Fetal Myocardial Expression of GLUT1: Roles of BPA Exposure and Cord Blood Exosomes in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Organotypic Cultures
2.3. Exosome Isolation and Characterization
2.4. RNA Isolation and Real Time PCR
2.5. Glucose Quantification
2.6. TG Quantification
2.7. Immunohistochemistry
2.8. Immunofluorescence
2.9. Transmission Electron Microscopy (TEM)
2.10. Nanoparticle Size Measurements
2.11. Western Blot
2.12. Antibodies Used in the Research
2.13. Statistical Analysis
3. Results
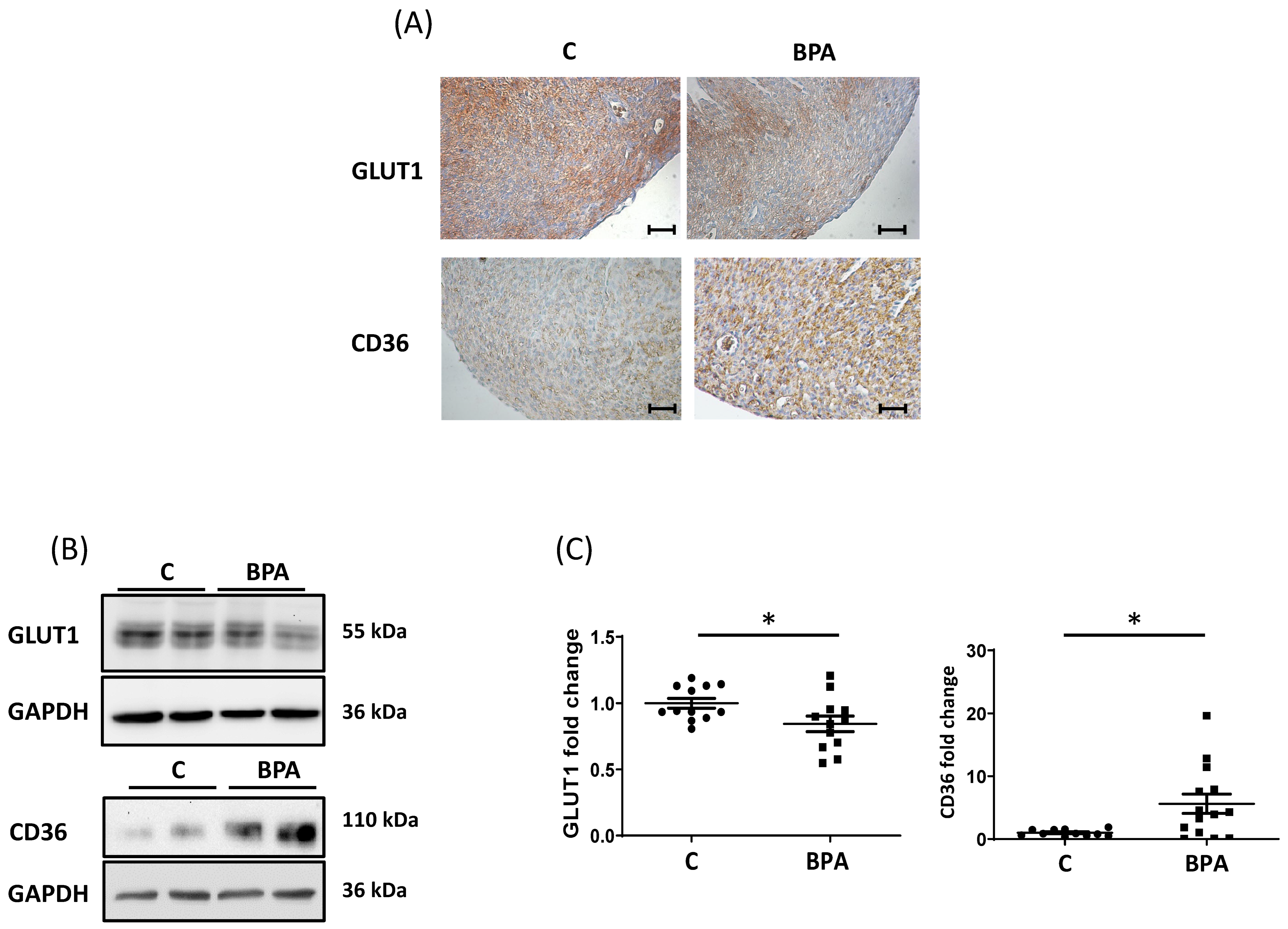
3.1. Maternal Dietary Exposure to BPA Alters Nutrient Transporter Expression in Fetal Heart Cardiomyocytes
3.2. Direct BPA Exposure Decreases GLUT1 Expression in Fetal Heart Cardiomyocytes
3.3. Maternal BPA Exposure Alters the Cargo of Cord Blood Nanovesicles to Antagonize its Effect on the Fetal Heart
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiraagni, I.A.; Mohd, M.A.; Rashid, R.B.A.; Haron, D.E.M. Validation of a simple extraction procedure for bisphenol A identification from human plasma. PLoS ONE 2019, 14, e0221774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.-L.; Zhang, J.; Goodyer, C.G.; Hayward, S.; Cooke, G.M.; Curran, I.H. Bisphenol A in human placental and fetal liver tissues collected from Greater Montreal area (Quebec) during 1998–2008. Chemosphere 2012, 89, 505–511. [Google Scholar] [CrossRef]
- Mørck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal–Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [Green Version]
- Manzan-Martins, C.; Paulesu, L. Impact of bisphenol A (BPA) on cells and tissues at the human materno-fetal interface. Tissue Cell 2021, 73, 101662. [Google Scholar] [CrossRef]
- Mannelli, C.; Ietta, F.; Carotenuto, C.; Romagnoli, R.; Szostek, A.Z.; Wasniewski, T.; Skarzynski, D.J.; Paulesu, L. Bisphenol A alters beta-hCG and MIF release by human placenta: An in vitro study to understand the role of en-dometrial cells. Mediat. Inflam. 2014, 2014, 635364. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, L.; Mandalà, M.; Paulesu, L.; Barberio, L.; Ietta, F. Prenatal Nutrition Containing Bisphenol A Affects Placenta Glucose Transfer: Evidence in Rats and Human Trophoblast. Nutrients 2020, 12, 1375. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Paulesu, L.; Mannelli, C.; Ermini, L.; Romagnoli, R.; Cintorino, M.; Ietta, F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell. Endocrinol. 2015, 412, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ermini, L.; Nuzzo, A.; Ietta, F.; Romagnoli, R.; Moretti, L.; Masturzo, B.; Paulesu, L.; Rolfo, A. Placental Glucose Transporters and Response to Bisphenol A in Pregnancies from of Normal and Overweight Mothers. Int. J. Mol. Sci. 2021, 22, 6625. [Google Scholar] [CrossRef] [PubMed]
- Barberio, L.; Paulesu, L.; Canesi, L.; Grasselli, E.; Mandalà, M. Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth. Int. J. Mol. Sci. 2021, 22, 6912. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tang, Y.; Xiong, Y.; Feng, L.; Li, X. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation ofWNT2. FASEB J. 2019, 33, 2732–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strakovsky, R.S.; Schantz, S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenetics 2018, 4, dvy022. [Google Scholar] [CrossRef] [Green Version]
