In Vitro Cell Death Mechanisms Induced by Dicoma anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Identification, and Extraction of D.A
2.2. Cell Culture
2.3. Photosensitizer (ZnPcS4) Co-Localization
2.4. Laser Parameters
2.5. Dose-Response Studies
2.5.1. Morphological Assessment
2.5.2. Trypan Blue Assay
2.5.3. Lactate Dehydrogenase Assay (Cytotoxicity)
2.5.4. Adenosine Triphosphate Assay (Proliferation)
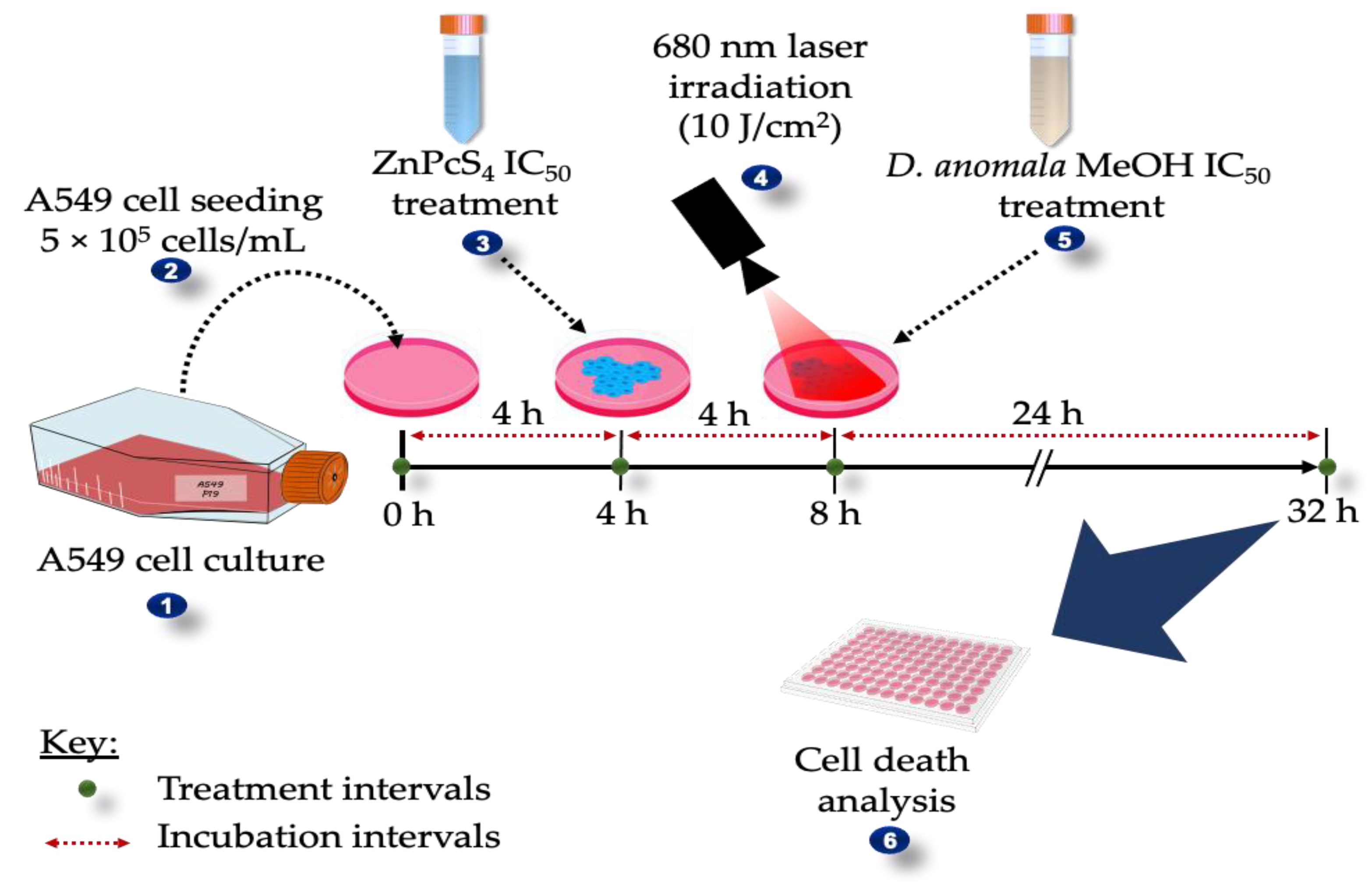
2.6. Combination Effects of D.A IC50 and ZnPcS4-Mediated PDT IC50
2.6.1. Analysis of Cell Death
Cell Viability/ Cytotoxicity (LIVE/DEADTM Assay)
Flow Cytometry (Annexin V-FITC/PI Assay)
Immunofluorescence and Fluorometric Measurement of Caspase 8 and 9
2.7. Statistical Analysis
3. Results
3.1. Photosensitizer (ZnPcS4) Co-Localization
3.2. Dose-Response Studies
