Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Astrocyte Subtype Gene-Sets
2.2. Mouse Disease Astrocyte Gene-Sets
2.3. Human Astrocyte Gene-Sets
2.4. Schizophrenia hiPSC-Derived Astrocytes
2.5. Disease-Disease Similarity
2.6. Gene Ontology (GO) Analysis
2.7. Density-Index
2.8. Drug-Target Enrichment Analysis
2.9. Rat Studies
2.10. qPCR Studies
2.11. “Look up” Studies
2.12. Identifying Small Molecules to Reverse Astrocyte-Disease Gene Signatures
2.13. Protein kinase Activity Array
3. Results
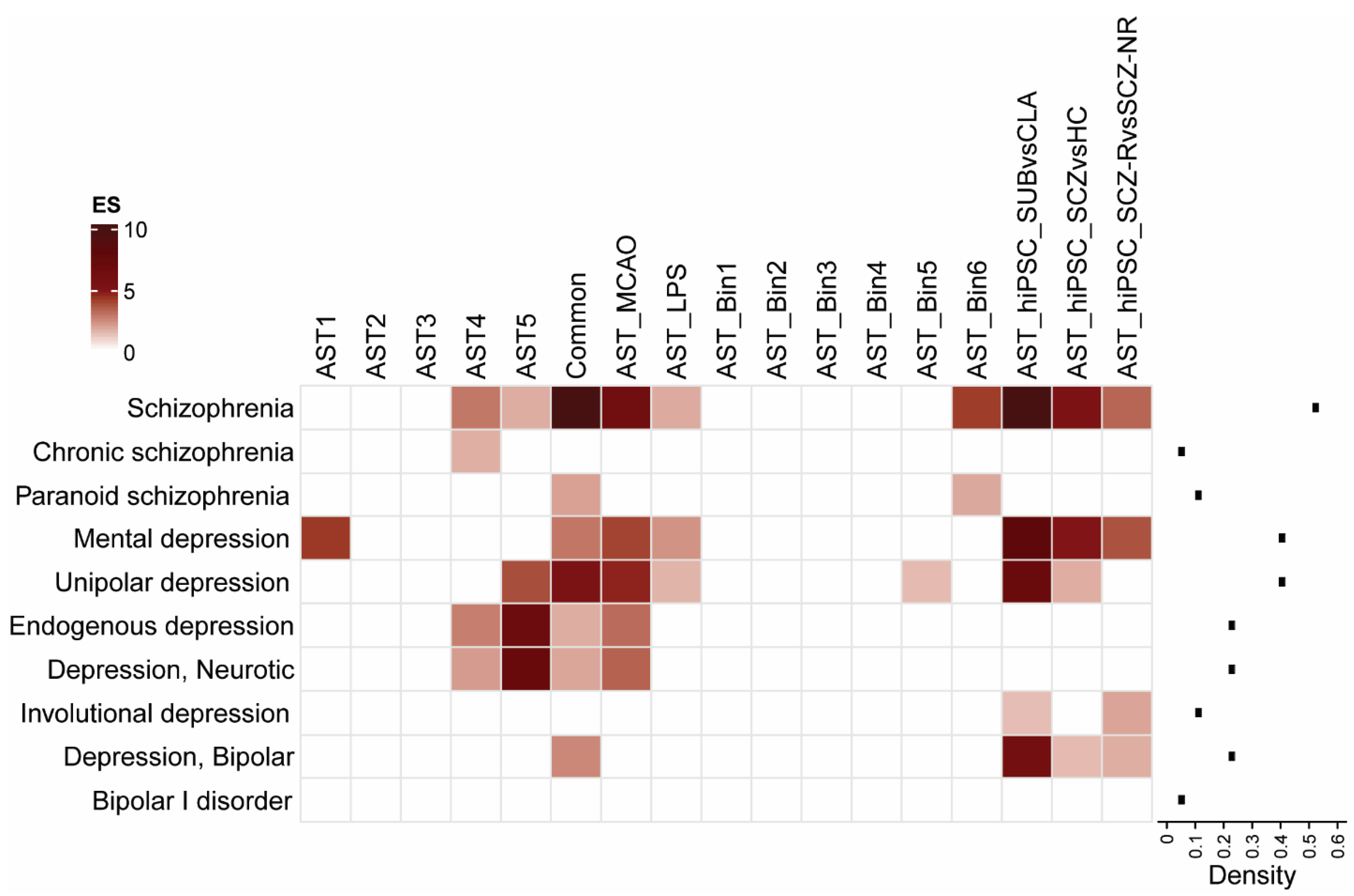
3.1. Astrocyte Subtype Enrichment in Psychiatric Disorders
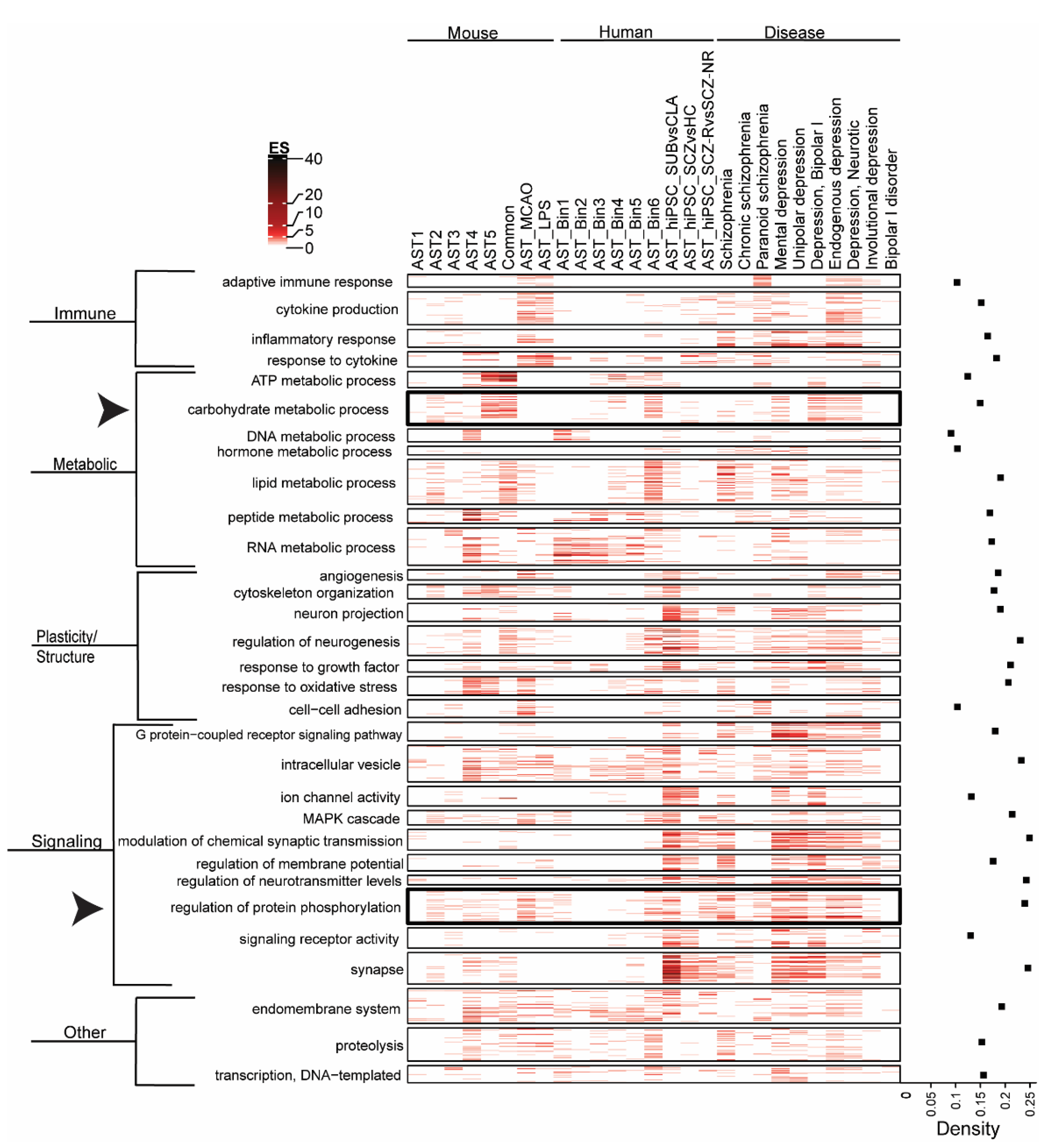
3.2. Biological Pathway Enrichment across Astrocyte Subtypes and Neuropsychiatric Disease
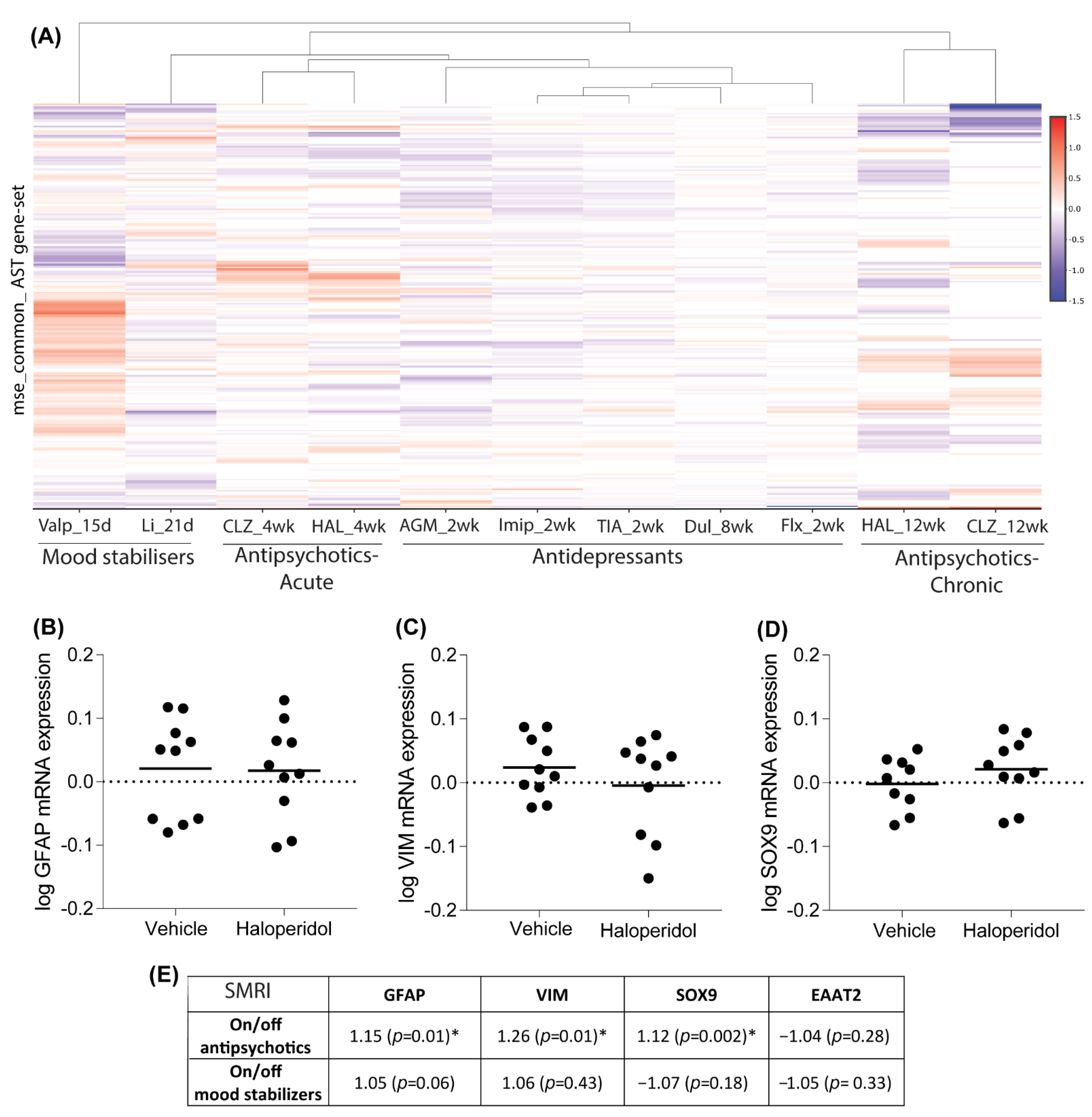
3.3. Psychotropic Medication Effect on Astrocyte Subtypes and Disease
3.4. Effect of Chronic Medication on Astrocyte Marker Expression in Rat Brain
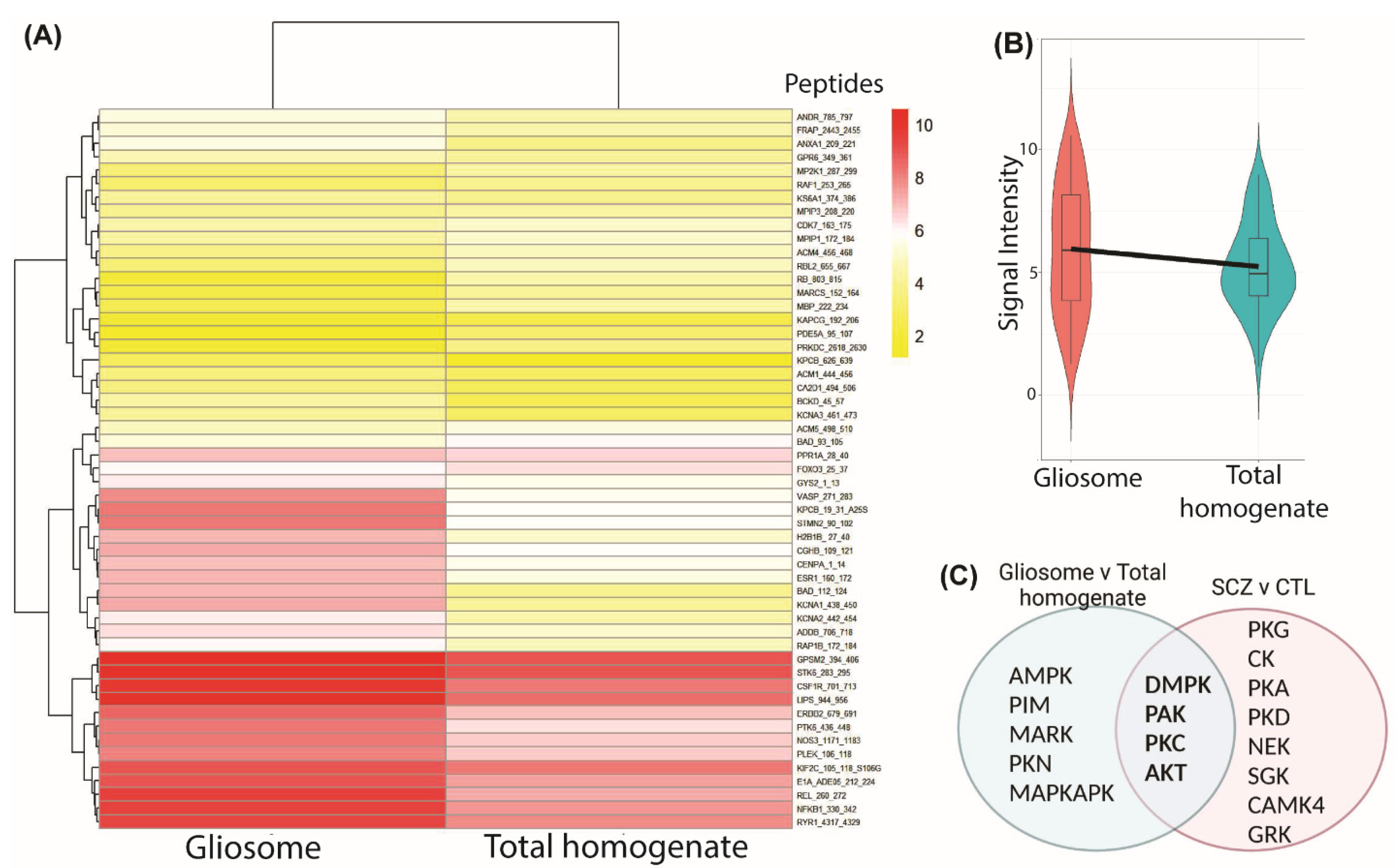
3.5. Exploratory Studies of Common Protein Kinase Activity Profiles in Postmortem Glial Cell Compartments and in Schizophrenia
3.6. Chemical Perturbagens That Reverse the hiPSC_SCZvHC Gene Signature
4. Discussion
4.1. Species, Astrocyte Subtype and Disease-Driven Changes in Gene Enrichment in Psychiatric Disorders
4.2. Limitations
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pekny, M.; Pekna, M.; Messing, A.; Steinhauser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Ransom, B.R. Neuroglia, 3rd ed.; Oxford University Press: New York, NY, USA, 2013; 930p. [Google Scholar]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.T.; Hallak, J.E.; Dunham, J.S.; Deakin, J.F. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci. Lett. 2006, 404, 276–281. [Google Scholar] [CrossRef]
- Markova, E.; Markov, I.; Revishchin, A.; Okhotin, V.; Sulimov, G. 3-D Golgi and image analysis of the olfactory tubercle in schizophrenia. Anal. Quant. Cytol. Histol. 2000, 22, 178–182. [Google Scholar]
- Feresten, A.H.; Barakauskas, V.; Ypsilanti, A.; Barr, A.M.; Beasley, C.L. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr. Res. 2013, 150, 252–257. [Google Scholar] [CrossRef]
- Qi, X.R.; Kamphuis, W.; Shan, L. Astrocyte Changes in the Prefrontal Cortex from Aged Non-suicidal Depressed Patients. Front. Cell. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef]
- Rao, J.S.; Harry, G.J.; Rapoport, S.I.; Kim, H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 2010, 15, 384–392. [Google Scholar] [CrossRef]
- Williams, M.R.; Hampton, T.; Pearce, R.K.; Hirsch, S.R.; Ansorge, O.; Thom, M.; Maier, M. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 41–52. [Google Scholar] [CrossRef]
- Williams, M.; Pearce, R.K.; Hirsch, S.R.; Ansorge, O.; Thom, M.; Maier, M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 357–362. [Google Scholar] [CrossRef]