- Vrachnis, N.; Loukas, N.; Vrachnis, D.; Antonakopoulos, N.; Zygouris, D.; Kοlialexi, A.; Pergaliotis, V.; Iavazzo, C.; Mastorakos, G.; Iliodromiti, Z. A Systematic Review of Bisphenol A from Dietary and Non-Dietary Sources during Pregnancy and Its Possible Connection with Fetal Growth Restriction: Investigating Its Potential Effects and the Window of Fetal Vulnerability. Nutrients 2021, 13, 2426. [Google Scholar] [CrossRef]
- Tsen, C.-M.; Liu, J.-H.; Yang, D.-P.; Chao, H.-R.; Chen, J.-L.; Chou, W.-C.; Ho, Y.-C.; Chuang, C.-Y. Study on the correlation of bisphenol A exposure, pro-inflammatory gene expression, and C-reactive protein with potential cardiovascular disease symptoms in young adults. Environ. Sci. Pollut. Res. 2021, 28, 32580–32591. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.H.; Hong, Y.-C. MicroRNA expression in response to bisphenol A is associated with high blood pressure. Environ. Int. 2020, 141, 105791. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.-T.; Wareham, N.J.; et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [Green Version]
- Chapalamadugu, K.C.; Vandevoort, C.A.; Settles, M.L.; Robison, B.D.; Murdoch, G.K. Maternal Bisphenol A Exposure Impacts the Fetal Heart Transcriptome. PLoS ONE 2014, 9, e89096. [Google Scholar] [CrossRef]
- Zhou, R.; Cheng, W.; Feng, Y.; Wang, W.; Liang, F.; Luo, F.; Yang, S.; Wang, Y. Combined effects of BPA and PFOS on fetal cardiac development: In vitro and in vivo experiments. Environ. Toxicol. Pharmacol. 2020, 80, 103434. [Google Scholar] [CrossRef] [PubMed]
- Rasdi, Z.; Kamaludin, R.; Rahim, S.A.; Fuad, S.B.S.A.; Othman, M.H.D.; Siran, R.; Nor, N.S.M.; Hasani, N.A.H.; Kadir, S.H.S.A. The impacts of intrauterine Bisphenol A exposure on pregnancy and expression of miRNAs related to heart development and diseases in animal model. Sci. Rep. 2020, 10, 5882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Magdalena, P.; Rivera, F.J.; Guerrero-Bosagna, C. Bisphenol-A and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ. Epigenetics 2016, 2, dvw022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudurí, E.; Marroqui, L.; Dos Santos, R.S.; Quesada, I.; Fuentes, E.; Alonso-Magdalena, P. Timing of Exposure and Bisphenol-A: Implications for Diabetes Development. Front. Endocrinol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, T.; Magdalena, P.A.; Vieira, E.; Amaral, M.E.C.D.; Cederroth, C.R.; Nef, S.; Quesada, I.; Carneiro, E.M.; Nadal, A. Short-Term Treatment with Bisphenol-A Leads to Metabolic Abnormalities in Adult Male Mice. PLoS ONE 2012, 7, e33814. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Ventura-Clapier, R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front. Physiol. 2018, 9, 959. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2015, 6, 331–351. [Google Scholar] [CrossRef] [Green Version]