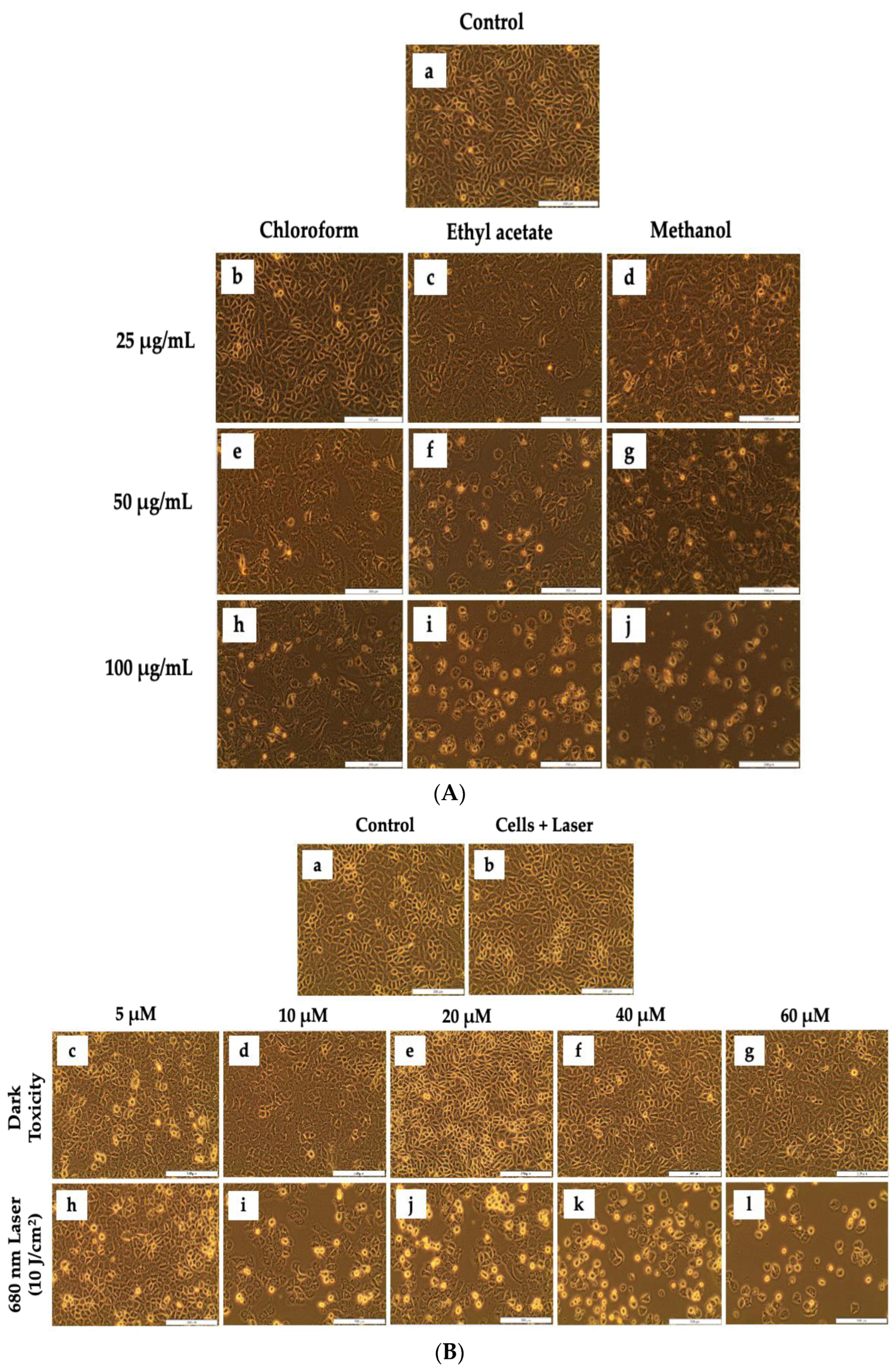
3.2.1. Morphological Assessment
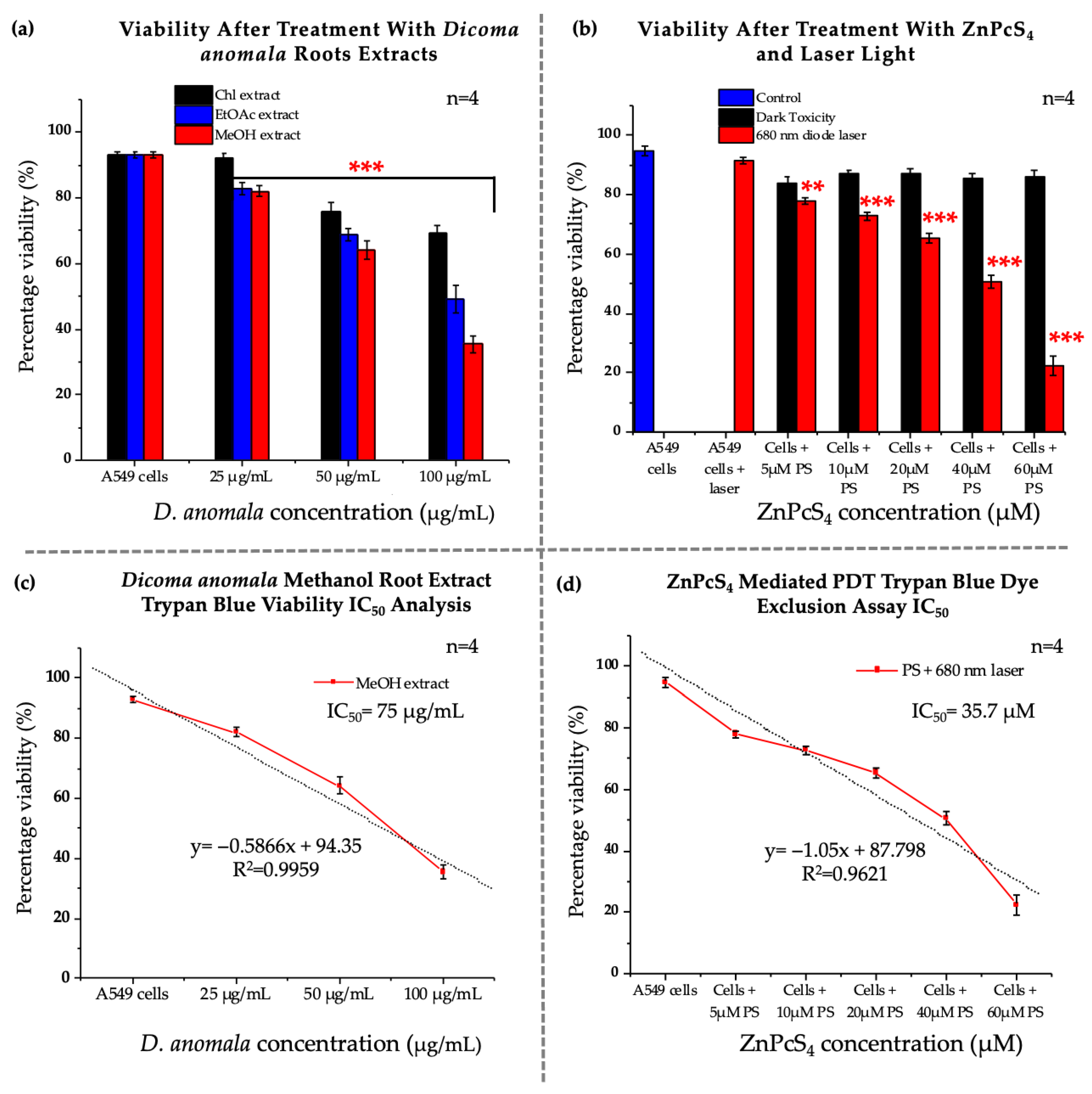
3.2.2. Trypan Blue Assay
3.2.3. Lactate Dehydrogenase Assay (Cytotoxicity)
3.2.4. Adenosine Triphosphate Assay (Proliferation)
3.3. Combination Effects of D.A IC50 and ZnPcS4 Mediated PDT IC50
3.3.1. Morphological, Viability, Cytotoxicity, and Proliferation Analysis
