Echinococcus granulosus Protoscoleces-Derived Exosome-like Vesicles and Egr-miR-277a-3p Promote Dendritic Cell Maturation and Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Parasite Infections
2.2. In Vitro Culture of Protoscoleces
2.3. Exosome-like Vesicle Purification and Characterization
2.4. Determination of the Total Protein Concentration of PSC-ELVs
2.5. Preparation and Treatment of Bone Marrow-Derived Dendritic Cells (BMDCs)
2.6. Uptake of PSC-ELVs by BMDCs
2.7. Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
2.8. Western Blotting Analysis
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Flow Cytometry
2.11. 3′ UTR Luciferase Reporter Constructs
2.12. Statistical Analysis
3. Results
3.1. Identification of E. granulosus Protoscoleces-Derived Exosome-like Vesicles (PSC-ELVs)
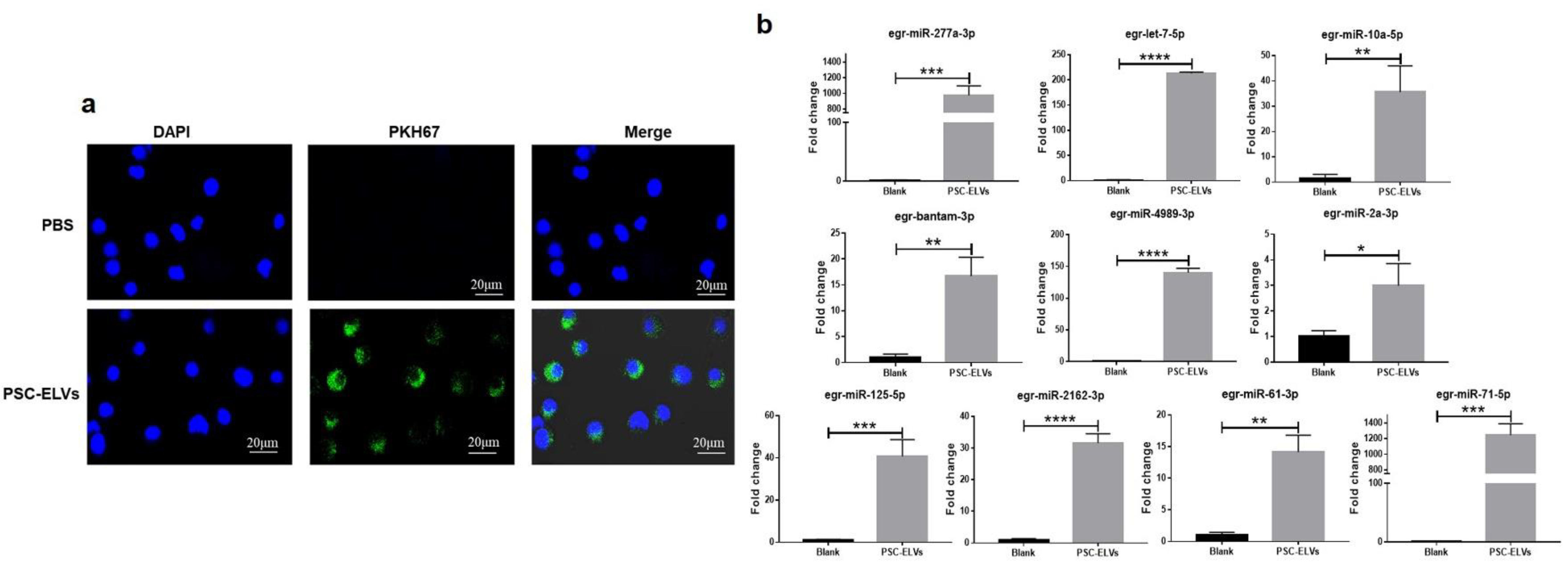
3.2. Internalization of E. granulosus miRNAs in Host BMDCs via PSC-ELVs
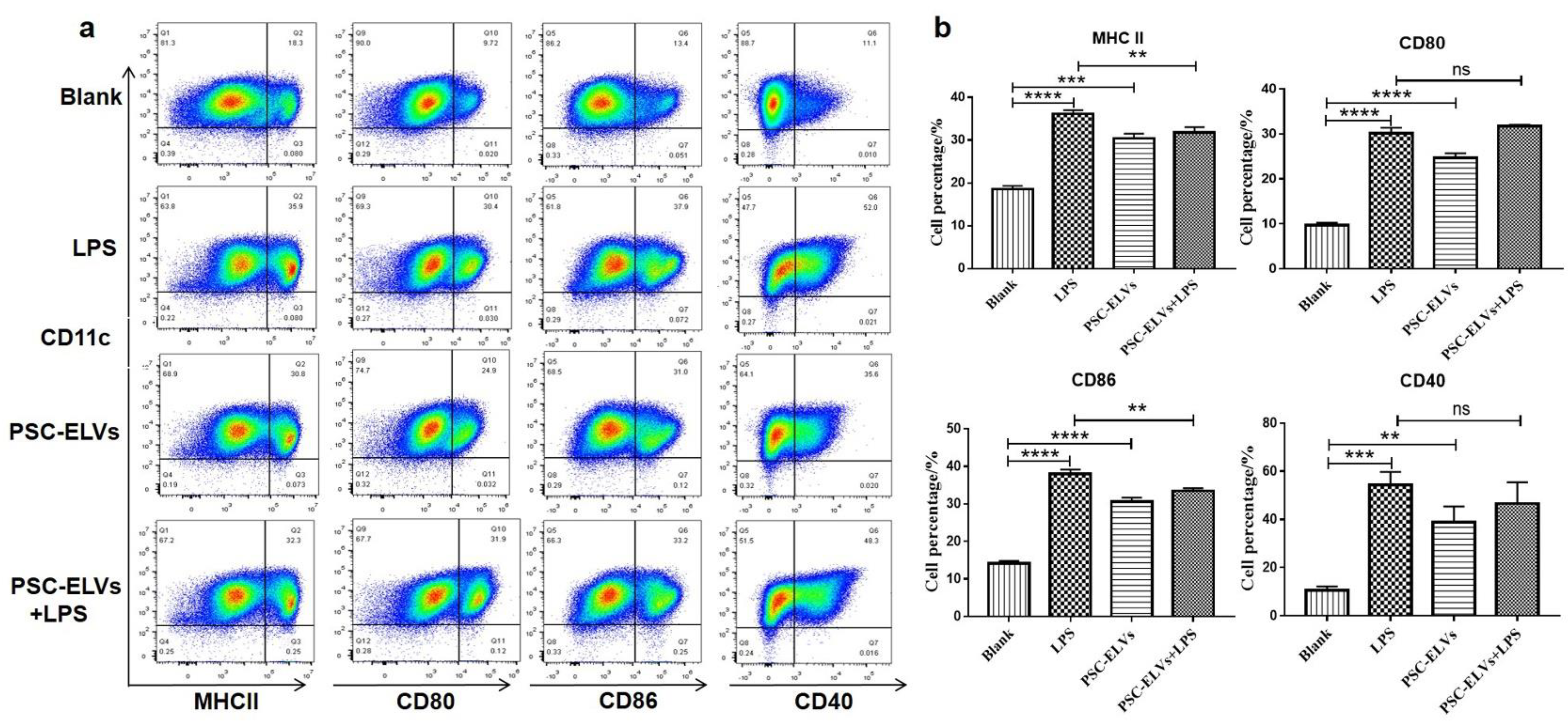
3.3. PSC-ELVs Promote the Differentiation and Maturation of BMDCs
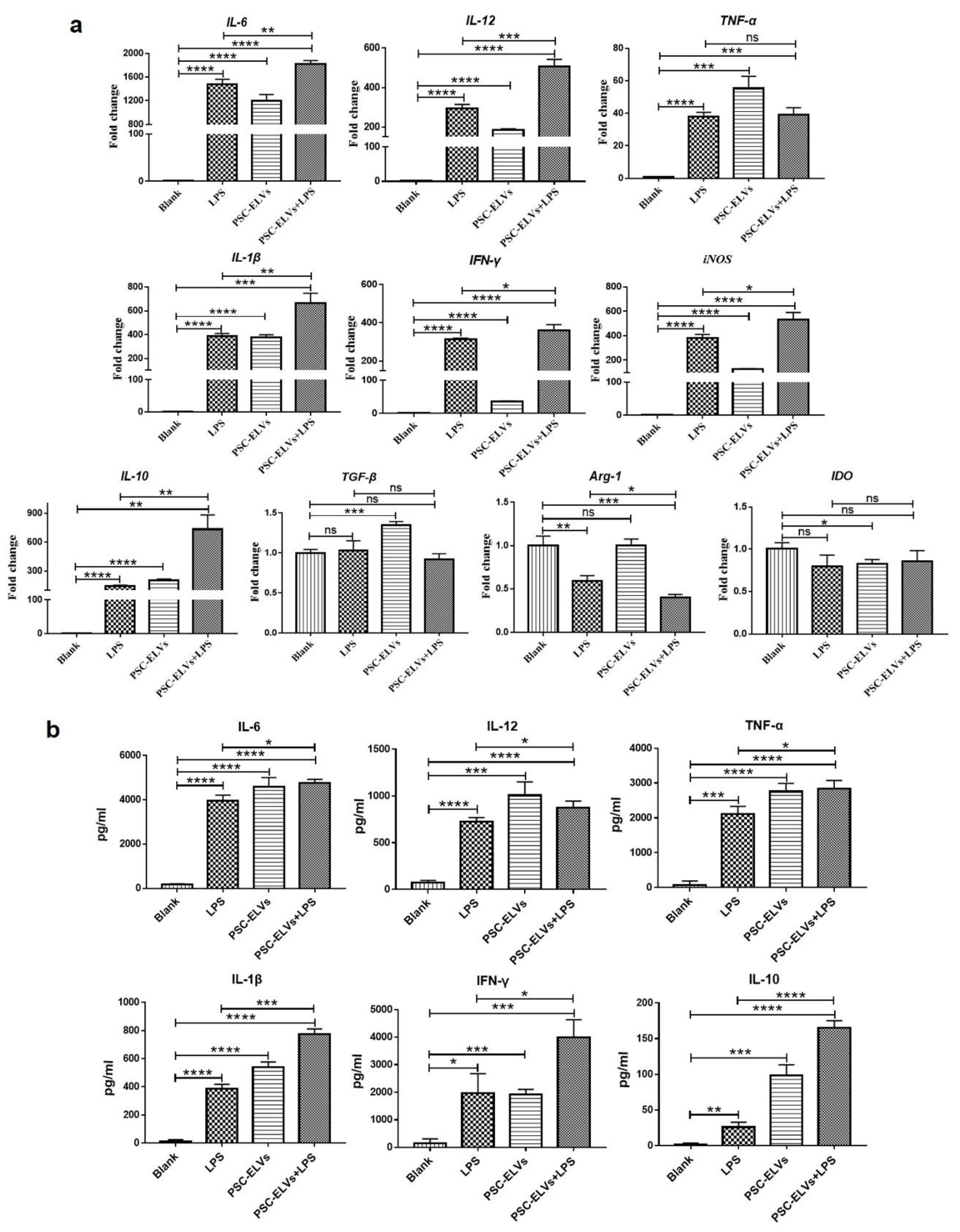
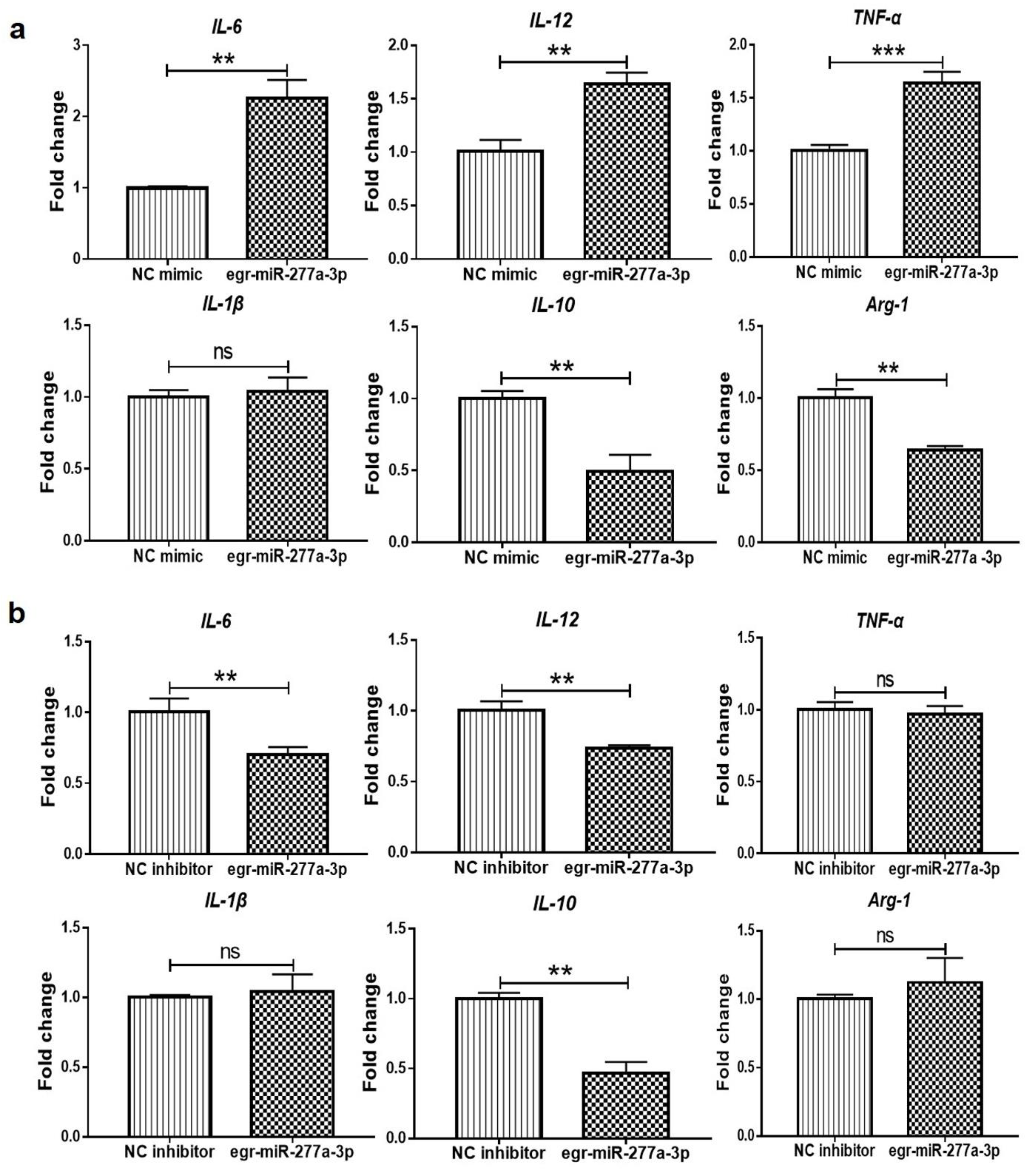
3.4. Egr-miR-277a-3p Induces BMDCs to Upregulate the Expression of Inflammatory Cytokines
3.5. Egr-miR-277a-3p Directly Targets Nfkb1 and Modifies the Nuclear NF-κB p65/p50 Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Gray, D.J.; Zhang, W.; Yang, Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012, 344, e3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
