Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
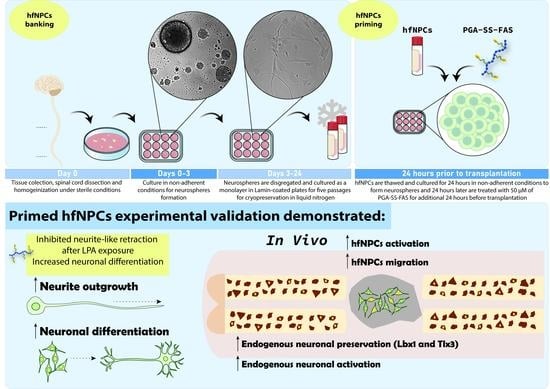
2.1. Isolation and Expansion of hfNPCs
2.2. Priming hfNPCs with PGA-SS-FAS Prior to Transplantation
2.3. hfNPC Proliferation Assay
2.4. Spontaneous hfNPC Differentiation Assay
2.5. Neurite Elongation Assays
2.6. Immunostaining
2.7. Spinal Cord Injury, hfNPC Transplantation, and Tissue Processing
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Human Fetal Spinal Cord NPCs Reside in the Ependymal Central Canal and the Spinal Parenchyma
3.2. hfNPCs Proliferate and Express Canonical Neural Markers in Vitro
3.3. PGA-SS-FAS Priming Enhances the Neuronal and Oligodendroglial Differentiation of hfNPCs
3.4. PGA-SS-FAS Priming Enhances the Ventral Engraftment of hfNPCs, Endogenous Neuronal Activation, and Neuronal Survival After Transplantation into the Injured Spinal Cord
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalamagkas, K.; Tsintou, M.; Seifalian, A.; Seifalian, A.M. Translational Regenerative Therapies for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, H.; Ohkawa, Y.; Saiwai, H.; Yamada, H.; Kubota, K.; Kobayakawa, K.; Akashi, K.; Okano, H.; Iwamoto, Y.; Okada, S. Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat. Commun. 2012, 3, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, S.; Yasuda, A.; Iwai, H.; Takano, M.; Kobayashi, Y.; Nori, S.; Tsuji, O.; Fujiyoshi, K.; Ebise, H.; Toyama, Y.; et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 2013, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Abematsu, M.; Tsujimura, K.; Yamano, M.; Saito, M.; Kohno, K.; Kohyama, J.; Namihira, M.; Komiya, S.; Nakashima, K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Invest. 2010, 120, 3255–3266. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef]
- Ferrari, D.; Gelati, M.; Profico, D.C.; Vescovi, A.L. Human fetal neural stem cells for neurodegenerative disease treatment. In Human Neural Stem Cells; Springer: Berlin/Heidelberg, Germany, 2018; pp. 307–329. [Google Scholar]
- Xu, N.; Xu, T.; Mirasol, R.; Holmberg, L.; Vincent, P.H.; Li, X.; Falk, A.; Benedikz, E.; Rotstein, E.; Seiger, A.; et al. Transplantation of Human Neural Precursor Cells Reverses Syrinx Growth in a Rat Model of Post-Traumatic Syringomyelia. Neurotherapeutics 2021, 18, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.S.; Yun, S.; Lee, H.; Jung, K.; Hwang, K.; Kim, M.; Lee, I.S.; et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural. Plast. 2015, 2015, 630932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15, 723–731. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Xie, Y.; Zhang, L.; Tang, P. Cell Transplantation for Spinal Cord Injury: Tumorigenicity of Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cells. Stem Cells Int. 2018, 2018, 5653787. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, E.; Nebot, V.J.; Dordevic, S.; Requejo-Aguilar, R.; Alastrue-Agudo, A.; Zagorodko, O.; Arminan, A.; Martinez-Rojas, B.; Vicent, M.J.; Moreno-Manzano, V. A rationally designed self-immolative linker enhances the synergism between a polymer-rock inhibitor conjugate and neural progenitor cells in the treatment of spinal cord injury. Biomaterials 2021, 276, 121052. [Google Scholar] [CrossRef]
- Torres-Espin, A.; Santos, D.; Gonzalez-Perez, F.; del Valle, J.; Navarro, X. Neurite-J: An image-J plug-in for axonal growth analysis in organotypic cultures. J. Neurosci. Methods 2014, 236, 26–39. [Google Scholar] [CrossRef]
- Boulland, J.L.; Lambert, F.M.; Zuchner, M.; Strom, S.; Glover, J.C. A neonatal mouse spinal cord injury model for assessing post-injury adaptive plasticity and human stem cell integration. PLoS ONE 2013, 8, e71701. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef] [PubMed]
- Namiki, J.; Tator, C.H. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J. Neuropathol. Exp. Neurol. 1999, 58, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Manzano, V.; Rodriguez-Jimenez, F.J.; Garcia-Rosello, M.; Lainez, S.; Erceg, S.; Calvo, M.T.; Ronaghi, M.; Lloret, M.; Planells-Cases, R.; Sanchez-Puelles, J.M.; et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 2009, 27, 733–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Manzano, V. Ependymal cells in the spinal cord as neuronal progenitors. Curr. Opin. Pharmacol. 2020, 50, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Cervello, C.; Soriano-Navarro, M.; Mirzadeh, Z.; Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Biciliated ependymal cell proliferation contributes to spinal cord growth. J. Comp. Neurol. 2012, 520, 3528–3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef] [Green Version]