- Altshuler, L.L.; Abulseoud, O.A.; Foland-Ross, L.; Bartzokis, G.; Chang, S.; Mintz, J.; Hellemann, G.; Vinters, H.V. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010, 12, 541–549. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Nagy, C.; Wakid, M.; Turecki, G.; Mechawar, N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol. Psychiatry 2016, 21, 509–515. [Google Scholar] [CrossRef]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E., Jr.; Akil, H.; Watson, S.J.; et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653–15658. [Google Scholar] [CrossRef]
- Zhao, J.; Verwer, R.W.; van Wamelen, D.J.; Qi, X.R.; Gao, S.F.; Lucassen, P.J.; Swaab, D.F. Prefrontal changes in the glutamate-glutamine cycle and neuronal/glial glutamate transporters in depression with and without suicide. J. Psychiatr. Res. 2016, 82, 8–15. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Laurence, J.A.; Araghi-Niknam, M.; Stary, J.M.; Schulz, S.C.; Lee, S.; Gottesman, I.I. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr. Res. 2004, 69, 317–323. [Google Scholar] [CrossRef]
- Arnold, S.E.; Franz, B.R.; Trojanowski, J.Q.; Moberg, P.J.; Gur, R.E. Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathol. 1996, 91, 269–277. [Google Scholar] [CrossRef]
- Muller, M.B.; Lucassen, P.J.; Yassouridis, A.; Hoogendijk, W.J.; Holsboer, F.; Swaab, D.F. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur. J. Neurosci. 2001, 14, 1603–1612. [Google Scholar] [CrossRef]
- Hercher, C.; Chopra, V.; Beasley, C.L. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. 2014, 39, 376–385. [Google Scholar] [CrossRef]
- Johnston-Wilson, N.L.; Sims, C.D.; Hofmann, J.P.; Anderson, L.; Shore, A.D.; Torrey, E.F.; Yolken, R.H. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol. Psychiatry 2000, 5, 142–149. [Google Scholar] [CrossRef]
- Pakkenberg, B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch. Gen. Psychiatry 1990, 47, 1023–1028. [Google Scholar] [CrossRef]
- Barley, K.; Dracheva, S.; Byne, W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr. Res. 2009, 112, 54–64. [Google Scholar] [CrossRef]
- Bowley, M.P.; Drevets, W.C.; Ongur, D.; Price, J.L. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 2002, 52, 404–412. [Google Scholar] [CrossRef]
- Ongur, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 2001, 58, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Catts, V.S.; Wong, J.; Fillman, S.G.; Fung, S.J.; Shannon Weickert, C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Aust. N. Z. J. Psychiatry 2014, 48, 722–734. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Hercher, C.; Davoli, M.A.; Maussion, G.; Labonte, B.; Turecki, G.; Mechawar, N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 2011, 36, 2650–2658. [Google Scholar] [CrossRef]
- Strakowski, S.M.; Adler, C.M.; Almeida, J.; Altshuler, L.L.; Blumberg, H.P.; Chang, K.D.; DelBello, M.P.; Frangou, S.; McIntosh, A.; Phillips, M.L.; et al. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord. 2012, 14, 313–325. [Google Scholar] [CrossRef]
- Steffek, A.E.; McCullumsmith, R.E.; Haroutunian, V.; Meador-Woodruff, J.H. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr. Res. 2008, 103, 71–82. [Google Scholar] [CrossRef]