- Camm, E.J.; Botting, K.J.; Sferruzzi-Perri, A.N. Near to One’s Heart: The Intimate Relationship between the Placenta and Fetal Heart. Front. Physiol. 2018, 9, 629. [Google Scholar] [CrossRef] [Green Version]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Maligianni, I.; Yapijakis, C.; Nousia, K.; Bacopoulou, F.; Chrousos, G.P. Exosomes and exosomal non-coding RNAs throughout human gestation (Review). Exp. Ther. Med. 2022, 24, 582. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Q.; Wang, Y.; Tang, Z. Clinical application of exosomes and circulating microRNAs in the diagnosis of pregnancy complications and foetal abnormalities. J. Transl. Med. 2020, 18, 32–39. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, N.A.; Moncayo-Arlandi, J.; Sepulveda, P.; Diez-Juan, A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc. Res. 2016, 109, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Guan, F.; Wu, J.; Wang, X.; Shi, C. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: A role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019, 33, 8530–8542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, P.; Qian, W.; Li, Y.; Zhao, J.; Huan, F.; Wang, J.; Xiao, H. Perinatal Bisphenol A Exposure and Adult Glucose Homeostasis: Identifying Critical Windows of Exposure. PLoS ONE 2013, 8, e64143. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Capitani, N.; Manganaro, N.; Tatangelo, V.; Libonati, F.; Panattoni, G.; Calaresu, I.; Ballerini, L.; Baldari, C.T.; Patrussi, L. Optimization of Organotypic Cultures of Mouse Spleen for Staining and Functional Assays. Front. Immunol. 2020, 11, 471. [Google Scholar] [CrossRef]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [Green Version]
- Ermini, L.; Ausman, J.; Melland-Smith, M.; Yeganeh, B.; Rolfo, A.; Litvack, M.L.; Todros, T.; Letarte, M.; Post, M.; Caniggia, I. A Single Sphingomyelin Species Promotes Exosomal Release of Endoglin into the Maternal Circulation in Preeclampsia. Sci. Rep. 2017, 7, 12172. [Google Scholar] [CrossRef] [Green Version]
- Ermini, L.; Farrell, A.; Alahari, S.; Ausman, J.; Park, C.; Sallais, J.; Melland-Smith, M.; Porter, T.; Edson, M.; Nevo, O.; et al. Ceramide-Induced Lysosomal Biogenesis and Exocytosis in Early-Onset Preeclampsia Promotes Exosomal Release of SMPD1 Causing Endothelial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 652651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Patrussi, L.; Capitani, N.; Martini, V.; Pizzi, M.; Trimarco, V.; Frezzato, F.; Marino, F.; Semenzato, G.; Trentin, L.; Baldari, C.T. Enhanced Chemokine Receptor Recycling and Impaired S1P1 Expression Promote Leukemic Cell Infiltration of Lymph Nodes in Chronic Lymphocytic Leukemia. Cancer Res. 2015, 75, 4153–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, E.D. Glucose transport in the heart. Front. Biosci. 2004, 9, 201–215. [Google Scholar] [CrossRef]
- Kim, T.T.; Dyck, J.R. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Taylor, J.A.; Sommerfeld-Sager, J.; Tillitt, D.E.; Ricke, W.A.; vom Saal, F.S. Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol. Environ. Epigenetics 2019, 5, dvz012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epp, N.; Rethmeier, R.; Krämer, L.; Ungermann, C. Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs. Eur. J. Cell Biol. 2011, 90, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef] [Green Version]
