín-Partido, G. Spatial and Temporal Patterns of Apoptosis during Differentiation of the Retina in the Turtle. Anat. Embryol. 2004, 208, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.; Farbman, A.I. Proliferation in the Rat Olfactory Epithelium: Age-Dependent Changes. J. Neurosci. 1997, 17, 3610–3622. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.; Farbman, A.I. Supporting Cell Proliferation in the Olfactory Epithelium Decreases Postnatally. Glia 1998, 22, 315–328. [Google Scholar] [CrossRef]
- Gibaja, A.; Aburto, M.R.; Pulido, S.; Collado, M.; Hurlé, J.M.; Varela-Nieto, I.; Magariños, M. TGFβ2-Induced Senescence during Early Inner Ear Development. Sci. Rep. 2019, 9, 5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva-Álvarez, S.; Guerra-Varela, J.; Sobrido-Cameán, D.; Quelle, A.; Barreiro-Iglesias, A.; Sánchez, L.; Collado, M. Developmentally-Programmed Cellular Senescence Is Conserved and Widespread in Zebrafish. Aging (Albany. NY) 2020, 12, 17895–17901. [Google Scholar] [CrossRef]
- Lorda-Díez, C.I.; García-Riart, B.; Montero, J.A.; Rodríguez-León, J.; García-Porrero, J.A.; Hurlé, J.M. Apoptosis during Embryonic Tissue Remodeling Is Accompanied by Cell Senescence. Aging (Albany. NY) 2015, 7, 974–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davaapil, H.; Brockes, J.P.; Yun, M.H. Conserved and Novel Functions of Programmed Cellular Senescence during Vertebrate Development. Development 2017, 144, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Villiard, É.; Denis, J.-F.; Hashemi, F.S.; Igelmann, S.; Ferbeyre, G.; Roy, S. Senescence Gives Insights into the Morphogenetic Evolution of Anamniotes. Biol. Open 2017, 6, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Da Silva-álvarez, S.; Lamas-González, O.; Ferreirós, A.; González, P.; Gómez, M.; García-Caballero, T.; Barcia, M.G.; García-González, M.A.; Collado, M. Pkd2 Deletion during Embryo Development Does Not Alter Mesonephric Programmed Cell Senescence. Int. J. Dev. Biol. 2018, 62, 637–640. [Google Scholar] [CrossRef]
- Robinson, A.M.; Conley, D.S.; Shinners, M.J.; Kern, R.C. Apoptosis in the Aging Olfactory Epithelium. Laryngoscope 2002, 112, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Deckner, M.L.; Risling, M.; Frisén, J. Apoptotic Death of Olfactory Sensory Neurons in the Adult Rat. Exp. Neurol. 1997, 143, 132–140. [Google Scholar] [CrossRef]
- Graziadei, G.A.M.; Stanley, R.S.; Graziadei, P.P.C. The Olfactory Marker Protein in the Olfactory System of the Mouse during Development. Neuroscience 1980, 5, 1239–1252. [Google Scholar] [CrossRef]
- Farbman, A.I.; Margolis, F.L. Olfactory Marker Protein during Ontogeny: Immunohistochemical Localization. Dev. Biol. 1980, 74, 205–215. [Google Scholar] [CrossRef]
- Chernova, T.; Nicotera, P.; Smith, A.G. Heme Deficiency Is Associated with Senescence and Causes Suppression of N-Methyl-D-Aspartate Receptor Subunits Expression in Primary Cortical Neurons. Mol. Pharmacol. 2006, 69, 697–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanu, U.M.; Mandraju, R.K.; Bhaskar, C.; Kondapi, A.K. Cultured Cerebellar Granule Neurons as an in Vitro Aging Model: Topoisomerase IIβ as an Additional Biomarker in DNA Repair and Aging. Toxicol. Vitr. 2010, 24, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.Q.; Guan, J.T.; Xu, X.H.; Fu, Y.C. Senescence-Associated Beta-Galactosidase Activity Expression in Aging Hippocampal Neurons. Biochem. Biophys. Res. Commun. 2010, 396, 866–869. [Google Scholar] [CrossRef]
- Dong, W.; Cheng, S.; Huang, F.; Fan, W.; Chen, Y.; Shi, H.; He, H. Mitochondrial Dysfunction in Long-Term Neuronal Cultures Mimics Changes with Aging. Med. Sci. Monit. 2011, 17, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Santos-Almeida, A.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Endogenous pH 6.0 β-Galactosidase Activity Is Linked to Neuronal Differentiation in the Olfactory Epithelium. Cells 2022, 11, 298. https://doi.org/10.3390/cells11020298
de Mera-Rodríguez JA, Álvarez-Hernán G, Gañán Y, Santos-Almeida A, Martín-Partido G, Rodríguez-León J, Francisco-Morcillo J. Endogenous pH 6.0 β-Galactosidase Activity Is Linked to Neuronal Differentiation in the Olfactory Epithelium. Cells. 2022; 11(2):298. https://doi.org/10.3390/cells11020298
Chicago/Turabian Stylede Mera-Rodríguez, José Antonio, Guadalupe Álvarez-Hernán, Yolanda Gañán, Ana Santos-Almeida, Gervasio Martín-Partido, Joaquín Rodríguez-León, and Javier Francisco-Morcillo. 2022. "Endogenous pH 6.0 β-Galactosidase Activity Is Linked to Neuronal Differentiation in the Olfactory Epithelium" Cells 11, no. 2: 298. https://doi.org/10.3390/cells11020298