- Trepanier, M.O.; Hopperton, K.E.; Mizrahi, R.; Mechawar, N.; Bazinet, R.P. Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Mol. Psychiatry 2016, 21, 1009–1026. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Woo, T.U.; Lim, M.P.; Lange, N.; Berretta, S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch. Gen. Psychiatry 2010, 67, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Damadzic, R.; Bigelow, L.B.; Krimer, L.S.; Goldenson, D.A.; Saunders, R.C.; Kleinman, J.E.; Herman, M.M. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: Absence of significant astrocytosis. Brain Res. Bull. 2001, 55, 611–618. [Google Scholar] [CrossRef]
- Dean, B.; Gray, L.; Scarr, E. Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Aust. N. Z. J. Psychiatry 2006, 40, 217–224. [Google Scholar] [CrossRef]
- Webster, M.J.; Knable, M.B.; Johnston-Wilson, N.; Nagata, K.; Inagaki, M.; Yolken, R.H. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav. Immun. 2001, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.M.; Davidson, J.; Bird, E.D. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch. Gen. Psychiatry 1986, 43, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bruton, C.J.; Crow, T.J.; Frith, C.D.; Johnstone, E.C.; Owens, D.G.; Roberts, G.W. Schizophrenia and the brain: A prospective clinico-neuropathological study. Psychol. Med. 1990, 20, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Makkos, Z.; Meltzer, H.; Overholser, J.; Stockmeier, C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2002, 57, 127–138. [Google Scholar] [CrossRef]
- Schmitt, A.; Steyskal, C.; Bernstein, H.G.; Schneider-Axmann, T.; Parlapani, E.; Schaeffer, E.L.; Gattaz, W.F.; Bogerts, B.; Schmitz, C.; Falkai, P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009, 117, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Parpura, V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017, 27, 629–644. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodriguez, J.J.; Steardo, L. Astrogliopathology: A central element of neuropsychiatric diseases? Neuroscientist 2014, 20, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Augusto-Oliveira, M.; Pivoriunas, A.; Popov, A.; Brazhe, A.; Semyanov, A. Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain. Pflüg. Arch. 2021, 473, 753–774. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Eddleston, M.; Mucke, L. Molecular profile of reactive astrocytes—Implications for their role in neurologic disease. Neuroscience 1993, 54, 15–36. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Rothstein, J.D. Mechanisms of Disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006, 2, 679–689. [Google Scholar] [CrossRef]
- Correa-Cerro, L.S.; Mandell, J.W. Molecular mechanisms of astrogliosis: New approaches with mouse genetics. J. Neuropathol. Exp. Neurol. 2007, 66, 169–176. [Google Scholar] [CrossRef]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 2009, 190, 159–170. [Google Scholar] [CrossRef]