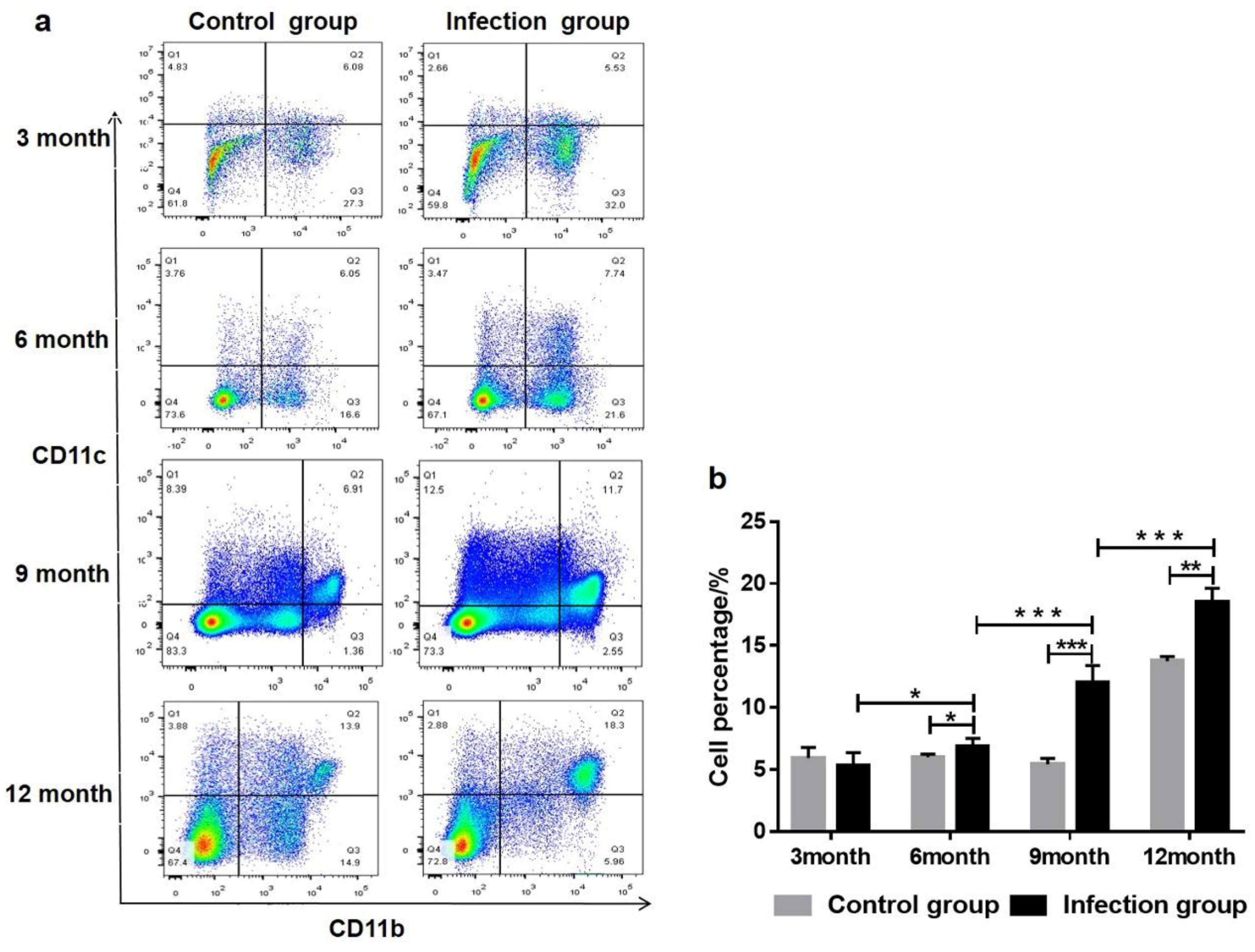
- Pan, W.; Zhou, H.J.; Shen, Y.J.; Wang, Y.; Xu, Y.X.; Hu, Y.; Jiang, Y.Y.; Yuan, Z.Y.; Ugwu, C.E.; Cao, J.P. Surveillance on the status of immune cells after Echinnococcus granulosus protoscoleces infection in Balb/c mice. PLoS ONE 2013, 8, e59746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Alvarez, V.; Folle, A.M.; Ramos, A.L.; Kitano, E.S.; Iwai, L.K.; Corraliza, I.; Corsico, B.; Ferreira, A.M. Echinococcus granulosus Antigen B binds to monocytes and macrophages modulating cell response to inflammation. Parasites Vectors 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Gong, W.; Zhang, X.; Xu, M.; Wang, Y.; Xu, Y.; Cao, J.; Shen, Y.; Chen, J. Arginase promotes immune evasion of Echinococcus granulosus in mice. Parasites Vectors 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Mourglia-Ettlin, G.; Marques, J.M.; Chabalgoity, J.A.; Dematteis, S. Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl. Trop. Dis. 2011, 5, e1293. [Google Scholar] [CrossRef] [PubMed]