- Sansom, S.N.; Griffiths, D.S.; Faedo, A.; Kleinjan, D.J.; Ruan, Y.; Smith, J.; van Heyningen, V.; Rubenstein, J.L.; Livesey, F.J. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 2009, 5, e1000511. [Google Scholar] [CrossRef] [Green Version]
- Canizares, M.A.; Albors, A.R.; Singer, G.; Suttie, N.; Gorkic, M.; Felts, P.; Storey, K.G. Multiple steps characterise ventricular layer attrition to form the ependymal cell lining of the adult mouse spinal cord central canal. J. Anat. 2020, 236, 334–350. [Google Scholar] [CrossRef]
- Shimada, I.S.; LeComte, M.D.; Granger, J.C.; Quinlan, N.J.; Spees, J.L. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J. Neurosci. 2012, 32, 7926–7940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, B.A.; Rietze, R.L. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods 2005, 2, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Zahir, T.; Santaguida, C.; Cook, D.; Tator, C.H. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS ONE 2011, 6, e27079. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Jimenez, F.J.; Clemente, E.; Moreno-Manzano, V.; Erceg, S. Organized Neurogenic-Niche-Like Pinwheel Structures Discovered in Spinal Cord Tissue-Derived Neurospheres. Front. Cell Dev. Biol. 2019, 7, 334. [Google Scholar] [CrossRef] [Green Version]
- Imayoshi, I.; Sakamoto, M.; Yamaguchi, M.; Mori, K.; Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010, 30, 3489–3498. [Google Scholar] [CrossRef] [Green Version]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Collignon, J.; Sockanathan, S.; Hacker, A.; Cohen-Tannoudji, M.; Norris, D.; Rastan, S.; Stevanovic, M.; Goodfellow, P.N.; Lovell-Badge, R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 1996, 122, 509–520. [Google Scholar] [CrossRef]
- Li, X.; Floriddia, E.M.; Toskas, K.; Chalfouh, C.; Honore, A.; Aumont, A.; Vallieres, N.; Lacroix, S.; Fernandes, K.J.L.; Guerout, N.; et al. FoxJ1 regulates spinal cord development and is required for the maintenance of spinal cord stem cell potential. Exp. Cell Res. 2018, 368, 84–100. [Google Scholar] [CrossRef]
- Sun, Y.; Nadal-Vicens, M.; Misono, S.; Lin, M.Z.; Zubiaga, A.; Hua, X.; Fan, G.; Greenberg, M.E. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001, 104, 365–376. [Google Scholar] [CrossRef]
- Brown, J.P.; Couillard-Despres, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Madura, T.; Yamashita, T.; Kubo, T.; Fujitani, M.; Hosokawa, K.; Tohyama, M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. 2004, 5, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranenburg, O.; Poland, M.; van Horck, F.P.; Drechsel, D.; Hall, A.; Moolenaar, W.H. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: Induction of neurite retraction. Mol. Biol. Cell 1999, 10, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.E. Genetic Reporters of Neuronal Activity: C-Fos and G-CaMP6. Methods Enzymol. 2018, 603, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.A.; Miranda, R.M.; Samina, M.C.; Dias, A.F.; Raposo, A.; Oliveira, P.; Reguenga, C.; Castro, D.S.; Lima, D. Tlx3 Exerts Direct Control in Specifying Excitatory Over Inhibitory Neurons in the Dorsal Spinal Cord. Front. Cell Dev. Biol. 2021, 9, 642697. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Knoll, B. CNS axon regeneration inhibitors stimulate an immediate early gene response via MAP kinase-SRF signaling. Mol. Brain 2014, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, C.P.; Holmstrom, N.A.; Lilja, J.A.; Schweinhardt, P.; Hao, J.; Spenger, C.; Wiesenfeld-Hallin, Z.; Kurpad, S.N.; Frisen, J.; Olson, L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005, 8, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Okubo, T.; Nagoshi, N.; Kohyama, J.; Tsuji, O.; Shinozaki, M.; Shibata, S.; Kase, Y.; Matsumoto, M.; Nakamura, M.; Okano, H. Treatment with a Gamma-Secretase Inhibitor Promotes Functional Recovery in Human iPSC- Derived Transplants for Chronic Spinal Cord Injury. Stem Cell Rep. 2018, 11, 1416–1432. [Google Scholar] [CrossRef]