- Simard, M.; Nedergaard, M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 2004, 129, 877–896. [Google Scholar] [CrossRef]
- Swanson, R.A.; Ying, W.; Kauppinen, T.M. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 2004, 4, 193–205. [Google Scholar] [CrossRef]
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Hamby, M.E.; Hewett, J.A.; Hewett, S.J. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia 2006, 54, 566–577. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodrigues, J.J.; Pivoriunas, A.; Zorec, R.; Semyanov, A. Astroglial atrophy in Alzheimer’s disease. Pflüg. Arch. 2019, 471, 1247–1261. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Norton, W.T.; Aquino, D.A.; Hozumi, I.; Chiu, F.C.; Brosnan, C.F. Quantitative aspects of reactive gliosis: A review. Neurochem. Res. 1992, 17, 877–885. [Google Scholar] [CrossRef]
- Zhang, X.; Alnafisah, R.S.; Hamoud, A.A.; Shukla, R.; Wen, Z.; McCullumsmith, R.E.; O’Donovan, S.M. Role of Astrocytes in Major Neuropsychiatric Disorders. Neurochem. Res. 2021, 46, 2715–2730. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Testen, A.; Kim, R.; Reissner, K.J. High-Resolution Three-Dimensional Imaging of Individual Astrocytes Using Confocal Microscopy. Curr. Protoc. Neurosci. 2020, 91, e92. [Google Scholar] [CrossRef]
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, R.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Tollefson, G.D.; Charles, C.; Zipursky, R.; Sharma, T.; Kahn, R.S.; Keefe, R.S.; Green, A.I.; Gur, R.E.; McEvoy, J.; et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry 2005, 62, 361–370. [Google Scholar] [CrossRef]
- Konopaske, G.T.; Dorph-Petersen, K.A.; Sweet, R.A.; Pierri, J.N.; Zhang, W.; Sampson, A.R.; Lewis, D.A. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol. Psychiatry 2008, 63, 759–765. [Google Scholar] [CrossRef]
- Konopaske, G.T.; Dorph-Petersen, K.A.; Pierri, J.N.; Wu, Q.; Sampson, A.R.; Lewis, D.A. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology 2007, 32, 1216–1223. [Google Scholar] [CrossRef]
- Martin, J.L.; Magistretti, P.J.; Allaman, I. Regulation of neurotrophic factors and energy metabolism by antidepressants in astrocytes. Curr. Drug Targets 2013, 14, 1308–1321. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Waltzer, R.; Whittom, A.A.; Austin, M.C.; Rajkowska, G.; Stockmeier, C.A. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J. Affect. Disord. 2010, 127, 230–240. [Google Scholar] [CrossRef]
- Rajkowska, G.; Clarke, G.; Mahajan, G.; Licht, C.M.; van de Werd, H.J.; Yuan, P.; Stockmeier, C.A.; Manji, H.K.; Uylings, H.B. Differential effect of lithium on cell number in the hippocampus and prefrontal cortex in adult mice: A stereological study. Bipolar Disord. 2016, 18, 41–51. [Google Scholar] [CrossRef]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Hribkova, H.; Grabiec, M.; Budinska, E.; Szabo, A.; Kasparek, T.; Andreassen, O.A.; Sun, Y.M.; Djurovic, S. Derivation and Molecular Characterization of a Morphological Subpopulation of Human iPSC Astrocytes Reveal a Potential Role in Schizophrenia and Clozapine Response. Schizophr. Bull. 2022, 48, 190–198. [Google Scholar] [CrossRef]