- Grover-McKay, M.; A Walsh, S.; Thompson, S.A. Glucose transporter 3 (GLUT3) protein is present in human myocardium. Biochim. Biophys. Acta Biomembr. 1999, 1416, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Membrez, M.; Hummler, E.; Beermann, F.; Haefliger, J.-A.; Savioz, R.; Pedrazzini, T.; Thorens, B. GLUT8 Is Dispensable for Embryonic Development but Influences Hippocampal Neurogenesis and Heart Function. Mol. Cell. Biol. 2006, 26, 4268–4276. [Google Scholar] [CrossRef] [Green Version]
- Lopaschuk, G.D.; Spafford, M.A.; Marsh, D.R. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am. J. Physiol. Circ. Physiol. 1991, 261, H1698–H1705. [Google Scholar] [CrossRef]
- Nakano, H.; Minami, I.; Braas, D.; Pappoe, H.; Wu, X.; Sagadevan, A.; Vergnes, L.; Fu, K.; Morselli, M.; Dunham, C.; et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 2017, 6, e29330. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gu, C.; Li, Y.; Huang, J. High Glucose Promotes CD36 Expression by Upregulating Peroxisome Proliferator-Activated Receptor gamma Levels to Exacerbate Lipid Deposition in Renal Tubular Cells. Biomed Res. Int. 2017, 2017, 1414070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimes, B.; Brandt, B. Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 1976, 98, 367–381. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Wu, J.; Yan, F.; Wang, Y.; Li, J. High glucoseinduced upregulation of CD36 promotes inflammation stress via NFkappaB in H9c2 cells. Mol. Med. Rep. 2021, 24, 764. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Heard, D.J.; Norby, P.L.; Holloway, J.; Vissing, H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: Tissue-specific isoforms are expressed during development and in the adult. Mol. Endocrinol. 2000, 14, 382–392. [Google Scholar]
- Sakamoto, T.; Matsuura, T.R.; Wan, S.; Ryba, D.M.; Kim, J.U.; Won, K.J.; Lai, L.; Petucci, C.; Petrenko, N.; Musunuru, K.; et al. A Critical Role for Estrogen-Related Receptor Signaling in Cardiac Maturation. Circ. Res. 2020, 126, 1685–1702. [Google Scholar] [CrossRef]
- Doshi, T.; D’Souza, C.; Dighe, V.; Vanage, G. Effect of neonatal exposure on male rats to bisphenol a on the expression of DNA methylation machinery in the postimplantation embryo. J. Biochem. Mol. Toxicol. 2012, 26, 337–343. [Google Scholar] [CrossRef]
- Tannetta, D.; Masliukaite, I.; Vatish, M.; Redman, C.; Sargent, I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2017, 119, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, M.; Mahdavi, R.; Jonoush, Z.A.; Sadeghi, M.; Ghadiri, N.; Farzaneh, M.; Salehi, A.M. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022, 20, 51. [Google Scholar] [CrossRef]