- Rigano, R.; Buttari, B.; Profumo, E.; Ortona, E.; Delunardo, F.; Margutti, P.; Mattei, V.; Teggi, A.; Sorice, M.; Siracusano, A. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect. Immun. 2007, 75, 1667–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, H.; Shen, Y.; Wang, Y.; Wu, W.; Liu, H.; Yuan, Z.; Xu, Y.; Hu, Y.; Cao, J. Impairment of dendritic cell function and induction of CD4+CD2+Foxp3+ T cells by excretory-secretory products: A potential mechanism of immune evasion adopted by Echinococcus granulosus. BMC Immunol. 2015, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.M.; Guo, K.; Hao, C.Y.; Zhan, B.; Huang, J.J.; Zhu, X. Trichinella spiralis excretory-secretory products stimulate host regulatory T cell differentiation through activating dendritic cells. Cells 2019, 8, 1404. [Google Scholar] [CrossRef] [Green Version]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and other extracellular vesicles: The new communicators in parasite infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef]
- Sarkies, P.; Miska, E.A. Molecular biology. Is there social RNA? Science 2013, 341, 467–468. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Fan, X.; Lei, N.; Tian, Y.; Zhang, D.; Pan, W. A schistosome miRNA promotes host hepatic fibrosis by targeting transforming growth factor beta receptor III. J. Hepatol. 2020, 72, 519–527. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Lei, N.; He, X.; Wang, X.; Luo, X.; Zhang, D.; Pan, W. A microrna derived from Schistosoma Japonicum promotes schistosomiasis hepatic fibrosis by targeting host secreted frizzled-related protein 1. Front. Cell. Infect. Microbiol. 