- Pinero, J.; Ramirez-Anguita, J.M.; Sauch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Williams, A.G.; Thomas, S.; Wyman, S.K.; Holloway, A.K. RNA-seq Data: Challenges in and Recommendations for Experimental Design and Analysis. Curr. Protoc. Hum. Genet. 2014, 83, 11.13.1–11.13.20. [Google Scholar] [CrossRef]
- Holmans, P. Statistical methods for pathway analysis of genome-wide data for association with complex genetic traits. Adv. Genet. 2010, 72, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Analysing biological pathways in genome-wide association studies. Nat. Rev. Genet. 2010, 11, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Fuxman Bass, J.I.; Diallo, A.; Nelson, J.; Soto, J.M.; Myers, C.L.; Walhout, A.J. Using networks to measure similarity between genes: Association index selection. Nat. Methods 2013, 10, 1169–1176. [Google Scholar] [CrossRef]
- Shukla, R.; Prevot, T.D.; French, L.; Isserlin, R.; Rocco, B.R.; Banasr, M.; Bader, G.D.; Sibille, E. The Relative Contributions of Cell-Dependent Cortical Microcircuit Aging to Cognition and Anxiety. Biol. Psychiatry 2019, 85, 257–267. [Google Scholar] [CrossRef]
- Smail, M.A.; Wu, X.; Henkel, N.D.; Eby, H.M.; Herman, J.P.; McCullumsmith, R.E.; Shukla, R. Similarities and dissimilarities between psychiatric cluster disorders. Mol. Psychiatry 2021, 26, 4853–4863. [Google Scholar] [CrossRef]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug signatures database for gene set analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Hasselfeld, K.; Bauer, D.; Simmons, M.; Roussos, P.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia. Transl. Psychiatry 2015, 5, e579. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Sullivan, C.; Koene, R.; Devine, E.; Hasselfeld, K.; Moody, C.L.; McCullumsmith, R.E. Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacology 2018, 43, 1667–1674. [Google Scholar] [CrossRef]
- Alganem, K.; Shukla, R.; Eby, H.; Abel, M.; Zhang, X.; McIntyre, W.B.; Lee, J.; Au-Yeung, C.; Asgariroozbehani, R.; Panda, R.; et al. Kaleidoscope: A New Bioinformatics Pipeline Web Application for In Silico Hypothesis Exploration of Omics Signatures. bioRxiv 2020. [Google Scholar] [CrossRef]
- Torrey, E.F.; Webster, M.; Knable, M.; Johnston, N.; Yolken, R.H. The stanley foundation brain collection and neuropathology consortium. Schizophr. Res. 2000, 44, 151–155. [Google Scholar] [CrossRef]
- Higgs, B.W.; Elashoff, M.; Richman, S.; Barci, B. An online database for brain disease research. BMC Genom. 2006, 7, 70. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Imami, A.; Eby, H.; Henkel, N.D.; Creeden, J.F.; Asah, S.; Zhang, X.; Wu, X.; Alnafisah, R.; Taylor, R.T.; et al. Identification of candidate repurposable drugs to combat COVID-19 using a signature-based approach. Sci. Rep. 2021, 11, 4495. [Google Scholar] [CrossRef]