3.3.2. Analysis of Cell Death
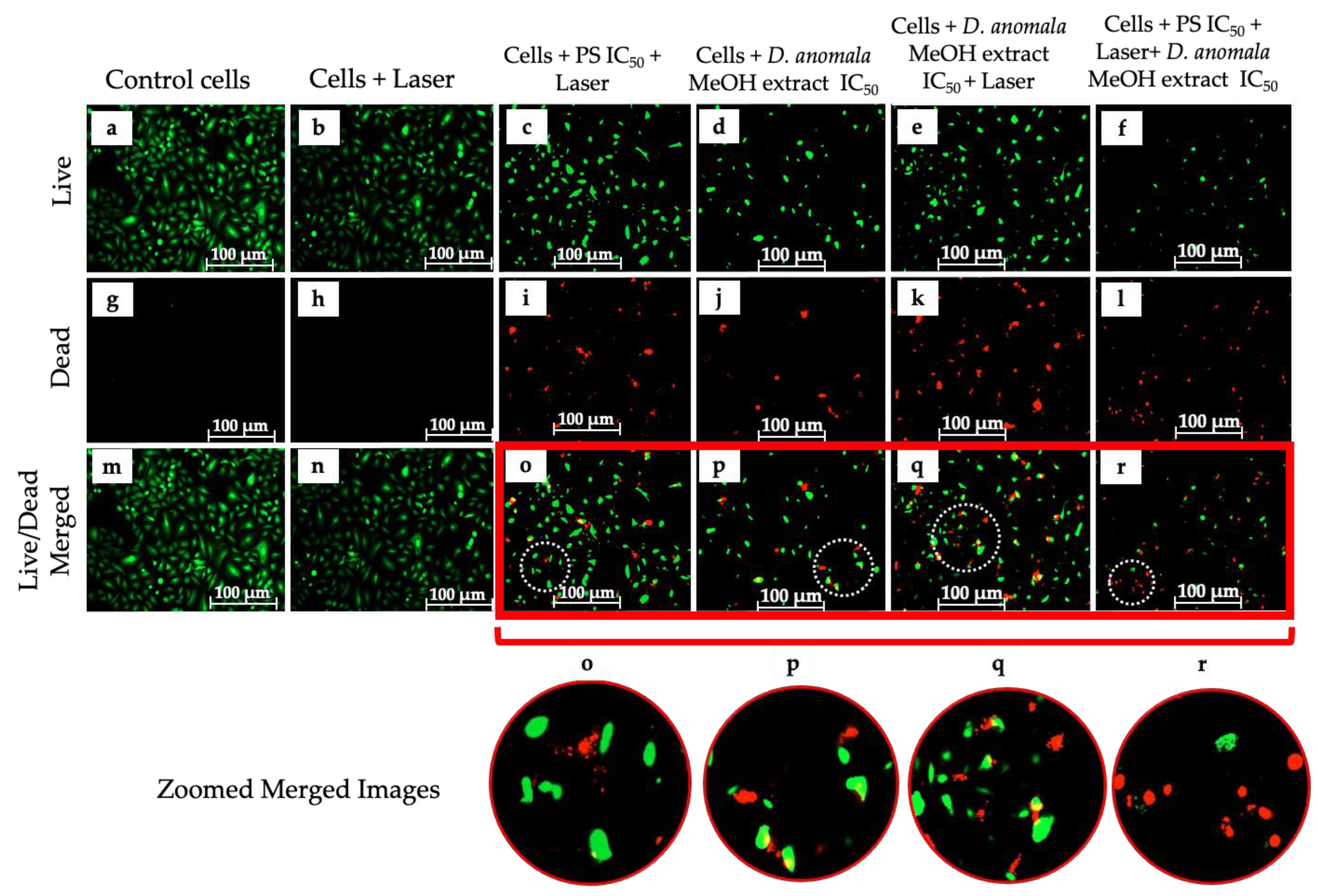
Cell Viability/Cytotoxicity (LIVE/DEADTM Assay)
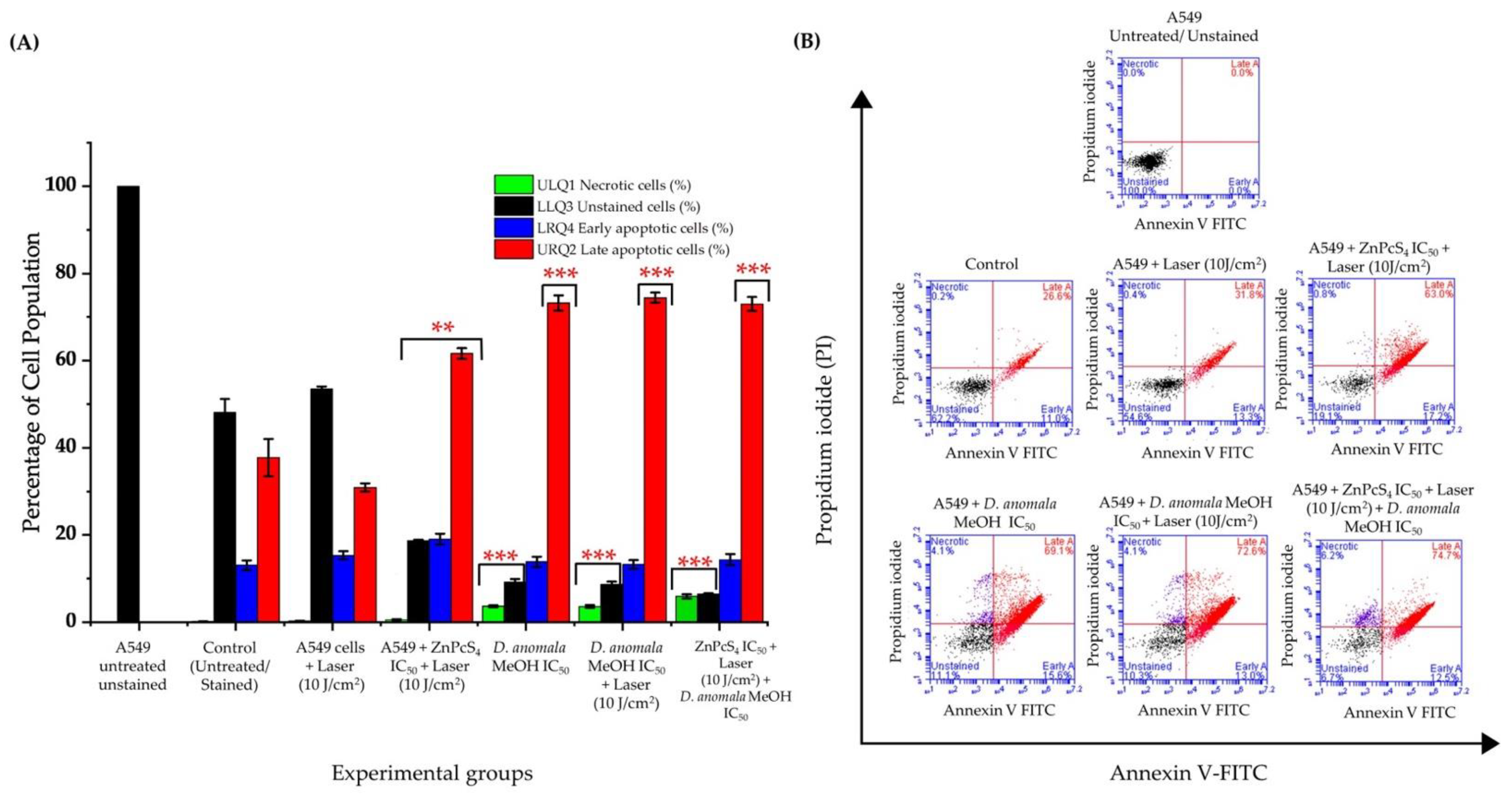
Cell Death Mechanism Analysis
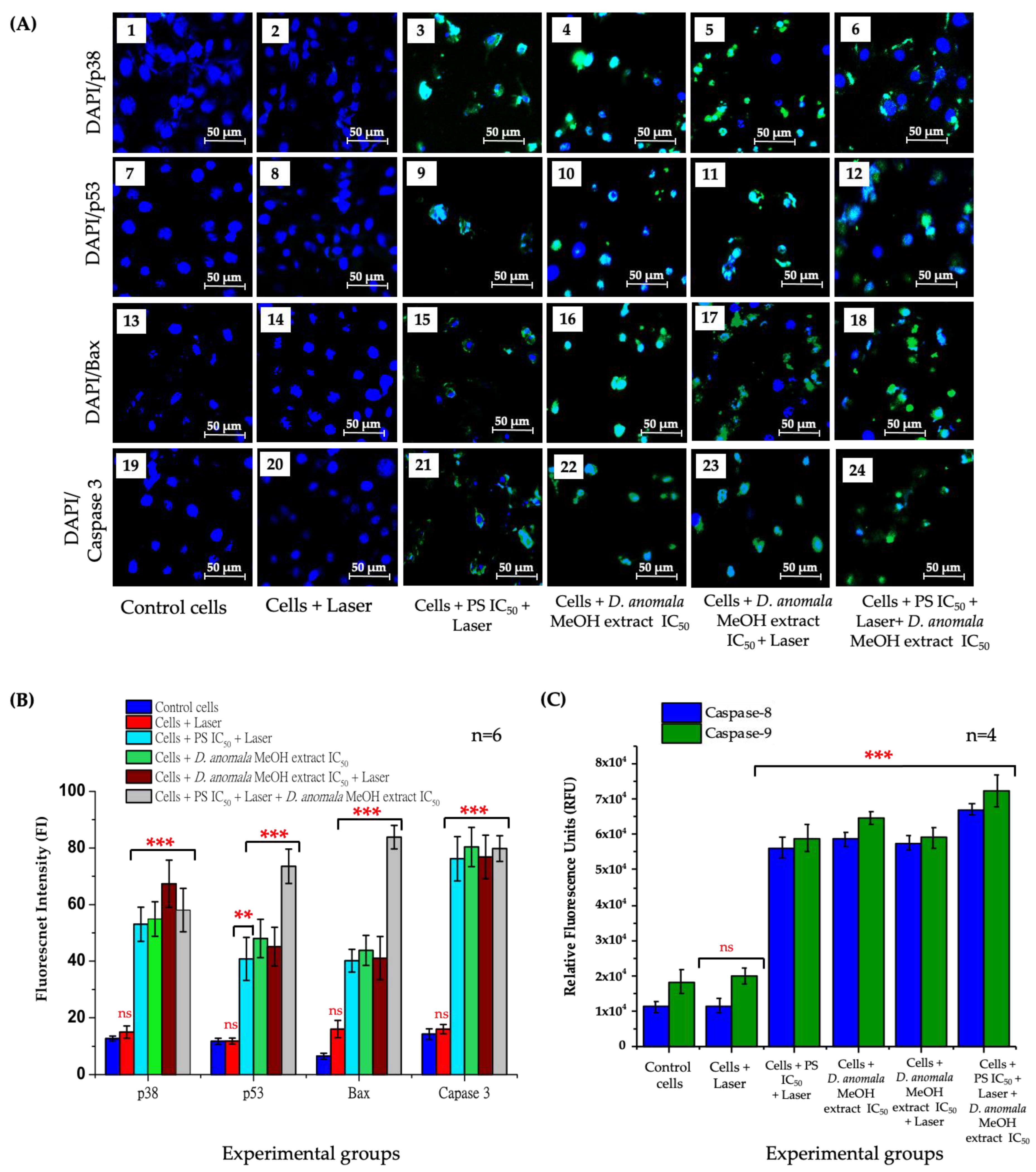
Immunofluorescence and Caspase 8 and 9 Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Ashour Ahmed, A. Growth Factors and Uncontrolled Proliferation. In Oxford Textbook of Oncology; Kerr, D.J., Haller, D.G., van de Velde, C.J.H., Baumann, M., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 11–22. ISBN 978-0-19-965610-3. [Google Scholar]
- Barrera-Rodriguez, R.; Morales-Fuentes, J. Lung Cancer in Women. Lung Cancer Targets Ther. 2012, 3, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Cancer Tommorow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 17 February 2022).