2020, 10, 101. [Google Scholar] [CrossRef]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory dendritic cells: There is more than just immune activation. Front. Immunol. 2012, 3, 274. [Google Scholar] [CrossRef] [Green Version]
- Asadirad, A.; Hashemi, S.M.; Baghaei, K.; Ghanbarian, H.; Mortaz, E.; Zali, M.R.; Amani, D. Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci. 2019, 219, 152–162. [Google Scholar] [CrossRef]
- Taghikhani, A.; Hassan, Z.M.; Ebrahimi, M.; Moazzeni, S.M. microRNA modified tumor-derived exosomes as novel tools for maturation of dendritic cells. J. Cell. Physiol. 2019, 234, 9417–9427. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Rodriguez Rodrigues, C.; Cumino, A.C. Extracellular vesicles from Echinococcus granulosus larval stage: Isolation, characterization and uptake by dendritic cells. PLoS Negl. Trop. Dis. 2019, 13, e0007032. [Google Scholar] [CrossRef] [Green Version]
- Siles-Lucas, M.; Sanchez-Ovejero, C.; Gonzalez-Sanchez, M.; Gonzalez, E.; Falcon-Perez, J.M.; Boufana, B.; Fratini, F.; Casulli, A.; Manzano-Roman, R. Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet. Parasitol. 2017, 236, 22–33. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, W.; Cao, S.; Yin, J.; Zhang, J.; Cao, J.; Shen, Y. Comprehensive analysis of non-coding rna profiles of exosome-like vesicles from the protoscoleces and hydatid cyst fluid of Echinococcus granulosus. Front. Cell. Infect. Microbiol. 2020, 10, 316. [Google Scholar] [CrossRef]
- Kang, X.; Jiao, Y.; Zhou, Y.; Meng, C.; Zhou, X.; Song, L.; Jiao, X.; Pan, Z. MicroRNA-5112 targets iKKgamma to dampen the inflammatory response and improve clinical symptoms in both bacterial infection and DSS-induced colitis. Front. Immunol. 2022, 13, 779770. [Google Scholar] [CrossRef]
- Lasser, C.; Alikhani, V.S.; Ekstrom, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjostrand, M.; Gabrielsson, S.; Lotvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gillan, V.; Simpson, D.M.; Kinnaird, J.; Maitland, K.; Shiels, B.; Devaney, E. Characterisation of infection associated microRNA and protein cargo in extracellular vesicles of Theileria annulata infected leukocytes. Cell. Microbiol. 2019, 21, e12969. [Google Scholar] [CrossRef] [Green Version]
- Silva-Gomez, J.A.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Miranda-Roblero, H.O.; Lucano-Landeros, S.; Santos, A.; Monroy-Ramirez, H.C.; Armendariz-Borunda, J. Hepatocarcinogenesis prevention by pirfenidone is ppargamma mediated and involves modification of nuclear NF-kB p65/p50 ratio. Int. J. Mol. Sci. 2021, 22, 1360. [Google Scholar] [CrossRef]