- Chadha, R.; Alganem, K.; McCullumsmith, R.E.; Meador-Woodruff, J.H. mTOR kinase activity disrupts a phosphorylation signaling network in schizophrenia brain. Mol. Psychiatry 2021, 26, 6868–6879. [Google Scholar] [CrossRef]
- Bentea, E.; Depasquale, E.A.K.; O’Donovan, S.M.; Sullivan, C.R.; Simmons, M.; Meador-Woodruff, J.H.; Zhou, Y.; Xu, C.; Bai, B.; Peng, J.; et al. Kinase network dysregulation in a human induced pluripotent stem cell model of DISC1 schizophrenia. Mol. Omics 2019, 15, 173–188. [Google Scholar] [CrossRef]
- McGuire, J.L.; Hammond, J.H.; Yates, S.D.; Chen, D.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Altered serine/threonine kinase activity in schizophrenia. Brain Res. 2014, 1568, 42–54. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Sullivan, C.R.; McCullumsmith, R.E. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr. 2017, 3, 32. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.L.; Funk, A.J.; Devine, E.; Homan, R.C.D.; Boison, D.; McCullumsmith, R.E.; O’Donovan, S.M. Adenosine Kinase Expression in the Frontal Cortex in Schizophrenia. Schizophr. Bull. 2020, 46, 690–698. [Google Scholar] [CrossRef]
- McGuire, J.L.; Depasquale, E.A.; Funk, A.J.; O’Donovan, S.M.; Hasselfeld, K.; Marwaha, S.; Hammond, J.H.; Hartounian, V.; Meador-Woodruff, J.H.; Meller, J.; et al. Abnormalities of signal transduction networks in chronic schizophrenia. NPJ Schizophr. 2017, 3, 30. [Google Scholar] [CrossRef]
- Stockmeier, C.A.; Mahajan, G.J.; Konick, L.C.; Overholser, J.C.; Jurjus, G.J.; Meltzer, H.Y.; Uylings, H.B.; Friedman, L.; Rajkowska, G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 2004, 56, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Stockmeier, C.A.; Rajkowska, G. Cellular abnormalities in depression: Evidence from postmortem brain tissue. Dialogues Clin. Neurosci. 2004, 6, 185–197. [Google Scholar] [CrossRef]
- Si, X.; Miguel-Hidalgo, J.J.; O’Dwyer, G.; Stockmeier, C.A.; Rajkowska, G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology 2004, 29, 2088–2096. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Pembroke, W.G.; Rexach, J.E.; Godoy, M.I.; Condro, M.C.; Alvarado, A.G.; Harteni, M.; Chen, Y.W.; Stiles, L.; et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 2021, 12, 3958. [Google Scholar] [CrossRef]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar] [CrossRef]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef]
- Sullivan, C.R.; O’Donovan, S.M.; McCullumsmith, R.E.; Ramsey, A. Defects in Bioenergetic Coupling in Schizophrenia. Biol. Psychiatry 2018, 83, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.R.; Koene, R.H.; Hasselfeld, K.; O’Donovan, S.M.; Ramsey, A.; McCullumsmith, R.E. Neuron-specific deficits of bioenergetic processes in the dorsolateral prefrontal cortex in schizophrenia. Mol. Psychiatry 2019, 24, 1319–1328. [Google Scholar] [CrossRef]
- Peng, L.; Li, B.; Verkhratsky, A. Targeting astrocytes in bipolar disorder. Expert Rev. Neurother. 2016, 16, 649–657. [Google Scholar] [CrossRef][Green Version]