- Miranda, J.; Paules, C.; Nair, S.; Lai, A.; Palma, C.; Scholz-Romero, K.; Rice, G.E.; Gratacos, E.; Crispi, F.; Salomon, C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction—Liquid biopsies to monitoring fetal growth. Placenta 2018, 64, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, Y.; Park, H.J.; Cantillon, A.; Getahun, D.; Menon, R.; Peltier, M.R. Effect of bisphenol-A (BPA) on placental biomarkers for inflammation, neurodevelopment and oxidative stress. J. Périnat. Med. 2019, 47, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Radnaa, E.; Arita, Y.; Getahun, D.; Jones, R.J.; Peltier, M.R.; Menon, R. Environmental pollutant induced cellular injury is reflected in exosomes from placental explants. Placenta 2020, 89, 42–49. [Google Scholar] [CrossRef]
- Tolosa, J.; Schjenken, J.; Clifton, V.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941. [Google Scholar] [CrossRef]
- Vargas, A.; Zhou, S.; Éthier-Chiasson, M.; Flipo, D.; Lafond, J.; Gilbert, C.; Barbeau, B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014, 28, 3703–3719. [Google Scholar] [CrossRef]
- Xu, P.; Ma, Y.; Wu, H.; Wang, Y.-L. Placenta-Derived MicroRNAs in the Pathophysiology of Human Pregnancy. Front. Cell Dev. Biol. 2021, 9, 646326. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, D.; Gao, C. Mir-150 Up-Regulates Glut1 and Increases Glycolysis in Osteosarcoma Cells. Asian Pac. J. Cancer Prev. 2017, 18, 1127–1131. [Google Scholar] [CrossRef]
- Song, R.; Hu, X.-Q.; Zhang, L. Mitochondrial MiRNA in Cardiovascular Function and Disease. Cells 2019, 8, 1475. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-Q.; Wang, S.-Q.; Chen, Y.; Fu, L.-Y.; Xu, Y.-N.; Li, L.; Tao, L.; Shen, X.-C. MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases. Front. Pharmacol. 2021, 12, 663322. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; A Kinkade, J.; Bivens, N.J.; Rosenfeld, C.S. miRNA changes in the mouse placenta due to bisphenol A exposure. Epigenomics 2021, 13, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Avissar-Whiting, M.; Veiga, K.R.; Uhl, K.M.; Maccani, M.A.; Gagne, L.A.; Moen, E.L.; Marsit, C.J. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010, 29, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwanath, V.A. Fatty Acid Beta-Oxidation Disorders: A Brief Review. Ann. Neurosci. 2016, 23, 51–55. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermini, L.; Mandalà, M.; Cresti, L.; Passaponti, S.; Patrussi, L.; Paulesu, L.; Thornburg, K.; Ietta, F. Fetal Myocardial Expression of GLUT1: Roles of BPA Exposure and Cord Blood Exosomes in a Rat Model. Cells 2022, 11, 3195. https://doi.org/10.3390/cells11203195
Ermini L, Mandalà M, Cresti L, Passaponti S, Patrussi L, Paulesu L, Thornburg K, Ietta F. Fetal Myocardial Expression of GLUT1: Roles of BPA Exposure and Cord Blood Exosomes in a Rat Model. Cells. 2022; 11(20):3195. https://doi.org/10.3390/cells11203195
Chicago/Turabian StyleErmini, Leonardo, Maurizio Mandalà, Laura Cresti, Sofia Passaponti, Laura Patrussi, Luana Paulesu, Kent Thornburg, and Francesca Ietta. 2022. "Fetal Myocardial Expression of GLUT1: Roles of BPA Exposure and Cord Blood Exosomes in a Rat Model" Cells 11, no. 20: 3195. https://doi.org/10.3390/cells11203195
APA StyleErmini, L., Mandalà, M., Cresti, L., Passaponti, S., Patrussi, L., Paulesu, L., Thornburg, K., & Ietta, F. (2022). Fetal Myocardial Expression of GLUT1: Roles of BPA Exposure and Cord Blood Exosomes in a Rat Model. Cells, 11(20), 3195. https://doi.org/10.3390/cells11203195