- Lecoq, L.; Raiola, L.; Chabot, P.R.; Cyr, N.; Arseneault, G.; Legault, P.; Omichinski, J.G. Structural characterization of interactions between transactivation domain 1 of the p65 subunit of NF-κB and transcription regulatory factors. Nucleic Acids Res. 2017, 45, 5564–5576. [Google Scholar] [CrossRef] [Green Version]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.X.; Zhou, X.C.; Xu, R.T.; Zhu, F.Y.; Bing, X.; Guo, N.; Shi, L.; Qi, W.W.; Liu, C.C.; Xia, M. Tfh exosomes derived from allergic rhinitis promote dc maturation through miR-142-5p/CDK5/STAT3 pathway. J. Inflamm. Res. 2022, 15, 3187–3205. [Google Scholar] [CrossRef]
- Benites, B.D.; Alvarez, M.C.; Saad, S.T.O. Small Particles, Big Effects: The Interplay between exosomes and dendritic cells in antitumor immunity and immunotherapy. Cells 2019, 8, 1648. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Xu, H.W.; Hao, W.T.; Sun, F.F.; Qin, Y.F.; Hao, S.S.; Liu, H.; Cao, J.P.; Shen, Y.J.; Zheng, K.Y. The excretory-secretory products of Echinococcus granulosus protoscoleces stimulated IL-10 production in B cells via TLR-2 signaling. BMC Immunol. 2018, 19, 29. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Hao, W.T.; Shen, Y.J.; Li, X.Y.; Wang, Y.J.; Sun, F.F.; Yin, J.H.; Zhang, J.; Tang, R.X.; Cao, J.P.; et al. The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells. Parasites Vectors 2017, 10, 348. [Google Scholar] [CrossRef]