- Rivera, A.D.; Butt, A.M. Astrocytes are direct cellular targets of lithium treatment: Novel roles for lysyl oxidase and peroxisome-proliferator activated receptor-gamma as astroglial targets of lithium. Transl. Psychiatry 2019, 9, 211. [Google Scholar] [CrossRef]
- Ravindran, N.; McKay, M.; Paric, A.; Johnson, S.; Chandrasena, R.; Abraham, G.; Ravindran, A.V. Randomized, Placebo-Controlled Effectiveness Study of Quetiapine XR in Comorbid Depressive and Anxiety Disorders. J. Clin. Psychiatry 2022, 83, 3. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Lovatt, D.; Xu, J.; Song, D.; Goldman, S.A.; Nedergaard, M.; Hertz, L.; Peng, L. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 2010, 6, 113–125. [Google Scholar] [CrossRef]
- Hertz, L.; Rothman, D.L.; Li, B.; Peng, L. Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: A potential paradigm shift. Front. Behav. Neurosci. 2015, 9, 25. [Google Scholar] [CrossRef]
- Sethi, P.; Virmani, G.; Gupta, K.; Thumu, S.C.R.; Ramanan, N.; Marathe, S. Automated morphometric analysis with SMorph software reveals plasticity induced by antidepressant therapy in hippocampal astrocytes. J. Cell Sci. 2021, 134, jcs258430. [Google Scholar] [CrossRef]
- Bouvier, M.L.; Fehsel, K.; Schmitt, A.; Meisenzahl-Lechner, E.; Gaebel, W.; von Wilmsdorff, M. Sex-dependent alterations of dopamine receptor and glucose transporter density in rat hypothalamus under long-term clozapine and haloperidol medication. Brain Behav. 2020, 10, e01694. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Ji, M.; Zhang, D.; Chen, B.; Gong, W.; Li, X.; Zhou, Y.; Dong, C.; Wen, G.; et al. The neuroprotective mechanism of lithium after ischaemic stroke. Commun. Biol. 2022, 5, 105. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Davoli, M.A.; Belliveau, C.; Tanti, A.; Ma, J.C.; Farmer, W.T.; Turecki, G.; Murai, K.K.; Mechawar, N. Characterization of Vimentin-Immunoreactive Astrocytes in the Human Brain. Front. Neuroanat. 2020, 14, 31. [Google Scholar] [CrossRef]
- Quist, E.; Ahlenius, H.; Canals, I. Transcription Factor Programming of Human Pluripotent Stem Cells to Functionally Mature Astrocytes for Monocultures and Cocultures with Neurons. Methods Mol. Biol. 2021, 2352, 133–148. [Google Scholar] [CrossRef]
- Balouch, B.; Funnell, J.L.; Ziemba, A.M.; Puhl, D.L.; Lin, K.; Gottipati, M.K.; Gilbert, R.J. Conventional immunomarkers stain a fraction of astrocytes in vitro: A comparison of rat cortical and spinal cord astrocytes in naive and stimulated cultures. J. Neurosci. Res. 2021, 99, 806–826. [Google Scholar] [CrossRef]
- Dragic, M.; Zaric, M.; Mitrovic, N.; Nedeljkovic, N.; Grkovic, I. Two Distinct Hippocampal Astrocyte Morphotypes Reveal Subfield-Different Fate during Neurodegeneration Induced by Trimethyltin Intoxication. Neuroscience 2019, 423, 38–54. [Google Scholar] [CrossRef]

| Drug | Score | LINCS ID | Cell Line | Dose | Timepoint | Drug Class |
|---|---|---|---|---|---|---|
| Antipsychotics | ||||||