- Elkhenany, H.; Bonilla, P.; Giraldo, E.; Alastrue Agudo, A.; Edel, M.J.; Vicent, M.J.; Roca, F.G.; Ramos, C.M.; Doblado, L.R.; Pradas, M.M.; et al. A Hyaluronic Acid Demilune Scaffold and Polypyrrole-Coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries. Biomedicines 2021, 9, 1928. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, E.; Wallace, K.; Grayden, K.; Kaplan, M. A Review of Neural Stem Cell Transplant Therapy for Traumatic Spinal Cord Injury. SN Compr. Clin. Med. 2021, 3, 1586–1592. [Google Scholar] [CrossRef]
- Dulin, J.N.; Adler, A.F.; Kumamaru, H.; Poplawski, G.H.D.; Lee-Kubli, C.; Strobl, H.; Gibbs, D.; Kadoya, K.; Fawcett, J.W.; Lu, P.; et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun. 2018, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Pathak, Z.; Kumar, H. Strategies to neutralize RhoA/ROCK pathway after spinal cord injury. Exp. Neurol. 2021, 343, 113794. [Google Scholar] [CrossRef]
- Boato, F.; Hendrix, S.; Huelsenbeck, S.C.; Hofmann, F.; Grosse, G.; Djalali, S.; Klimaschewski, L.; Auer, M.; Just, I.; Ahnert-Hilger, G.; et al. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J. Cell Sci. 2010, 123, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, S.; Adamson, C.; Sankar, V.; Gibbs, K.M.; Kane-Goldsmith, N.; Ayer, J.; Babiarz, J.; Kalinski, H.; Ashush, H.; Alpert, E.; et al. Delayed intrathecal delivery of RhoA siRNA to the contused spinal cord inhibits allodynia, preserves white matter, and increases serotonergic fiber growth. J. Neurotrauma 2011, 28, 1063–1076. [Google Scholar] [CrossRef]
- Stern, S.; Hilton, B.J.; Burnside, E.R.; Dupraz, S.; Handley, E.E.; Gonyer, J.M.; Brakebusch, C.; Bradke, F. RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 2021, 109, 3436–3455. [Google Scholar] [CrossRef]
- Shimomura, A.; Patel, D.; Wilson, S.M.; Koehler, K.R.; Khanna, R.; Hashino, E. Tlx3 promotes glutamatergic neuronal subtype specification through direct interactions with the chromatin modifier CBP. PLoS ONE 2015, 10, e0135060. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.K.; Dottori, M.; Goulding, M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 2002, 34, 535–549. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.; Kalita, K.; Knapska, E. c-Fos and neuronal plasticity: The aftermath of Kaczmarek’s theory. Acta Neurobiol. Exp. 2018, 78, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Hu, X.; Zhong, J.F. Mesenchymal Stem Cells: Characteristics, Function, and Application. Stem Cells Int. 2019, 2019, 8106818. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.C.; Tang, R.H.; Yu, Z.Y.; Xie, M.J.; Wang, W.; Luo, X. The Rho-associated kinase inhibitors Y27632 and fasudil promote microglial migration in the spinal cord via the ERK signaling pathway. Neural. Regen. Res. 2018, 13, 677–683. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo, E.; Bonilla, P.; Mellado, M.; Garcia-Manau, P.; Rodo, C.; Alastrue, A.; Lopez, E.; Moratonas, E.C.; Pellise, F.; Đorđević, S.; et al. Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury. Cells 2022, 11, 3304. https://doi.org/10.3390/cells11203304
Giraldo E, Bonilla P, Mellado M, Garcia-Manau P, Rodo C, Alastrue A, Lopez E, Moratonas EC, Pellise F, Đorđević S, et al. Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury. Cells. 2022; 11(20):3304. https://doi.org/10.3390/cells11203304
Chicago/Turabian StyleGiraldo, Esther, Pablo Bonilla, Mara Mellado, Pablo Garcia-Manau, Carlota Rodo, Ana Alastrue, Eric Lopez, Elena Carreras Moratonas, Ferran Pellise, Snežana Đorđević, and et al. 2022. "Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury" Cells 11, no. 20: 3304. https://doi.org/10.3390/cells11203304
APA StyleGiraldo, E., Bonilla, P., Mellado, M., Garcia-Manau, P., Rodo, C., Alastrue, A., Lopez, E., Moratonas, E. C., Pellise, F., Đorđević, S., Vicent, M. J., & Moreno Manzano, V. (2022). Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury. Cells, 11(20), 3304. https://doi.org/10.3390/cells11203304