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung Cancer in Never Smokers: The Role of Different Risk Factors Other than Tobacco Smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895. [Google Scholar] [CrossRef] [PubMed]
- Guloksuz, S.; Pries, L.-K.; van Os, J. Application of Network Methods for Understanding Mental Disorders: Pitfalls and Promise. Psychol. Med. 2017, 47, 2743–2752. [Google Scholar] [CrossRef]
- Zhi, X.-Y.; Wu, Y.-L.; Bu, H.; Cheng, G.; Cheng, Y.; Du, X.; Han, B.; Jiang, G.-N.; Jiao, S.-C.; Liu, D.-R.; et al. Chinese Guidelines on the Diagnosis and Treatment of Primary Lung Cancer (2011). J. Thorac. Dis. 2012, 4, 88–101. [Google Scholar] [CrossRef]
- Palumbo, M.O.; Kavan, P.; Miller, W.H.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic Cancer Therapy: Achievements and Challenges That Lie Ahead. Front. Pharmacol. 2013, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Betz, C.S.; Rauschning, W.; Stranadko, E.P.; Riabov, M.V.; Albrecht, V.; Nifantiev, N.E.; Hopper, C. Optimization of Treatment Parameters for Foscan®-PDT of Basal Cell Carcinomas. Lasers Surg. Med. 2008, 40, 300–311. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, e2006742. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; Melo, B.L.; Alves, C.G.; Moreira, A.F.; Mendonça, A.G.; Correia, I.J.; de Melo-Diogo, D. Combining Photothermal-Photodynamic Therapy Mediated by Nanomaterials with Immune Checkpoint Blockade for Metastatic Cancer Treatment and Creation of Immune Memory. Adv. Funct. Mater. 2021, 31, 2010777. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhang, C.; Ye, J.-J.; Zou, M.-Z.; Liu, C.-J.; Zhang, X.-Z. Near-Infrared Light Responsive Nanoreactor for Simultaneous Tumor Photothermal Therapy and Carbon Monoxide-Mediated Anti-Inflammation. ACS Cent. Sci. 2020, 6, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Portilho, F.A.; Cavalcanti, C.E.d.; Miranda-Vilela, A.L.; Estevanato, L.L.C.; Longo, J.P.F.; Santos, M.d.A.; Bocca, A.L.; Martins, O.P.; Simioni, A.R.; Morais, P.C.; et al. Antitumor Activity of Photodynamic Therapy Performed with Nanospheres Containing Zinc-Phthalocyanine. J. Nanobiotechnol. 2013, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapathy, G.J.; George, B.P.; Abrahamse, H. Enhancement of Phthalocyanine Mediated Photodynamic Therapy by Catechin on Lung Cancer Cells. Molecules 2020, 25, 4874. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E. The Potential of South African Plants in the Development of New Medicinal Products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Maroyi, A. Dicoma Anomala Sond.: A Review of Its Botany, Ethnomedicine, Phytochemistry and Pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Chota, A.; George, B.P.; Abrahamse, H. Dicoma Anomala Enhances Phthalocyanine Mediated Photodynamic Therapy in MCF-7 Breast Cancer Cells. Front. Pharmacol. 2022, 13, 892490. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Moghissi, K.; Dixon, K.; Gibbins, S. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 1, e1–e15. [Google Scholar] [CrossRef] [Green Version]
- An, Y.-W.; Jin, H.-T.; Yuan, B.; Wang, J.-C.; Wang, C.; Liu, H.-Q. Research Progress of Berberine Mediated Photodynamic Therapy (Review). Oncol. Lett. 2021, 21, 359. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Straten, D.v.; Mashayekhi, V.; Bruijn, H.S.d.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Paczyński, R.; Dubin, G.; Pereira, M.M.; Arnaut, L.G.; Dąbrowski, J.M. Properties of Halogenated and Sulfonated Porphyrins Relevant for the Selection of Photosensitizers in Anticancer and Antimicrobial Therapies. PLoS ONE 2017, 12, e0185984. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Hernández, F.; Granada-Ramírez, D.A.; Arias-Cerón, J.S.; Rodriguez-Fragoso, P.; Mendoza-Álvarez, J.G.; Ramón-Gallegos, E.; Cruz-Orea, A.; Luna-Arias, J.P. 20-Use of Nanostructured Materials in Drug Delivery. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 503–549. ISBN 978-0-08-100716-7. [Google Scholar]
- Montes de Oca, M.N.; Vara, J.; Milla, L.; Rivarola, V.; Ortiz, C.S. Physicochemical Properties and Photodynamic Activity of Novel Derivatives of Triarylmethane and Thiazine. Arch. Pharm. 2013, 346, 255–265. [Google Scholar] [CrossRef]
- Tynga, I.M.; Houreld, N.N.; Abrahamse, H. The Primary Subcellular Localization of Zinc Phthalocyanine and Its Cellular Impact on Viability, Proliferation and Structure of Breast Cancer Cells (MCF-7). J. Photochem. Photobiol. B 2013, 120, 171–176. [Google Scholar] [CrossRef]
- Mondal, B.; Dutta, T.; Padhy, A.; Das, S.; Sen Gupta, S. Lysosome-Targeting Strategy Using Polypeptides and Chimeric Molecules. ACS Omega 2021, 7, 5–16. [Google Scholar] [CrossRef]