- Artis, D.; Kane, C.M.; Fiore, J.; Zaph, C.; Shapira, S.; Joyce, K.; Macdonald, A.; Hunter, C.; Scott, P.; Pearce, E.J. Dendritic cell-intrinsic expression of NF-kappa B1 is required to promote optimal Th2 cell differentiation. J. Immunol. 2005, 174, 7154–7159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtois, G.; Fauvarque, M.O. The many roles of ubiquitin in NF-kappaB signaling. Biomedicines 2018, 6, 43. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gong, W.; Duan, C.; Cai, H.; Shen, Y.; Cao, J. Echinococcus granulosus Protoscoleces-Derived Exosome-like Vesicles and Egr-miR-277a-3p Promote Dendritic Cell Maturation and Differentiation. Cells 2022, 11, 3220. https://doi.org/10.3390/cells11203220
Zhang X, Gong W, Duan C, Cai H, Shen Y, Cao J. Echinococcus granulosus Protoscoleces-Derived Exosome-like Vesicles and Egr-miR-277a-3p Promote Dendritic Cell Maturation and Differentiation. Cells. 2022; 11(20):3220. https://doi.org/10.3390/cells11203220
Chicago/Turabian StyleZhang, Xiaofan, Wenci Gong, Chaohui Duan, Huixia Cai, Yujuan Shen, and Jianping Cao. 2022. "Echinococcus granulosus Protoscoleces-Derived Exosome-like Vesicles and Egr-miR-277a-3p Promote Dendritic Cell Maturation and Differentiation" Cells 11, no. 20: 3220. https://doi.org/10.3390/cells11203220