| Promazine | −0.656 | LINCSCP_235798 | MCF7 | 10 uM | 6 h | antipsychotic |
| Chlorpromazine | −0.493 | LINCSCP_21936 | HEPG2 | 10 uM | 6 h | antipsychotic |
| Trifluoperazine | −0.474 | LINCSCP_64508 | VCAP | 5 uM | 24 h | antipsychotic |
| Phenothiazine | −0.464 | LINCSCP_209948 | HA1E | 10 uM | 24 h | antipsychotic |
| Fluphenazine | −0.460 | LINCSCP_22209 | HEPG2 | 10 uM | 6 h | antipsychotic |
| Thioridazine | −0.511 | LINCSCP_50729 | RKO | 10 uM | 6 h | antipsychotic |
| Trifluoperazine | −0.474 | LINCSCP_64508 | VCAP | 5 uM | 24 h | antipsychotic |
| Kinase inhibitors | ||||||
| Neratinib | −0.673 | LINCSCP_163133 | SKBR3 | 10 uM | 3 h | kinase inhibitor |
| Vemurafenib | −0.658 | LINCSCP_162826 | SKBR3 | 1.11 uM | 24 h | kinase inhibitor |
| Tivozanib | −0.653 | LINCSCP_128031 | HUES3 | 0.37 uM | 24 h | kinase inhibitor |
| Everolimus | −0.618 | LINCSCP_121310 | HT29 | 0.37 uM | 24 h | kinase inhibitor |
| Dacomitinib | −0.602 | LINCSCP_84161 | ASC | 3.33 uM | 24 h | kinase inhibitor |
| Other drugs | ||||||
| Oxandrolone | −0.689 | LINCSCP_34288 | MCF7 | 10 uM | 24 h | Anabolic steroid |
| Vorinostat | −0.636 | LINCSCP_67113 | HT29 | 10 uM | 6 h | HDAC inhibitor |
| Cholic acid | −0.631 | LINCSCP_258416 | NEU | 10 uM | 24 h | Bile acid |
| Aminosalicylic acid | −0.631 | LINCSCP_141713 | MCF7 | 10 uM | 24 h | Antitubercular agent |
| Tadalafil | −0.623 | LINCSCP_144322 | MCF7 | 0.04 uM | 24 h | Phosphodiesterase 5 inhibitor |
| Omacetaxine mepesuccinate | −0.623 | LINCSCP_126799 | HT29 | 0.37 uM | 24 h | protein synthesis inhibitor |
| Abiraterone acetate | −0.613 | LINCSCP_153115 | PC3 | 10 uM | 24 h | antiandrogen |
| Mebendazole | −0.611 | LINCSCP_27148 | HT29 | 10 uM | 6 h | anthelmintic |
| Bisacodyl | −0.609 | LINCSCP_30211 | MCF7 | 10 uM | 6 h | stimulant laxative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wolfinger, A.; Wu, X.; Alnafisah, R.; Imami, A.; Hamoud, A.-r.; Lundh, A.; Parpura, V.; McCullumsmith, R.E.; Shukla, R.; et al. Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets. Cells 2022, 11, 3315. https://doi.org/10.3390/cells11203315
Zhang X, Wolfinger A, Wu X, Alnafisah R, Imami A, Hamoud A-r, Lundh A, Parpura V, McCullumsmith RE, Shukla R, et al. Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets. Cells. 2022; 11(20):3315. https://doi.org/10.3390/cells11203315
Chicago/Turabian StyleZhang, Xiaolu, Alyssa Wolfinger, Xiaojun Wu, Rawan Alnafisah, Ali Imami, Abdul-rizaq Hamoud, Anna Lundh, Vladimir Parpura, Robert E. McCullumsmith, Rammohan Shukla, and et al. 2022. "Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets" Cells 11, no. 20: 3315. https://doi.org/10.3390/cells11203315
APA StyleZhang, X., Wolfinger, A., Wu, X., Alnafisah, R., Imami, A., Hamoud, A.-r., Lundh, A., Parpura, V., McCullumsmith, R. E., Shukla, R., & O’Donovan, S. M. (2022). Gene Enrichment Analysis of Astrocyte Subtypes in Psychiatric Disorders and Psychotropic Medication Datasets. Cells, 11(20), 3315. https://doi.org/10.3390/cells11203315