- Huang, P.; Xu, M.; Wu, Y.; Rizvi Syeda, A.K.; Dong, X.-P. Lysosomal Potassium Channels. Handb. Exp. Pharmacol. 2022, 102, 102536. [Google Scholar] [CrossRef]
- Qin, L.; Crawford, J.M. 1-Anatomy and Cellular Functions of the Liver. In Zakim and Boyer’s Hepatology (Seventh Edition); Sanyal, A.J., Boyer, T.D., Lindor, K.D., Terrault, N.A., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 2–19.e4. ISBN 978-0-323-37591-7. [Google Scholar]
- Schwarz, D.S.; Blower, M.D. The Endoplasmic Reticulum: Structure, Function and Response to Cellular Signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Rambani, V.; Hromnikova, D.; Gasperikova, D.; Skopkova, M. Mitochondria and Mitochondrial Disorders: An Overview Update. Endocr. Regul. 2022, 56, 232–248. [Google Scholar] [CrossRef]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Peña-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The Interplay between BAX and BAK Tunes Apoptotic Pore Growth to Control Mitochondrial-DNA-Mediated Inflammation. Mol. Cell. 2022, 82, 933–949.e9. [Google Scholar] [CrossRef]
- Grashei, M.; Biechl, P.; Schilling, F.; Otto, A.M. Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment. Cancers 2022, 14, 1845. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Carbajal-García, A.; Di Mise, A.; Zheng, Y.-M.; Wang, X.; Wang, Y.-X. Important Functions and Molecular Mechanisms of Mitochondrial Redox Signaling in Pulmonary Hypertension. Antioxidants 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef]
- Bouhamdani, N.; Comeau, D.; Turcotte, S. A Compendium of Information on the Lysosome. Front. Cell Dev. Biol. 2021, 9, 798262. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-Specific Drug Release and Reactive Oxygen Species Burst Induced by Polyprodrug Nanoreactors Can Enhance Chemotherapy. Nat. Commun. 2019, 10, 1704. [Google Scholar] [CrossRef] [Green Version]
- Manoto, S.L.; Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Localization and Phototoxic Effect of Zinc Sulfophthalocyanine Photosensitizer in Human Colon (DLD-1) and Lung (A549) Carcinoma Cells (in Vitro). Photodiagn. Photodyn. Ther. 2012, 9, 52–59. [Google Scholar] [CrossRef]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted Photodynamic Therapy as Potential Treatment Modality for the Eradication of Colon Cancer and Colon Cancer Stem Cells. Tumor Biol. 2017, 39, 1010428317734691. [Google Scholar] [CrossRef] [Green Version]
- Khajah, M.A.; Luqmani, Y.A. Involvement of Membrane Blebbing in Immunological Disorders and Cancer. Med. Princ. Pract. 2016, 25, 18–27. [Google Scholar] [CrossRef]
- Tripathy, S.; Rademan, S.; Matsabisa, M.G. Effects of Silver Nanoparticle from Dicoma Anomala Sond. Root Extract on MCF-7 Cancer Cell Line and NF54 Parasite Strain: An In Vitro Study. Biol. Trace Elem. Res. 2020, 195, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Houreld, N.N.; Abrahamse, H. Resistance of Lung Cancer Cells Grown as Multicellular Tumour Spheroids to Zinc Sulfophthalocyanine Photosensitization. Int. J. Mol. Sci. 2015, 16, 10185–10200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Sagbo, I.J.; Otang-Mbeng, W. Plants Used for the Traditional Management of Cancer in the Eastern Cape Province of South Africa: A Review of Ethnobotanical Surveys, Ethnopharmacological Studies and Active Phytochemicals. Molecules 2021, 26, 4639. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Chikezie, P.C.; Chikezie, C.M.; Enyoh, C.E. Anticancer Activity of Nigerian Medicinal Plants: A Review. Future J. Pharm. Sci. 2021, 7, 70. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-Derived Anticancer Agents: A Green Anticancer Approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- George, B.P.; Abrahamse, H.; Parimelazhagan, T. Caspase Dependent Apoptotic Activity of Rubus Fairholmianus Gard. on MCF-7 Human Breast Cancer Cell Lines. J. Appl. Biomed. 2016, 14, 211–219. [Google Scholar] [CrossRef]
- Puangpraphant, S.; Berhow, M.A.; Vermillion, K.; Potts, G.; Gonzalez de Mejia, E. Dicaffeoylquinic Acids in Yerba Mate (Ilex Paraguariensis St. Hilaire) Inhibit NF-ΚB Nucleus Translocation in Macrophages and Induce Apoptosis by Activating Caspases-8 and -3 in Human Colon Cancer Cells. Mol. Nutr. Food Res. 2011, 55, 1509–1522. [Google Scholar] [CrossRef]
- Yang, P.-F.; Feng, Z.-M.; Yang, Y.-N.; Jiang, J.-S.; Zhang, P.-C. Neuroprotective Caffeoylquinic Acid Derivatives from the Flowers of Chrysanthemum Morifolium. J. Nat. Prod. 2017, 80, 1028–1033. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, X.; Guan, Y.; Gong, M.; He, K.; Huang, B. 1,3-Dicaffeoylquinic Acid Targeting 14-3-3 Tau Suppresses Human Breast Cancer Cell Proliferation and Metastasis through IL6/JAK2/PI3K Pathway. Biochem. Pharmacol. 2020, 172, 113752. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkan, H. The Program Cell Death (Apoptosis) and the Therapy of Cancer; IntechOpen: London, UK, 2021; ISBN 978-1-83969-194-2. [Google Scholar]
- Abbas, R.; Larisch, S. Killing by Degradation: Regulation of Apoptosis by the Ubiquitin-Proteasome-System. Cells 2021, 10, 3465. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.M.; Zhan, Y.; Brady, J.L.; Zhang, J.-G.; Sutherland, R.M.; Anstee, N.S.; Schenk, R.L.; Vikstrom, I.B.; Delconte, R.B.; Segal, D.; et al. Anti-Apoptotic Proteins BCL-2, MCL-1 and A1 Summate Collectively to Maintain Survival of Immune Cell Populations Both in Vitro and in Vivo. Cell. Death Differ. 2017, 24, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. In Vitro Combined Effect of Doxorubicin and Sulfonated Zinc Phthalocyanine–Mediated Photodynamic Therapy on MCF-7 Breast Cancer Cells. Tumor Biol. 2017, 39, 1010428317727278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydemir, E.A.; Simsek, E.; Imir, N.; Göktürk, R.S.; Yesilada, E.; Fiskin, K. Cytotoxic and Apoptotic Effects of Ebenus Boissieri Barbey on Human Lung Cancer Cell Line A549. Pharmacogn. Mag. 2015, 11, S37–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell. Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Torcia, M.; Chiara, G.D.; Nencioni, L.; Ammendola, S.; Labardi, D.; Lucibello, M.; Rosini, P.; Marlier, L.N.J.L.; Bonini, P.; Sbarba, P.D.; et al. Nerve Growth Factor Inhibits Apoptosis in Memory B Lymphocytes via Inactivation of P38 MAPK, Prevention of Bcl-2 Phosphorylation, and Cytochrome c Release *. J. Biol. Chem. 2001, 276, 39027–39036. [Google Scholar] [CrossRef] [Green Version]
- Gräb, J.; Rybniker, J. The Expanding Role of P38 Mitogen-Activated Protein Kinase in Programmed Host Cell Death. Microbiol. Insights 2019, 12, 1178636119864594. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular Biology of Bax and Bak Activation and Action. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Vringer, E.; Tait, S.W.G. Mitochondria and Inflammation: Cell Death Heats Up. Front. Cell Dev. Biol. 2019, 7, 100. [Google Scholar] [CrossRef]
- Blandino, G.; Di Agostino, S. New Therapeutic Strategies to Treat Human Cancers Expressing Mutant P53 Proteins. J. Exp. Clin. Cancer Res. 2018, 37, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Towards DNA-Damage Induced Autophagy: A Boolean Model of P53-Induced Cell Fate Mechanisms. DNA Repair 2020, 96, 102971. [Google Scholar] [CrossRef]
- Shi, T.; van Soest, D.M.K.; Polderman, P.E.; Burgering, B.M.T.; Dansen, T.B. DNA Damage and Oxidant Stress Activate P53 through Differential Upstream Signaling Pathways. Free Radic. Biol. Med. 2021, 172, 298–311. [Google Scholar] [CrossRef]
- Liu, S.; Luo, L.; Zuo, F.; Geng, Y.; Ou, Y.; Chen, D.; Yang, S.; Luo, W.; Wang, Y.; Wang, J.; et al. Immunosuppression and Apoptosis Activation Mediated by P53-Bcl2/Bax Signaling Pathway-The Potential Mechanism of Goldfish (Carassius Auratus Linnaeus) Gill Disease Caused by Myxobolus Ampullicapsulatus. Front. Immunol. 2022, 13, 998975. [Google Scholar] [CrossRef] [PubMed]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, R.; Wang, X.; Zhang, H.; Zhu, X.; Chen, J. Lobaplatin-Induced Apoptosis Requires P53-Mediated P38MAPK Activation Through ROS Generation in Non-Small-Cell Lung Cancer. Front. Oncol. 2019, 9, 538. [Google Scholar] [CrossRef]




| Parameter Name | Description |
|---|---|
| Laser type | Semiconductor (Diode) |
| Wave emission | Continuous |
| Wavelength | 680 nm |
| Spectrum | Visible light (Red) |
| Fluency | 10 J/cm2 |
| Output power | 193 mW |
| Spot size | 9.1 cm2 |
| Irradiation time | 8 min, 6 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chota, A.; George, B.P.; Abrahamse, H. In Vitro Cell Death Mechanisms Induced by Dicoma anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells. Cells 2022, 11, 3288. https://doi.org/10.3390/cells11203288
Chota A, George BP, Abrahamse H. In Vitro Cell Death Mechanisms Induced by Dicoma anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells. Cells. 2022; 11(20):3288. https://doi.org/10.3390/cells11203288
Chicago/Turabian StyleChota, Alexander, Blassan P. George, and Heidi Abrahamse. 2022. "In Vitro Cell Death Mechanisms Induced by Dicoma anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells" Cells 11, no. 20: 3288. https://doi.org/10.3390/cells11203288
APA StyleChota, A., George, B. P., & Abrahamse, H. (2022). In Vitro Cell Death Mechanisms Induced by Dicoma anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells. Cells, 11(20), 3288. https://doi.org/10.3390/cells11203288









